Abstract
A feature of fear memory is its persistence, which could be a factor for affective disorders. Memory retrieval destabilizes consolidated memories, and then rapid molecular cascades contribute to early stabilization of reactivated memories. However, persistence of reactivated memories has been poorly understood. Here, we discover that late Arc (also known as Arg3.1) expression in the mouse basolateral amygdala (BLA) is involved in persistence of newly-acquired and reactivated fear memories. After both fear learning and retrieval, Arc levels increased at 2 h, returned to basal levels at 6 h but increased again at 12 h. Inhibiting late Arc expression impaired memory retention 7 d, but not 2 d, after fear learning and retrieval. Moreover, blockade of NR2B-containing N-methyl-D-aspartate receptors (NMDARs) prevented memory destabilization and inhibited late Arc expression. These findings indicate that NR2B-NMDAR and late Arc expression plays a critical role in the destabilization and persistence of reactivated memories.
A persistent fear memory could be a factor for affective disorders. For example, the characteristics of post-traumatic disorder (PTSD) is that the patients suffer flashbacks or avoidance of memories of traumatic events for more than a month after the events occurred. Erasing the memories of traumatic events is expected to be a novel approach to treating PTSD1. Although early memory formation is built on rapid molecular cascades within a brief time window after learning2,3,4,5,6,7, the mechanisms for memory persistence are less understood. A few recent studies have found that inhibition of brain-derived neurotrophic factor (BDNF) or c-Fos at 12–24 h after learning impairs memory retention at 7 d but not 2 d after learning8,9,10. They have proposed that the delayed biochemical changes are possible regulators of memory persistence.
Reactivation of memories destabilizes once consolidated memories, and their early stabilization is based on de novo gene expression11,12,13. It is possible that persistence of reactivated memories, as well as new memories, requires additional cellular signaling. In fact, we have previously shown that inhibition of protein synthesis at 12 h after memory retrieval impaired memory retention at 7 d but not 2 d after memory retrieval14. However, the precise molecular mechanisms for persistence of reactivated memories are unclear.
Arc (also known as Arg3.1) is an immediate early gene that is required for the consolidation of synaptic plasticity and long-term memory15,16,17. Arc expression is rapidly upregulated following both initial learning and memory retrieval, and is required for early stabilization of newly acquired and reactivated memories15,16,17,18,19. We previously found that late-phase Arc expression in the hippocampus after learning is involved in the persistence of newly-acquired memories20. Thus, we hypothesized that late phase of Arc expression contributes to persistence of reactivated memories as well as newly acquired memories. In this study, we characterized Arc expression in the basolateral amygdala (BLA) after contextual fear conditioning and fear memory retrieval, and revealed the role of late Arc expression in newly acquired and reactivated memories. Then, we investigated the mechanisms that prime late Arc expression.
Results
Early and late Arc expression after contextual fear learning and retrieval
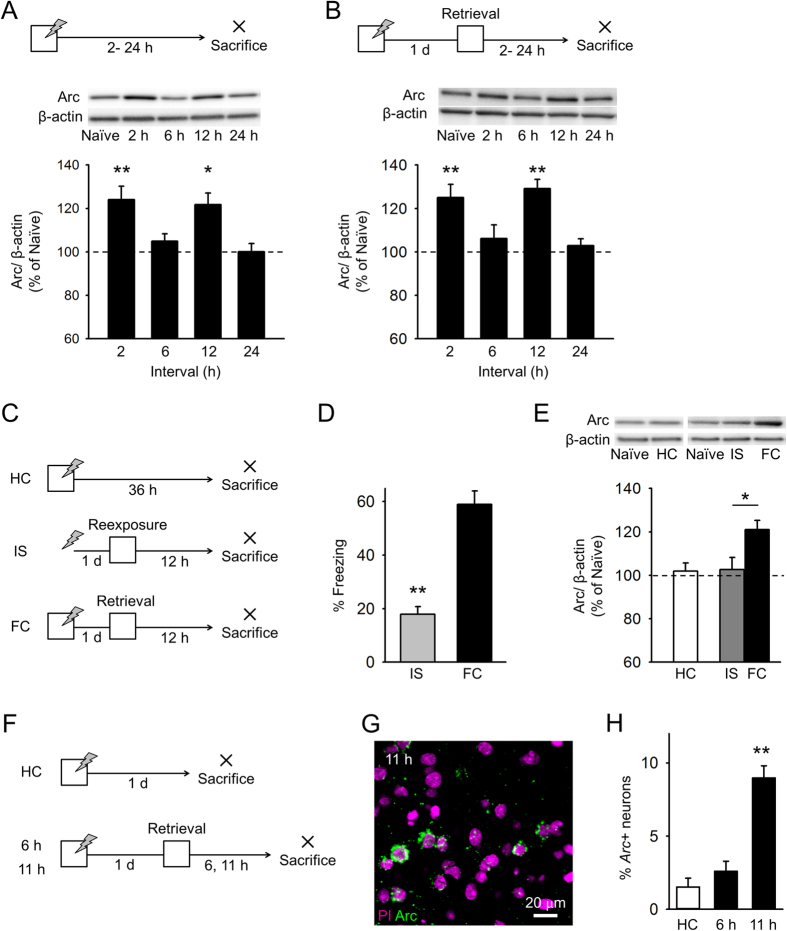
We measured BLA Arc levels following contextual fear conditioning. Mice were sacrificed 2, 6, 12, and 24 h after fear conditioning, and BLA lysates were subjected to western blotting with an anti-Arc antibody (Fig. 1A). Compared to those of naïve control mice, following fear conditioning, BLA Arc levels increased at 2 h, returned to basal levels at 6 h, increased again at 12 h, and returned to basal levels at 24 h (Fig. 1A).
Figure 1.
Early and late Arc expression following contextual fear learning and retrieval (A) Arc levels in the BLA increased 2 and 12 h after learning, but not 6 and 24 h after learning, compared to control levels, n = 10 mice per group. (B) Arc levels increased 2 and 12 h after context re-exposure, but not 6 and 24 h after, compared to control levels. n = 10 mice per group. (C) The experimental procedure for (D,E) (HC, n = 10 mice; IS, n = 13 mice; FC, n = 13 mice). (D) The IS group exhibited less freezing behavior than the FC group. (E) BLA Arc levels in the HC group were comparable to those in naïve mice (n = 10 mice). BLA Arc levels in the IS group were comparable to those in naïve mice (n = 13 mice) and were lower than those in the FC group. (F) The experimental procedure for (G,H) (HC, n = 6 mice; 6 h, n = 3 mice; 11 h, n = 6 mice). (G) Representative image of Arc RNA expression in the BLA 11 h after fear memory retrieval. (H) The proportion of Arc + neurons in the BLA increased 12 h after fear memory retrieval. **P < 0.01, *P < 0.05.
Next, we characterized Arc expression after fear memory retrieval. We subjected mice to contextual fear conditioning on day 1 and re-exposure to a conditioned context on day 2 (Fig. 1B). Mice exhibited freezing behavior upon exposure to the conditioned context (freezing time; 2 h, 63.4 ± 4.9%; 6 h, 57.5 ± 4.3%; 12 h, 59.4 ± 2.5%; 24 h, 59.0 ± 3.2%), indicating that they recalled a fear memory. Mice were sacrificed 2, 6, 12, or 24 h after re-exposure. Compared to those of naïve control mice, following re-exposure, BLA Arc levels increased at 2 h, returned to basal levels at 6 h, increased again at 12 h, and returned to basal levels at 24 h (Fig. 1B). When mice received fear conditioning and were sacrificed 36 h later without re-exposure to the conditioned context (Fig. 1C, homecage (HC) group), Arc levels at this time point were comparable to basal levels (Fig. 1E). In addition, Arc expression was measured in mice subjected to an immediate shock session instead of a fear conditioning session (Fig. 1C, IS group). The immediate shock protocol temporally dissociated the context and shock exposure in mice and resulted in poor memory retention21. Freezing time of mice receiving the immediate shock was shorter than that of mice given standard fear conditioning (Fig. 1D), although it was still longer than the freezing time reported in previous studies22 possibly because the overall duration of shock was longer (6 s). Arc levels 12 h after re-exposure to the context were comparable to basal levels (Fig. 1E). These data indicate that late Arc expression depends on retrieval of an associative fear memory.
In addition, we investigated whether late Arc protein expression (at 12 h) was accompanied by an increase in Arc transcription. We prepared different mice and sacrificed 6 or 11 h after re-exposure to the conditioned context (Fig. 1F). Brain slices, including the BLA, were subjected to fluorescent in situ hybridization (Fig. 1G). We measured the proportion of neurons expressing cytoplasmic Arc, which is detected for longer time than nuclear Arc. The proportion of Arc+ neurons in the BLA at 11 h, but not at 6 h, was greater than that at basal levels (Fig. 1H). These results suggest that fear memory retrieval induces late Arc expression in the BLA through increased transcription.
Late Arc expression contributes to the persistence of newly-acquired fear memory
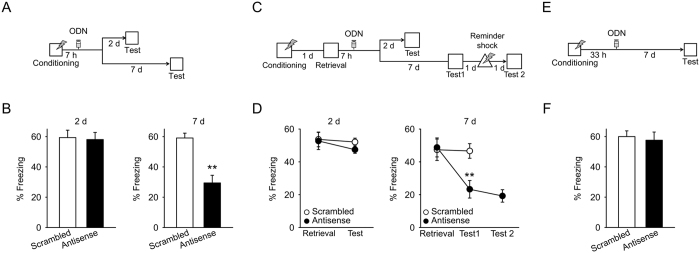
To investigate the effects of late Arc expression on newly-acquired fear memories, we employed Arc antisense oligodeoxynucleotides (ODNs). We previously confirmed that infusion of an Arc antisense ODN (400 pmol/hemisphere) into the dorsal hippocampus inhibits hippocampal Arc expression (5 h after infusion) and has no effect on the expression of other immediate-early genes23. We also confirmed that infusion of an Arc antisense ODN inhibited late Arc expression following memory retrieval in the BLA (Supplementary Fig. S1). Then, we infused Arc antisense or scrambled ODNs into the BLA 7 h after fear conditioning (Supplementary Fig. S2), and 2 d later, mice were placed into the conditioned context to test memory retention (Fig. 2A). In contrast to a previous study reporting that inhibition of early Arc expression impairs freezing behavior in a test performed 1 d after infusion of an antisense ODN15, inhibition of late Arc expression did not affect freezing behavior 2 d after infusion (Fig. 2B, left). Next, we prepared different mice and tested memory retention 7 d later, instead of 2 d after receiving Arc antisense or scrambled ODN infusions (Fig. 2A). Inhibition of late Arc expression disrupted freezing behavior in the 7 d test (Fig. 2B, right). These results indicate that late Arc expression in the BLA contributes to the persistence but not formation of newly-acquired memories.
Figure 2. Late Arc expression contributes to persistence of newly-acquired and reactivated fear memories.
(A) The experimental procedure for (B). (B) (Left) Arc antisense ODN treatment 7 h after conditioning did not affect freezing behavior in the 2 d test (n = 9 mice). (Right) Arc antisense ODN treatment 7 h after conditioning disrupted freezing behavior in the 7 d test (n = 8, 9 mice). (C) The experimental procedure for (D). (D) (Left) Arc antisense ODN treatment 7 h after re-exposure had no effect on freezing behavior in the 2 d test (n = 8 mice). (Right) Arc antisense ODN treatment 7 h after re-exposure impaired freezing behavior in the 7 d test (n = 7 mice). The reminder shock did not affect freezing behavior in a subsequent test. (E) The experimental procedure for (F). (F) Arc antisense ODN treatment in the absence of memory retrieval did not affect freezing behavior in the 7 d test (n = 7 mice). Histological verification of cannula placements are shown in supplementary Fig. S1. **P < 0.01, *P < 0.05.
Late Arc expression contributes to the persistence of reactivated fear memory
To test whether late Arc expression after memory retrieval, as well as after initial learning, contributes to memory persistence, we infused Arc antisense or scrambled ODN into the BLA 7 h after re-exposure to the conditioned context (Fig. 2C). Memory retention was tested either 2 d or 7 d later. Inhibition of late Arc expression had no effect on freezing behavior during the 2 d test but disrupted freezing behavior in the 7 d test (Fig. 2D). This result suggests that late Arc expression after memory retrieval contributes to memory persistence. However, it is possible that decrease in freezing behavior observed after inhibition of late Arc expression was the result of increased fear extinction rather than memory impairment. If this was the case, a weak immediate shock (reminder shock) would reinstate conditioned fear24,45. To test this possibility, we gave a reminder shock to mice receiving the Arc antisense ODN 1 d after testing memory retention, and examined memory retention again 1 d later (Fig. 2C). The reminder shock did not increase freezing behavior (Fig. 2D, right), suggesting that the observed decrease in freezing behavior was not due to increased fear extinction. Furthermore, we tested whether the memory impairment resulting from infusion of Arc antisense ODN depended on memory retrieval. Mice were infused with Arc antisense or scrambled ODNs into the BLA 31 h after fear conditioning (without memory retrieval), and memory retention was tested 7 d later (Fig. 2E). Infusions of Arc antisense ODN in the absence of memory retrieval did not affect freezing behavior in the 7 d test (Fig. 2F). These results indicate that late Arc expression following memory retrieval contributes to the persistence of reactivated memories.
NR2B-NMDARs are involved in memory destabilization that entails early and late Arc expression
Our finding that inhibition of late Arc expression impaired retention of reactivated memories 7 d after treatment suggests that reactivated memories are destabilized and then stabilized in a late Arc expression-dependent manner. However, the destabilization process that entails late Arc expression is unclear. Previous studies have shown that inhibition of NR2B-containing N-methyl-D-aspartate receptors (NMDARs) prevents memory impairment resulting from inhibition of protein synthesis following memory reactivation26,27. The studies suggested that activation of NR2B-NMDARs is involved in memory destabilization that entails early protein synthesis after memory reactivation. Thus, NR2B-NMDAR activation could be involved in memory destabilization that entails late Arc expression, as well as early Arc expression.
First, we tested whether blockade of NR2B-NMDARs prevents memory impairment resulting from inhibiting protein synthesis in our experimental conditions. We infused ifenprodil, an NR2B-NMDAR antagonist, or vehicle into the BLA 5 min before memory retrieval, and also infused anisomycin, a protein synthesis inhibitor, immediately after memory retrieval (Supplementary Fig. S3a). Consistent with previous studies, blockade of NR2B-NMDARs prevented memory impairment resulting from inhibition of early protein synthesis following memory reactivation (Supplementary Fig. S3b). We also prepared different mice and infused ifenprodil or vehicle into the BLA before memory retrieval and anisomycin 10 h after memory retrieval. Infusions of ifenprodil prevented memory impairment resulting from inhibition of late protein synthesis (Supplementary Fig. S3c,d).
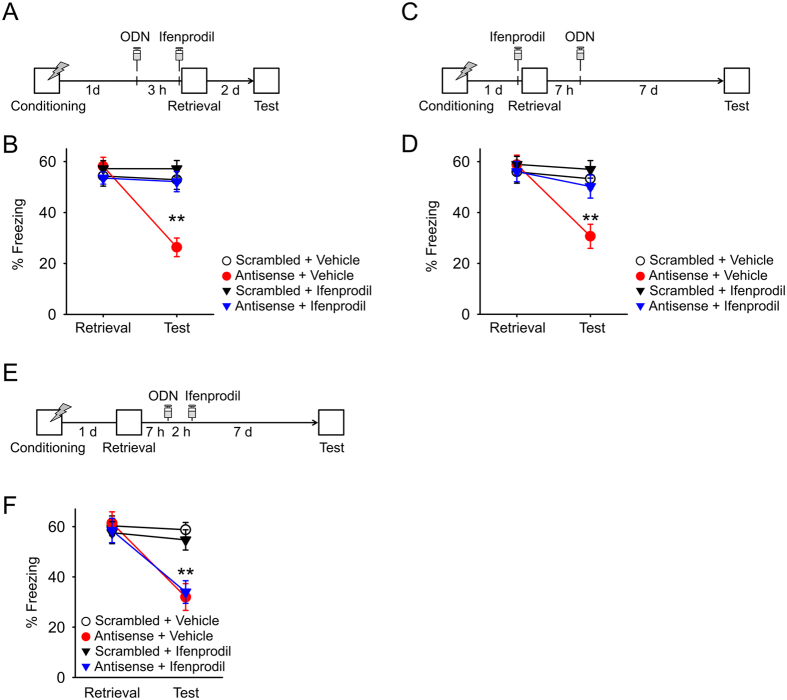
Next, we determined whether blockade of NR2B-NDMARs prevents memory impairment resulting from inhibition of early Arc expression. We infused Arc antisense or scrambled ODNs into the BLA 3 h before memory retrieval, and also infused ifenprodil or vehicle 5 min before memory retrieval (Fig. 3A). Memory retention was tested 2 d later. While inhibition of early Arc expression impaired freezing behavior in the 2 d test (Fig. 3B; Antisense + Vehicle group), this impairment was prevented by a combinatorial infusion of ifenprodil (Antisense + Ifenprodil). Infusions of ifenprodil alone did not affect freezing behavior in this test (Scrambled + Ifenprodil group). All behavioral groups showed comparable freezing behavior in a retrieval session (Fig. 3B). These results suggest that NR2B-NMDAR activation is involved in memory destabilization that entails early Arc expression.
Figure 3.
NR2B-NMDARs are involved in memory destabilization that entails early and late Arc expression (A) The experimental procedure for (B) (scrambled + Vehicle: n = 11 mice, antisense + Vehicle: n = 12 mice, scrambled + Ifenprodil: n = 11 mice, antisense + Ifenprodil: n = 12 mice). (B) Arc antisense ODN treatment disrupted freezing behavior 2 d after memory retrieval. Combinatorial infusions of ifenprodil prevented this memory impairment. (C) The experimental procedure for (D) (scrambled + Vehicle: n = 11 mice, antisense + Vehicle: n = 11 mice, scrambled + Ifenprodil: n = 11 mice, antisense + Ifenprodil: n = 12 mice). (D) Infusions of Arc antisense ODNs disrupted freezing behavior 7 d after memory retrieval. Combinatorial infusions of ifenprodil prevented this memory impairment. (E) The experimental procedure for (F) (Scrambled + Vehicle: n = 11 mice, Antisense + Vehicle: n = 11 mice, Scrambled + Ifenprodil: n = 12 mice, Antisense + Ifenprodil: n = 12 mice). (F) Infusions of Arc antisense ODN disrupted freezing behavior 7 d after memory retrieval. Combinatorial infusions of ifenprodil did not affect this memory impairment. Histological verification of cannula placements are shown in supplementary Fig. S3. **P < 0.01, *P < 0.05.
To test whether blockade of NR2B-NDMAR prevents memory impairment resulting from inhibition of late Arc expression, we infused ifenprodil or vehicle into the BLA 5 min before memory retrieval and also infused Arc antisense or scrambled ODNs 7 h after memory retrieval (Fig. 3C). Memory retention was tested 7 d later. Consistent with the previous result (Fig. 2D), inhibition of late Arc expression impaired freezing behavior in the 7 d test (Fig. 3D; Antisense + Vehicle group). However, combinatorial infusions of ifenprodil prevented this impairment (Antisense + Ifenprodil). Infusions of ifenprodil alone did not affect freezing behavior (Scrambled + Ifenprodil group).
When mice received infusions of ifenprodil 9 h after retrieval, ifenprodil infusions did not prevent memory impairment resulting from inhibition of late Arc expression (Fig. 3E,F). These results indicate that activation of NR2B-NMDARs at the time of memory retrieval is involved in memory destabilization that entails late Arc expression.
Milton et al. showed that NR2A-NMDARs are involved in restabilization of cued fear memory26. We asked whether restabilization of contextual fear memory requires NR2A-NMDARs activation. Mice were given infusions of NVP-AAM077, NR2A-preferring NMDAR antagonist, into the BLA 5 min before memory retrieval (Supplementary Fig. S5a). NVP-AAM077 infusions disrupted freezing behavior at the test, indicating that NR2A-NMDARs are involved in memory restabilization of contextual fear memory.
NR2B-NMDARs are essential for early and late Arc expression
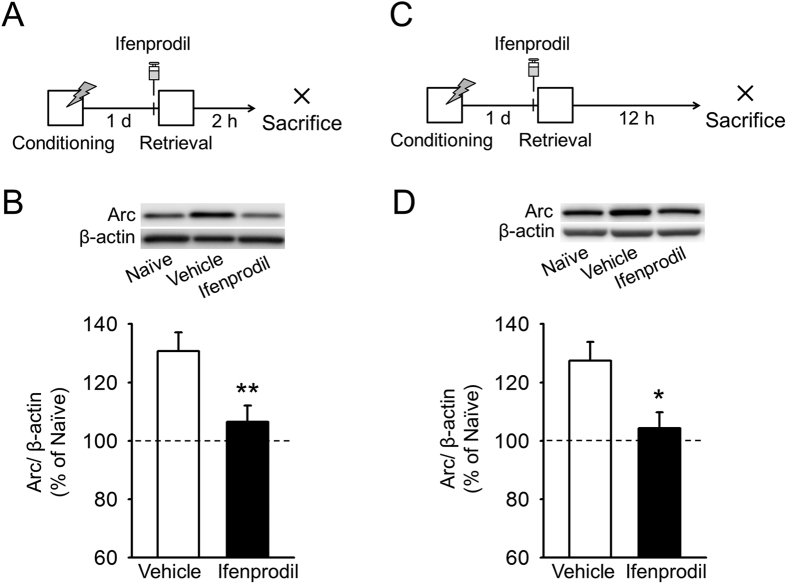
Finally, we investigated whether early and late Arc expression depends on NR2B-NMDAR activation. Mice received infusions of ifenprodil or vehicle into the BLA 5 min before memory retrieval and were sacrificed either 2 h or 12 h later (Fig. 4A,C). Consistent with the previous results (Fig. 1D), Arc levels in the BLA increased at 2 h and 12 h in vehicle-treated mice (Fig. 4B,D). In ifenprodil-treated mice, however, Arc levels at 2 h and 12 h were comparable to basal levels (Fig. 4B,D). These results indicate that NR2B-NMDAR activation primes early and late Arc expression, which contributes to early and late memory stabilization.
Figure 4. Early and late Arc expression after memory retrieval depend on activation of NR2B-NMDARs.
(A) The experimental procedure for (B) (n = 8 mice for each group). (B) Ifenprodil infusions inhibited Arc expression at the 2 h time point. (C) The experimental procedure for (D) (n = 8 mice for each group). (D) Ifenprodil infusions inhibited Arc expression at the 12 h time point. Histological verification of cannula placements are shown in supplementary Fig. S6. **P < 0.01, *P < 0.05.
Discussion
In the present study, we found that late Arc expression following fear learning and retrieval is essential for persistence of newly-acquired and reactivated fear memories, respectively. We also found that blockade of NR2B-NMDARs prior to memory retrieval prevented memory destabilization that entails early and late Arc expression and that this blockade also inhibited early and late Arc expression. Therefore, NR2B-NMDAR signaling is likely to be involved in both destabilization and restabilization of reactivated fear memories.
Similarities between the stabilization of newly-acquired memories (consolidation) and restabilization of reactivated memories (reconsolidation) are under debate2,12. Although memory consolidation and reconsolidation utilize many common mechanisms2,12, some studies reported that distinct molecular cascades and brain regions are involved in consolidation and reconsolidation12,28,29. In our current study, unlike previous studies, we focused on persistence of newly-acquired and reactivated memories. We found that BLA Arc levels were upregulated 12 h after initial learning and 12 h after memory retrieval and that late Arc expression is required for persistence of both new and reactivated memories. Therefore, persistence of newly-acquired and reactivated memories may require, at least in part, common molecular mechanisms.
It is difficult to exactly separate processes involved in persistence and formation of newly-acquired memories. Using antisense ODN approach, we found that early and late Arc expression following fear conditioning are required for formation and persistence of fear memory, respectively. However, early alterations in Arc expression could be reflective both of conditioning (e.g. context-shock convergence) itself and of memory formation. Therefore, early Arc expression might also contribute to the conditioning itself.
NR2B-NMDAR signaling is involved in destabilization of reactivated memories. A previous study found that ifenprodil infusions prevented memory impairments induced by anisomycin, a protein synthesis inhibitor26,27. In the present study, ifenprodil prevented memory impairment resulting from inhibition of early Arc expression following memory retrieval. This result indicates that reactivated memories that are destabilized through NR2B-NMDARs are restabilized through early Arc expression. Furthermore, we found that ifenprodil infusions administered prior to memory retrieval prevented memory impairment resulting from inhibition of late Arc expression. These findings indicate that activation of NR2B-NMDARs at the time of memory retrieval triggers memory destabilization that entails both early memory stabilization and memory persistence. NR2B-NMDARs strongly interact with Ca2+/calmodulin-dependent protein kinase II (CaMKII), which recruits proteasomes to dendritic spines30,31. Thus, activation of NR2B-NMDA receptors may induce protein degradation, which leads to memory destabilization32.
NMDAR signaling is essential for Arc expression following memory retrieval, which is implicated in memory restabilization. A previous study demonstrated that although NR2A-NMDAR signaling is involved in restabilization but not destabilization of reactivated memories, NR2B-NMDAR signaling is involved in memory destabilization26. However, it was not determined whether activation of NR2B-NMDARs is important for memory restabilization. In our study, ifenprodil infusions inhibited early and late Arc expression following memory retrieval, which are implicated in early and late stabilization of reactivated memories, respectively. These results suggest that NR2B-NMDA receptor signaling is involved in the restabilization of reactivated memories as well as their destabilization. Ifenprodil infusions had no effect on memory retention, probably because ifenprodil prevented memory destabilization. NR2B-NMDARs interact with nitric oxide synthase (NOS)33,34, which is implicated in the epigenetic regulation of Arc35. Therefore, NR2B-NMDAR activation could contribute to late Arc expression through epigenetic priming of Arc transcription.
A possible mechanism by which late Arc expression contribute to memory persistence is refinement of neuronal circuit through pruning of dendritic spines. We previously have shown that persistence of newly-acquired memory is associated with late Arc-dependent pruning of small mushroom spines in hippocampal CA1 neurons 7 d following fear conditioning20. Because the strengthening of specific synaptic connections underlies a memory trace, elimination of redundant synapses could refine functional circuits for memory. Although it remains unknown whether spine pruning in the BLA is following memory retrieval, the similar synaptic mechanisms to newly-acquired memory could be involved in persistence of reactivated memory. Stabilization of 7-d but not 2-d new and reactivated memories may require late Arc-dependent spine pruning.
The different roles of the lateral (LA) and basal amygdala (BA) are of interest. Classically, the BA is thought to be implicated in contextual fear and the LA in cued fear. However, contextual fear is also encoded in the LA36,37. In this study, we did not distinguish these areas. Further studies are helpful for identifying more specific role of the LA and BA.
In conclusion, we report that late Arc expression is essential for the persistence of newly-acquired and reactivated memories. Persistent fear memories could be a factor in post-traumatic stress disorder1. Therefore, pharmacological manipulation of delayed cellular signaling following initial learning and memory retrieval could be a possible therapeutic intervention for these disorders.
Materials and Methods
Mice
All experiments performed were approved by the animal experiment ethics committee at the University of Tokyo (approval number 24–10), and were in accordance with the University of Tokyo guidelines for the care and use of laboratory animals. Adult male C57BL/6 J mice (Japan SLC Inc., Shizuoka, Japan), weighing 20–30 g and aged 8–13 weeks, were housed 2–4 per cage, and kept on a 12-h light/dark cycle (lights on from 7 a.m. to 7 p.m.). All mice were given free access to food and water, and acclimated to daily handling for 1 week prior to the start of the study.
Behavioral procedures
Contextual fear conditioning and subsequent testing were performed in a conditioning chamber (18 cm wide, 15 cm deep and 27 cm high) that had a stainless steel grid floor38. The chamber was cleaned with 70% ethanol before each session. A conditioning session consisted of placing the mice in the chamber and delivering a 2-s footshock (1 mA) after 148 s. The mice then received 2 additional shocks every 148 s. They were kept in the chamber for an additional 60 s and were then returned to their home cages. An immediate shock session consisted of delivering 2-s footshocks 3 times to the mice immediately after placing them in the chamber. The mice then remained in the chamber for 500 s. The retrieval and testing sessions consisted of exposing the mice to the conditioning chamber for 5 min, in the absence of a footshock. We employed 5 min of exposure, since we preliminary confirmed that 5 min of exposure reliably induced memory destabilization. In the reminder shock session, mice received mild footshock (0.6 mA, 2 s) in a neutral chamber (triangle, 21 × 21 cm and 26 cm) immediately after being placed in the chamber. The mice were then returned to their home cages immediately after receiving footshock. We previously confirmed that this mild immediate shock reinstates extinguished fear25. All sessions were performed between 8 a.m. and 12 p.m., and each session was video-recorded for automatic scoring of freezing, according to a previously described method39. Images were captured at two frames per second. When the amount of area within the mouse moved was below a certain threshold for more than 1 s, behavior was judged as ‘freezing.’ The optimal threshold by which we judged freezing was determined by adjusting it to the amount of freezing measured by human observation. In the human observation, freezing was defined as the absence of all movements, except those related to breathing.
Western blotting
Mice were decapitated after diethyl- ether anesthesia, and their brains were rapidly removed and frozen at −80 °C. Coronal brain sections (300 μm) were prepared using a cryostat (HM520; Thermo Fisher Scientific, Waltham, MA, USA). The basolateral amygdala (0.90 to 2.00 mm posterior to bregma) was punched out and homogenized in RIPA buffer (08714-04, Nacalai Tesque, Kyoto, Japan). Protein concentrations were normalized across homogenates using the BCA method (Thermo Scientific). Equal amounts of protein were electrophoresed on 5–20% SDS polyacrylamide gels and transferred to nitrocellulose membranes. Western blots were blocked in blocking buffer (03953-95, Nacalai Tesque, JAPAN) and then incubated with an anti-Arc/Arg3.1 antibody (sc-17839, Santa Cruz Biotechnology) at a 1:1000 dilution or with an anti-β-actin antibody (A3854, Sigma) at a 1:10,000 dilution. After incubation with anti-Mouse IgG (A9044, Sigma) at a 1:100,000 dilution, bands were developed with a chemiluminescent substrate (RPN2132, GE Healthcare). The immunopositive signals were quantified by ImageQuant LAS 4000 (GE Healthcare). Arc/Arg3.1 signals were normalized with β-actin signals.
Fluorescent in situ hybridization
After the mice were sacrificed, the brains were quick-frozen and stored at −80 °C until further processing. Coronal brain sections (20 μm) were prepared using a cryostat and mounted on slides. Slides were air-dried and stored at −80 °C until use. Regions containing amygdala were selected for in situ hybridization. Antisense riboprobes for Arc, conjugated to digoxigenin-UTP (Roche Applied Science, Penzberg, Germany), were generated from cDNA plasmid (provided by Dr. Paul Worley) containing an almost full-length cDNA of the Arc transcript using MAXIScript (Ambion, Austin, TX USA). In situ hybridization was performed as previously published protocols40. Slide-mounted brain sections were fixed in 4% buffered paraformaldehyde, acetylated with 0.5% acetic anhydride/1.5% triethanolamine, dehydrated through 50% methanol/50% acetone solutions, and equilibrated in 2 × saline sodium citrate buffer (SSC). Slides were incubated in prehybridization buffer for 30 min. The antisense riboprobe was diluted to 150 μl in hybridization buffer, heat denatured, chilled on ice, and applied to each slide. Hybridization was carried out at 56 °C for 16 h. Slides were washed to a final stringency of 0.5 × SSC at 56 °C, which included an earlier wash step at 37 °C in 2 × SSC with RNase A (10 μg/ml). Endogenous peroxidase activity was quenched with 2% H2O2 in 1 × SSC. Slides were blocked with TSA blocking reagent (PerkinElmer, Waltham, MA USA) and incubated with an anti-digoxigenin horseradish peroxidase (HRP)-conjugate (1:500, Roche) for 2 h. Slides were washed 3 times in Tris-buffered saline with 0.05% Tween-20, and the conjugate was detected using biotin-labeled tyramide and Alexa Fluor 488 streptavidin (0.5 μg/mL; Life Technologies, Carlsbad, CA USA)41. Slides were washed in PBS, and the nuclei were counterstained with propidium iodide (PI, 10 μM, Life Technologies) for 10 min. Finally, the sections were washed with PBS and mounted in Permafluor (Thermo Fisher Scientific, Waltham, MA USA).
Immunohistochemistry
After anesthesia with diethyl ether, mice were transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were post-fixed in 4% PFA for 12 h. Free-floating coronal sections (40 μm) were prepared using a cryostat. Arc was visualized with an anti-Arc/Arg3.1 primary antibody (156 003, 1:1,000; Synaptic Systems, Gottingen, Germany), biotinylated anti-rabbit secondary antibody (BA-1000, 1:500; Vector Laboratories, Burlingame, CA, USA), VECTASTAIN ABC Kit (Vector Laboratories), and TSA Plus Cyanine 3 System (NEL744001KT, 1:100; Perkin-Elmer, Waltham, MA, USA). Nuclei were counterstained with Hoechst dye (1:1,000; Life Technologies).
Confocal microscopy and image analysis following immunohistochemistry
Images of BLA neurons (1.00 to 1.50 mm posterior to bregma, 4–6 slices per mouse) were acquired using a confocal microscope (CV1000; YOKOGAWA, Tokyo, Japan) at 40 × under an oil-immersion lens (NA, 1.3). Areas of analysis were z-sectioned in 1.6-μm optical sections. The same laser and scanning settings were used for images within an experiment to allow for comparison across groups. Fluorescence images were analyzed using ImageJ software (NIH). The nuclei in the BLA area were traced in the Hoechst channel using ImageJ software. Only cells that were presumptive neurons, with large nuclei stained diffusely with Hoechst, were included in the analysis. The designation “Arc positive (Arc+)” was assigned to cells containing perinuclear labeling over three optical sections.
Confocal microscopy and image analysis following in situ hybridization
Images were collected using a confocal microscope (LSM 510; Carl Zeiss AG, Oberkochen, Germany) at 40× under a water-immersion lens (NA 1.2). Photomultiplier tube assignments and pinhole size were kept constant, while laser power and gain were optimized on each slide to detect relatively weaker cytoplasmic Arc signals. Images of the basolateral amygdala were acquired by collecting z-stacks (1-μm-thick optical sections). Images were analyzed on the MetaMorph 6.0 program (Molecular Devices, LLC, Sunnyvale, CA USA). Only cells containing whole nuclei were included in the analysis. The PI stains revealed nuclei of 2 distinct morphologies. The majority of nuclei in the amygdala were large and stained diffusely with PI. Only these presumptive neurons were included in the analysis. The remainder of the cells, presumably glial cells, had much smaller nuclei, stained strongly with PI, and did not express Arc mRNA. The designation “Arc+” was given to cells containing perinuclear/cytoplasmic labeling over more than 3 optical sections.
Surgery
Mice were anesthetized with pentobarbital (2.5 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and 26-gauge stainless-steel guide cannulas (Plastics One) were implanted in the amygdala (−1.3, ±3.1, −4.6 mm relative to bregma). Cannulas were secured to the skull using a mixture of acrylic and dental cement, and 33-gauge dummy cannulas were then inserted into each guide cannula to prevent clogging. Mice were given at least 7 d of postoperative recovery time.
Oligodeoxynucleotide design
Arc/Arg3.1 antisense ODN and scrambled ODN were designed in reference to a previous study17. The Arc/Arg3.1 ODN encoded an antisense sequence for the Arc/Arg3.1 mRNA sequence near the translation start site42. The scrambled ODN does not show significant homology to any sequences in the GenBank database17. Both ODNs contained phosphorothioate linkages on the three terminal bases of both the 5′ and 3′ ends and phosphodiester internal bonds. This design is reported as more stable than unmodified phosphorothioated ODNs in vivo and less toxic than fully phosphorothioated ODNs17. The following sequences were used (“~” denotes a phosphorothioate linkage): 5′-G~T~C~CAGCTCCATCTGGT~C~G~T-3′ (antisense) and 5′-C~G~T~GCACCTCTCGCAGG~T~T~T-3′ (scrambled). This antisense sequence has been shown to effectively inhibit Arc/Arg3.1 protein expression in the hippocampus and to exhibit a high degree of specificity for Arc relative to other immediate early genes17,23 .
Drugs and microinfusions
Mice received intra-amygdala infusion (0.5 μL per side) of ODN (400 pmol), threo ifenprodil hemitartrate (2.5 μg, Tocris Bioscience), anisomycin (62.5 μg, Sigma) or NVP-AAM077 (0.2 μg, Sigma). The volume of drugs that we used spreads within only the BLA in previous studies43,44. The solutions were dissolved in PBS or 100 mM DMSO (WAKO, JAPAN), and PBS, DMSO was used as vehicle solution. Infusions were made over 2 min, and the infusion cannulas (28 gauge, extending 0.5 mm below the guide cannula) were left in place for at least 2 min afterwards in order to facilitate the diffusion of solutions throughout the amygdala.
Data analysis
All values were reported as means ± SEMs. Statistical analysis was performed using two-tailed Student’s t-test, paired t-test, one-way analysis of variance (ANOVA), repeated-measures ANOVA and Tukey’s test, where appropriate. P < 0.05 is considered statistically significant. Statistical results were shown in Supplementary Table 1.
Additional Information
How to cite this article: Nakayama, D. et al. Late Arc/Arg3.1 expression in the basolateral amygdala is essential for persistence of newly-acquired and reactivated contextual fear memories. Sci. Rep. 6, 21007; doi: 10.1038/srep21007 (2016).
Supplementary Material
Acknowledgments
We thank Dr. Paul Worley for the Arc cDNA. This work was supported by a Grant-in-Aid for Young Scientists (B) (25830002, to HN), Grant-in-Aid for Scientific Research on Innovative Areas, “Memory Dynamism” (No. 26115509 to HN), “The Science of Mental Time” (No. 26119507 to HN and No. 25119004 to YI) and “Mesoscopic Neurocircuitry” (No. 23115101, to HN).
Footnotes
Author Contributions D.N. and Y.H.Y. performed experiments. D.N. and H.N. analyzed the data and wrote the manuscript. H.N. designed the study. Y.I. and N.M. supervised the project.
References
- Steckler T. & Risbrough V. Pharmacological treatment of PTSD—established and new approaches. Neuropharmacology 62, 617–627 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. P., Cain C. K., Ostroff L. E. & LeDoux J. E. Molecular mechanisms of fear learning and memory. Cell 147, 509–524 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Bernal I. Learning and memory consolidation: linking molecular and behavioral data. Neuroscience 176, 12–19 (2011). [DOI] [PubMed] [Google Scholar]
- Richter J. D. & Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev 23, 1–11 (2009). [DOI] [PubMed] [Google Scholar]
- Schafe G. E. & LeDoux J. E. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 20, RC96 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G. E., Nadel N. V., Sullivan G. M., Harris A. & LeDoux J. E. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem 6, 97–110 (1999). [PMC free article] [PubMed] [Google Scholar]
- Schafe G. E., Nader K., Blair H. T. & LeDoux J. E. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci 24, 540–546 (2001). [DOI] [PubMed] [Google Scholar]
- Bekinschtein P. et al. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron 53, 261–277 (2007). [DOI] [PubMed] [Google Scholar]
- Bekinschtein P. et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA 105, 2711–2716 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C. et al. Delayed wave of c-Fos expression in the dorsal hippocampus involved specifically in persistence of long-term memory storage. Proc Natl Acad Sci USA 107, 349–354 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. & Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10, 224–234 (2009). [DOI] [PubMed] [Google Scholar]
- Alberini C. M. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci 28, 51–56 (2005). [DOI] [PubMed] [Google Scholar]
- Nader K., Schafe G. E. & Le Doux J. E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000). [DOI] [PubMed] [Google Scholar]
- Nakayama D., Yamasaki Y., Matsuki N. & Nomura H. Post-retrieval late process contributes to persistence of reactivated fear memory. Learn Mem 20, 307–310 (2013). [DOI] [PubMed] [Google Scholar]
- Ploski J. E. et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci 28, 12383–12395 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N. et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52, 437–444 (2006). [DOI] [PubMed] [Google Scholar]
- Guzowski J. F. et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20, 3993–4001 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox S. A. & Schafe G. E. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J Neurosci 31, 7073–7082 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia C. & Otto T. Hippocampal Arc (Arg3.1) expression is induced by memory recall and required for memory reconsolidation in trace fear conditioning. Neurobiol Learn Mem 106, 48–55 (2013). [DOI] [PubMed] [Google Scholar]
- Nakayama D. et al. et al. Long-delayed expression of the immediate early gene Arc/Arg3.1 refines neuronal circuits to perpetuate fear memory. J Neurosci 35, 819–830 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M. S. Conditioned fear-induced opiate analgesia: a competing motivational state theory of stress analgesia. Ann N Y Acad Sci 467, 40–54 (1986). [DOI] [PubMed] [Google Scholar]
- Rao-Ruiz P. et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci 14, 1302–1308 (2011). [DOI] [PubMed] [Google Scholar]
- Onoue K., Nakayama D., Ikegaya Y., Matsuki N. & Nomura H. Fear extinction requires Arc/Arg3.1 expression in the basolateral amygdala. Mol Brain 7, 30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C. et al. Neuronal circuits of fear extinction. Eur J Neurosci 31, 599–612 (2010). [DOI] [PubMed] [Google Scholar]
- Shen H., Igarashi H., Imamura N., Matsuki N. & Nomura H. N-methyl-D-aspartate receptors and protein synthesis are necessary for reinstatement of conditioned fear. Neuroreport 24, 763–767 (2013). [DOI] [PubMed] [Google Scholar]
- Milton A. L. et al. Double Dissociation of the Requirement for GluN2B- and GluN2A-Containing NMDA Receptors in the Destabilization and Restabilization of a Reconsolidating Memory. J Neurosci 33, 1109–1115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mamou C., Gamache K. & Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9, 1237–1239 (2006). [DOI] [PubMed] [Google Scholar]
- Kelly A., Laroche S. & Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci 23, 5354–5360 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. L., Everitt B. J. & Thomas K. L. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304, 839–843 (2004). [DOI] [PubMed] [Google Scholar]
- Bingol B. et al. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell 140, 567–578 (2010). [DOI] [PubMed] [Google Scholar]
- Barria A. & Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48, 289–301 (2005). [DOI] [PubMed] [Google Scholar]
- Lee S. H. et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319, 1253–1256 (2008). [DOI] [PubMed] [Google Scholar]
- Martel M. A. et al. The subtype of GluN2 C-terminal domain determines the response to excitotoxic insults. Neuron 74, 543–556 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X. et al. Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci 27, 6103–6114 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J. et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 156, 261–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues S. M., Bauer E. P., Farb C. R., Schafe G. E. & LeDoux J. E. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci 22, 5219–5229 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson Y. M. & Murphy M. A discrete population of neurons in the lateral amygdala is specifically activated by contextual fear conditioning. Learn Mem 16, 357–361 (2009). [DOI] [PubMed] [Google Scholar]
- Nomura H., Nonaka A., Imamura N., Hashikawa K. & Matsuki N. Memory coding in plastic neuronal subpopulations within the amygdala. Neuroimage 60, 153–161 (2012). [DOI] [PubMed] [Google Scholar]
- Nomura H. & Matsuki N. Ethanol enhances reactivated fear memories. Neuropsychopharmacology 33, 2912–2921 (2008). [DOI] [PubMed] [Google Scholar]
- Toyoda T., Nomura H., Hashikawa K., Nonaka A. & Matsuki N. Persistent neural activity regulates Arc/Arg3.1 transcription in the dentate gyrus. J Neurosci Res. 88, 3060–3066 (2010). [DOI] [PubMed] [Google Scholar]
- Imamura N., Nonaka A., Yamamoto H., Matsuki N. & Nomura H. Experience-dependent Homer1a expression in excitatory and inhibitory neurons. Neuroreport 22, 353–357 (2011). [DOI] [PubMed] [Google Scholar]
- Lyford G. L. et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14, 433–445 (1995). [DOI] [PubMed] [Google Scholar]
- Dias B. G. et al. Amygdala-dependent fear memory consolidation via miR-34a and Notch signaling. Neuron 83, 906–918 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asede D., Bosch D., Lüthi A., Ferraguti F. & Ehrlich I. Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron 86, 541–554 (2015). [DOI] [PubMed] [Google Scholar]
- Hitora-Imamura N., Miura Y., Teshirogi C., Ikegaya Y., Matsuki N. & Nomura H. Prefrontal dopamine regulates fear reinstatement through the downregulation of extinction circuits. Elife 4, e08274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.