Abstract
A large number of articles have reported substantia nigra hyperechogenicity in Parkinson’s disease (PD) and have assessed the diagnostic accuracy of transcranial sonography (TCS); however, the conclusions are discrepant. Consequently, this systematic review and meta-analysis aims to consolidate the available observational studies and provide a comprehensive evaluation of the clinical utility of TCS in PD. Totally, 31 studies containing 4,386 participants from 13 countries were included. A random effects model was utilized to pool the effect sizes. Meta-regression and sensitivity analysis were performed to explore potential heterogeneity. Overall diagnostic accuracy of TCS in differentiating PD from normal controls was quite high, with a pooled sensitivity of 0.83 (95% CI: 0.81–0.85) and a pooled specificity of 0.87 (95% CI: 0.85–0.88). The positive likelihood ratio, the negative likelihood ratio and diagnostic odds ratio were calculated 6.94 (95% CI: 5.09–9.48), 0.19 (95% CI: 0.16–0.23), and 42.89 (95% CI: 30.03–61.25) respectively. Our systematic review of the literature and meta-analysis suggest that TCS has high diagnostic accuracy in the diagnosis of PD when compared to healthy control.
Parkinson’s disease (PD) is the second most common neurodegenerative disease and is clinically characterized by resting tremor, rigidity, bradykinesia, and abnormal gait and posture. The gold standard for the diagnosis of PD is post-mortem neuropathological examination, which unfortunately precludes impactful clinical decision making to alleviate a PD patient’s symptoms1. Consequently, the diagnosis of PD is mostly based on clinical manifestations and expertise, which results in a large cohort of PD patients unidentified2. Therefore, a reliable and convenient test that recapitulates the clinical diagnosis of PD and identifies subclinical PD patients is needed in order to facilitate early disease management and delay or prevent the progression of PD.
Ultrasonography has been well-established as a diagnostic method in general medicine for over five decades. However, ultrasonography had not been applied to movement disorders due to the impenetrability of intact skull bones, until Becker first reported a specific high echogenic area within the substantia nigra (SN) in PD patients3. Since then, numerous studies have focused on the echogenicity of the SN and the diagnostic accuracy of transcranial sonography (TCS) in distinguishing PD patients from healthy controls, or other movement disorders. Nevertheless, the sensitivity and specificity of TCS in PD varied widely due to racial differences, sample size and diverse ultrasound devices. In a cross-sectional study conducted in Italy, using a 2–4 MHz probe, researchers found the sensitivity and specificity of TCS in diagnosing PD to be 62.71% and 76.92%, respectively4, while the value reported by Maria Sierria et al. was 95.50% and 84.78%, respectively5. Unfortunately, the lack of a comprehensive evaluation of the clinical utility of TCS has prevented the application of this non-invasive, non-radioactive and convenient technique in routine clinical practice. Therefore, the purpose of the present study is to perform a systematic literature review and meta-analysis to assess the overall diagnostic accuracy of TCS in the diagnosis of PD.
Methods
Search strategy
A systematic and comprehensive literature search using Pubmed, ISI Web of Science, EMBASE, Cochrane Library databases, and CNKI (a Chinese database), from 1966 until March 2015, was conducted for all the existing literatures regarding the diagnostic accuracy of TCS in the diagnosis of PD. The Medical Subjective Heading (MeSH) terms or keywords “transcranial sonography” and “Parkinson’s disease” were used. Subsequently, only studies published in English or Chinese were evaluated. Repeat articles were manually deleted. If an article did not present complete data, a request for raw data was sent to the original authors via e-mail. In addition, an earnest attempt to acquire unpublished data was made but no studies were appropriate for inclusion. This work was performed by two independent authors (Li and He).
Eligibility and Exclusion criteria
Two authors carefully read and evaluated all of the articles independently. Studies were included in the current review if they met the following criteria: 1) Cross-sectional study that evaluated the ability of TCS of the SN to distinguish PD patients from healthy controls; 2) Cross-sectional study that compared SN echogenicity between patients with PD, essential tremor, or other movement disorders. Review articles, conference reports, letters, editorial comments, opinions, preface, and articles not published in English or Chinese were excluded. Other exclusion criteria for the current systematic review were: 1) articles focused on therapy and management of PD; 2) articles on Parkinsonism or other diseases, but not idiopathic PD; 3) studies that did not contain a healthy control group; 4) studies investigating the pathogenesis of SN echogenicity; 5) epidemiological studies of TCS in community dwelling elders. Two independent investigators evaluated the eligibility of all included studies.
Data extraction, Quality assessment and Statistical analysis
All relevant data of the 31 studies, including: the first author, the year when the study was carried out, diagnostic criteria of PD, ultrasound device, number of true positives, false negatives, true negatives, and false positives were extracted in a unified form. Any divergence in this procedure was resolved by discussion. The revised version of the Quality Assessment of studies of Diagnostic Accuracy Studies (QUADAS-2), with 4 key domains containing 11 items6, was used to assess the quality of all included studies. Each domain facilitates assessment of the risk of bias and applicability of the primary investigation. Two authors performed the quality assessment independently, with disagreements resolved by discussion or appealing to a third author.
The statistical software Meta-Disc, version 1.4 for windows (XI Cochrane Colloquium, Barcelona, Spain) and STATA, version 12.0 (Stata Corporation, College Station, TX, USA) were used in the present study. To explore potential heterogeneity arising from the threshold effect, we computed Spearman correlation coefficients between sensitivity and 1-specificity. For any possible non-threshold heterogeneity, we applied the chi-square-based Q test and the inconsistency index I2. A significant Q test (I2 value > 50%) identifies a moderate or high degree of heterogeneity7. Subsequently, a random-effect model (DerSimonian Laird method) was used to calculate the pooled sensitivity, specificity, diagnostic odds ratio (DOR), and other related indexes. Otherwise, the Mantel-Haenszel fixed effect model was utilized. In order to assess the source of heterogeneity, we used subgroup analysis according to different threshold variables when heterogeneity arose from the threshold effect, and sensitivity analysis was chosen for non-threshold heterogeneity. Furthermore, meta-regression was implemented to investigate the source of heterogeneity within the included studies. We produced Deeks’ funnel plot to test the potential publication bias in our study, with a p value < 0.1 suggesting significance8.
Results
Characteristics and quality of the included studies
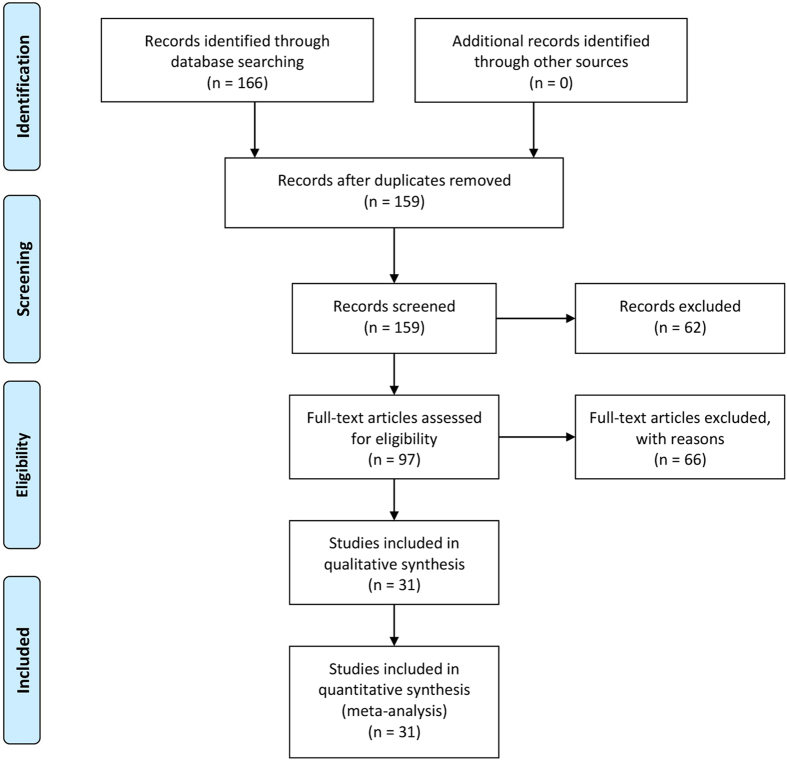
The inclusion and exclusion criteria for article selection are illustrated in Fig. 1. Ultimately, 31 studies4,5,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 containing 1,926 idiopathic PD patients and 2,460 healthy controls from 13 countries, were included in our meta-analysis. The main characteristics of the included studies are summarized in Table 1.
Figure 1. Flow chart of the selection process of included studies.
Table 1. Characteristics of included studies.
| Author | Year | Country | PD cases | Age (Ave.) | Diagnostic Criteria | TCS device | Cut-off value | TP | FP | FN | TN | QUADAS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stenc Bradvica I | 2015 | Italy | 59 | 67.2 | UK Brain Criteria | 2–4 MHz | 20 mm2 | 37 | 6 | 22 | 20 | 11 |
| Maria Sierra | 2013 | Spain | 68 | 68.93 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 65 | 7 | 3 | 39 | 10 |
| Sinem Tunc | 2015 | Germany | 53 | 73.92 | UK Brain Criteria | 2–2.5 MHz | 25 mm2 | 40 | 21 | 13 | 207 | 10 |
| M. O. Izawa | 2011 | Japan | 33 | 64.8 | UK Brain Criteria | 2 MHz | 16 mm2 | 26 | 2 | 7 | 30 | 9 |
| Hee Young Shin | 2011 | Korea | 24 | 62.3 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 21 | 4 | 3 | 21 | 11 |
| Tobias Bottcher | 2013 | Germany | 12 | 60.9 | UK Brain Criteria | 2.5 MHz | 24 mm2 | 10 | 4 | 2 | 28 | 10 |
| Christoph Schmidauer | 2005 | Austria | 20 | 64 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 19 | 5 | 1 | 15 | 10 |
| Pavel Ressner | 2007 | Czech | 47 | 64.7 | UK Brain Criteria | 2–3 MHz | 19 mm2 | 41 | 2 | 6 | 37 | 11 |
| Heike Stochner | 2007 | Austria | 100 | 65.2 | UK Brain Criteria | 2.5 MHz | 24 mm2 | 75 | 3 | 25 | 97 | 10 |
| Panteha Fathinia | 2012 | Germany | 31 | 63.5 | UK Brain Criteria | 3 MHz | 20 mm2 | 26 | 3 | 5 | 70 | 10 |
| Kristina Lauckaitel | 2012 | Lithuania | 71 | 63.8 | UK Brain Criteria | 1.3–4 MHz | 20 mm2 | 66 | 8 | 5 | 63 | 11 |
| Edson Bor–Seng–Shu | 2014 | Brazil | 20 | 62.5 | UK Brain Criteria | 2–3 MHz | 22 mm2 | 20 | 2 | 0 | 7 | 10 |
| U. Walter | 2001 | Germany | 30 | 68.9 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 30 | 7 | 0 | 23 | 10 |
| Philipp Mahlknecht | 2013 | Austria | 17 | 81.8 | UK Brain Criteria | 2.5 MHz | 18 mm2 | 15 | 103 | 2 | 344 | 9 |
| Do–Young Kwon | 2010 | Korea | 63 | 64.6 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 51 | 5 | 12 | 35 | 11 |
| Yu–Wen Huang | 2007 | Chinese Taipei | 80 | 59.1 | UK Brain Criteria | 2.25 MHz | 20 mm2 | 54 | 6 | 26 | 114 | 11 |
| Rita de Cassia | 2011 | Brazil | 17 | 66.9 | UK Brain Criteria | 1.6–2.5 MHz | 20 mm2 | 15 | 2 | 2 | 9 | 9 |
| Sabine Mehnert | 2010 | Germany | 183 | 66 | UK Brain Criteria | 1.8–3.6 MHz | 20 mm2 | 173 | 8 | 10 | 193 | 10 |
| Nikola Kresojevi | 2012 | Germany | 54 | 61.5 | None | 2.5 MHz | 19 mm2 | 46 | 5 | 8 | 48 | 11 |
| Wei–Feng Luo | 2011 | China | 110 | 58.7 | UK Brain Criteria | None | 20 mm2 | 88 | 11 | 22 | 99 | 10 |
| Kristina Lauckaite | 2014 | Lithuania | 141 | 64.4 | UK Brain Criteria | None | 20 mm2 | 106 | 18 | 35 | 83 | 10 |
| Li Chen | 2013 | China | 170 | 61.3 | UK Brain Criteria | 1–3 MHz | 20 mm2 | 139 | 12 | 31 | 91 | 9 |
| Sheng Yujing | 2011 | China | 78 | 62.2 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 66 | 5 | 12 | 55 | 11 |
| Zhang Yingchun | 2010 | China | 80 | 60.7 | UK Brain Criteria | 2–2.5 MHz | 20 mm2 | 58 | 10 | 22 | 70 | 10 |
| Ahmad Chitsaz | 2013 | Iran | 43 | 63.39 | UK Brain Criteria | 2–4 MHz | 20 mm2 | 39 | 4 | 4 | 46 | 11 |
| Jurgen Prestel | 2006 | Germany | 42 | 64.6 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 36 | 6 | 6 | 29 | 11 |
| Ji Youn Kim | 2007 | Korea | 35 | 56.7 | UK Brain Criteria | 2–5 MHz | 20 mm2 | 29 | 2 | 6 | 25 | 10 |
| Jung Ho Ryu | 2011 | Korea | 19 | 68.5 | UK Brain Criteria | 2.5 MHz | 20 mm2 | 16 | 24 | 3 | 11 | 10 |
| Wang Rong | 2011 | China | 34 | 64.11 | UK Brain Criteria | 1–5 MHz | 20 mm2 | 31 | 4 | 3 | 34 | 9 |
| Araceli | 2014 | Germany | 97 | 67 | UK Brain Criteria | 2.5 MHz | 21 mm2 | 80 | 15 | 17 | 117 | 11 |
| Alonso | ||||||||||||
| Canovas | ||||||||||||
| W. Ambrosius | 2014 | Poland | 95 | 62 | UK Brain Criteria | 2.5–3.5 MHz | 19 mm2 | 78 | 10 | 17 | 85 | 11 |
Age (Ave.): average of age of included PD Patients; TP: true positive; FP: false positive; FN: false negative; TN: true negative.
Diagnostic accuracy
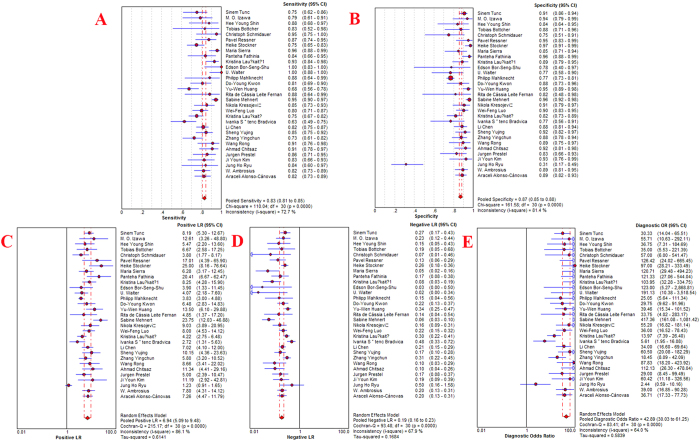
Statistical analysis revealed no heterogeneity secondary to the threshold effect, as the ROC plane did not have the typical “shoulder arm” pattern (Fig. 2) and the Spearman correlation coefficient of sensitivity and 1-specificity was 0.289 (p = 0.115). However, there was significant heterogeneity across the studies in sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and Diagnostic Odds Ratio (DOR), with an I2 index of 72.7% (p < 0.0001), 81.4% (p < 0.0001), 86.1% (p < 0.0001), 67.9% (p < 0.0001) and 64.0% (p < 0.0001), respectively. Overall, the diagnostic accuracy of TCS for the diagnosis of PD among patients versus healthy controls was measured based on the pooled sensitivity of 0.83 (95% CI: 0.81–0.85), pooled specificity of 0.87 (95% CI: 0.85–0.88), pooled PLR of 6.94 (95% CI: 5.09–9.48), pooled NLR of 0.19 (95% CI: 0.16–0.23) and pooled DOR of 42.89 (95% CI: 30.03–61.25) using the random effects model. The forest plots of all the indices are displayed in Fig. 3. The overall high level of accuracy is reflected by the symmetric SROC curve with an AUC of 0.9306 (standard error: 0.0095) and Q-value of 0.8658 (standard error: 0.0114) (Fig. 4).
Figure 2. Sensitivity versus 1-specificity in receiver operating characteristic (ROC) plane for each eligible study.
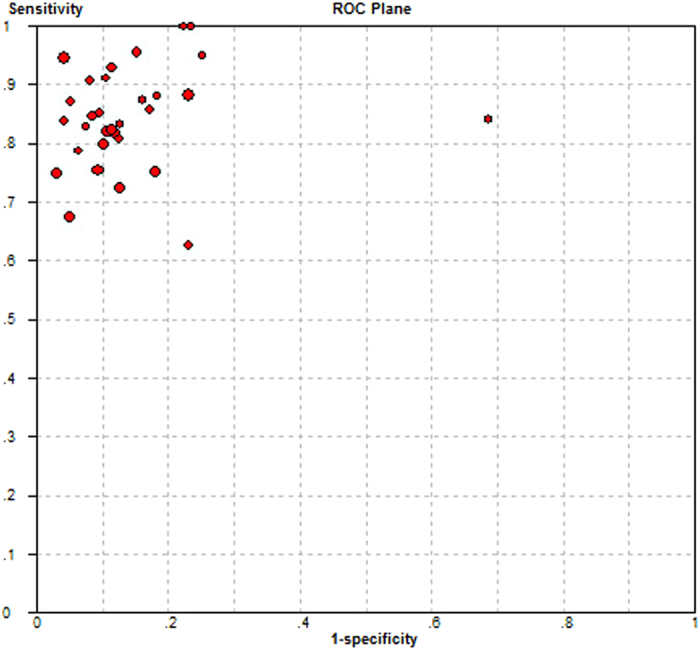
Figure 3. Forest plots of the diagnostic accuracy of the transcranial sonongraphy of the substantia nigra in Parkinson’s diseases.
A = Sensitivity; B = Specificity; C = Positive LR; D = Negative LR; E = Diagnostic OR. CI = confidence interval; LR = likelihood ratio; OR = odds ratio.
Figure 4. Summary receiver operating characteristic (SROC) curve for transcranial sonography of the substantia nigra in the diagnosis of Parkinson’s disease for all studies.
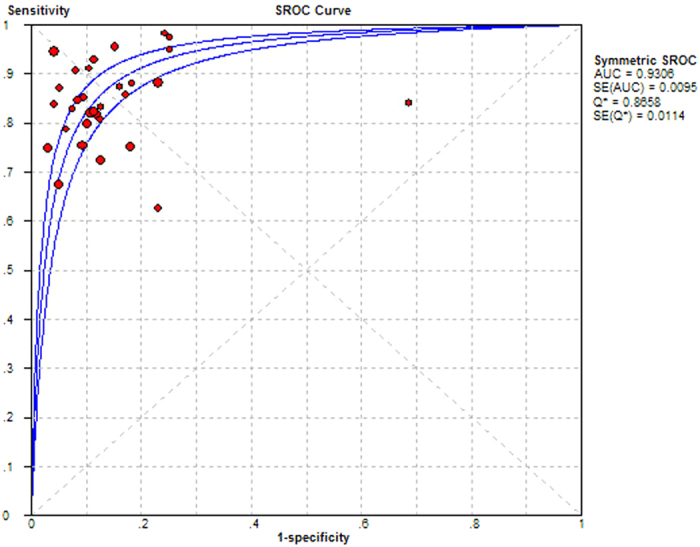
AUC = area under curve; SE = standard error; Q* = point at which sensitivity and specificity are equal.
Meta-regression analysis
Meta-regression analysis was utilized to investigate potential reasons for inter-study heterogeneity based on geographical location (Europe, Asia or America), sample size (<50 or ≥ 50), age of PD patients (<65 or ≥ 65), ultrasound equipment (<2.5 MHz or ≥ 2.5 MHz), and QUADAS-2 scores (<10 or ≥ 10). However, none of the above covariates were found to be significant sources of heterogeneity, as all p values were > 0.05.
Sensitivity analyses
Sensitivity analyses were performed to explore the possible heterogeneity and verify the consistency of the results from our meta-analysis by applying the leave-one-out method in which the first of the K studies is left out on repeat meta-analysis of the resulting subgroup containing K−1 studies. This analysis is repeated for the next K studies until all distinct meta-analyses are performed, each leaving out one study. Overall, no substantial alterations of the results were found in our investigation, with the pooled sensitivity ranging from 0.82 (95% CI: 0.80–0.84) with omission of the study by Maria Sierra 20135 to 0.84 (95% CI: 0.82–0.85) with omission of the study by Yu-Wen 200722, and the pooled specificity ranging from 0.86 (95% CI: 0.85–0.88) by removing the study by SinemTunc 20159 to 0.89 (95% CI: 0.88–0.90) by removing the study by Philipp Mahlknecht 201320. These sensitivity analyses indicate statistically consistent results with a high level of overall accuracy using TCS in the diagnosis of PD. Moreover, among the included studies, no single study was found to be the source of heterogeneity.
Evaluation of publication bias
Deeks’ funnel plots were produced to explore the potential presence of publication bias. Based on the symmetric shape of the funnel plot of pooled DOR (Fig. 5) and the Deeks’ test non-significant value (p = 0.29), there is no potential publication bias in the current meta-analysis.
Figure 5. Funnel plot for the assessment of the potential publication bias of the 31 included studies.
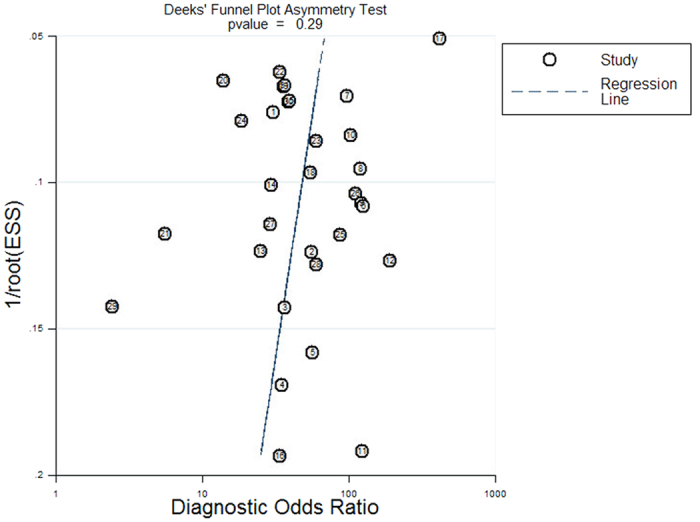
Each solid circle represents each study in the meta-analysis. The line indicates the regression line.
Discussion
The results of our meta-analysis, which included 1,926 PD patients and 2,460 healthy controls from 13 countries, demonstrated a high clinical utility of TCS in the diagnosis of PD, with a pooled sensitivity (83%) and specificity (87%). The AUC (0.9306) and DOR (42.89) further indicate an excellent overall accuracy. In addition, a PLR value of 6.94 (95% CI: 5.09–9.48), which is more clinically meaningful for our measures of diagnostic accuracy38, suggests that patients with SN hyperechogenicity have a moderate increase in the likelihood of having PD.
For all meta-analyses, heterogeneity is a potential problem when interpreting the results. One major source of heterogeneity is the threshold effect in which different cut-offs are used in the studies included in a meta-analysis. The Spearman correlation coefficient in our study indicates that there is no threshold effect related heterogeneity. Furthermore, meta-regression analysis to find other possible sources of heterogeneity, including geographical location (Europe, Asia or America), sample size (<50 or ≥ 50), age of PD patients (<65 or ≥ 65), ultrasound equipment (<2.5 MHz or ≥ 2.5 MHz), and QUADAS-2 scores (<10 or ≥ 10), revealed that none of the variables were substantial sources of heterogeneity. Therefore, we subsequently performed sensitivity analyses to explore the possibility of significant overall inter-study heterogeneity and to verify the consistency of our results. No obvious alterations were detected, indicating no conceivable source of heterogeneity and statistically consistent results.
In recent years, applications of TCS in the clinical differentiation of PD patients from the healthy population have shown great value. Investigations into the differential diagnosis of PD from atypical parkinsonian syndrome (APS), essential tremor (ET), restless leg syndrome (RLS), or other neurological diseases utilizing TCS suggest that normal SN echogenicity was correlated with multiple system atrophy (MSA)39 and ET4,17,26,27. Furthermore, SN hypoechogenicity was detected in patients with RLS21. More interestingly, abnormal SN hyperechogenic areas were also discovered in 67% of amyotrophic lateral sclerosis (ALS) patients16, a disease that might be related to impairment of the nigrostriatal system based on neuroimaging data40,41. Additionally, lenticular nucleus hyperechogenicity in combination with third-ventricle dilatation of more than 10 mm by TCS helps differentiate progressive supranuclear palsy (PSP) from PD39. Moreover, the combination of TCS and olfactory test42 or MIBG myocardial scintigraphy10 has been identified to improve the differential diagnostic power for identifying PD. All of these investigations demonstrated that the clinical application of TCS may not only help identify PD patients, but also differentiate PD patients from other movement disorders, which suggests great value for TCS in routine clinical practice.
The origin of SN hyperechogenicity, assessed by animal and postmortem studies, has been shown to be related to midbrain iron deposition43. Furthermore, the levels of H- and L-ferritins44, iron metabolizing protein45, plasma ferroxidase activity46, and serum CRP47 were abnormal in PD patients with SN hyperechogenicity, which further bolsters the concept that SN hyperechogenicity is related to alterations in iron metabolism in PD. Other sources of SN hyperechogenicity include microglia activation48 and gliosis49, which were found in brain tissue with SN echogenicity after correction for iron and neuromelanin contents. The LRRK2 gene, an autosomal-dominant PD gene, participates in the regulation of neuroinflammation50 and microglia activation51, and has been found to correlate with SN echogenicity as well. Specifically, carriers of the LRRK2 mutation with no clinical manifestation of PD have a similar proportion of SN hyperechogenicity when compared with idiopathic PD patients5. Other PD related gene mutation loci, such as PINK152, GBA53 have been also reported to correlate with diverse echogenicity. In the previous research54, we explored the potential correlation between SN hyperechogenicity with dopaminergic function represented by DAT-SEPCT, however the results consistent with other study55, demonstrated SN echogenicity was not based on dopaminergic pathomechanisms.
Ever since Becker G, et al.3 first reported a specific high echogenic area within the SN of PD patients over 20 years ago, midbrain echo-features of PD patients have been confirmed and further investigated by numerous groups. However, the utility of TCS in the clinical diagnosis of PD is not universally accepted for several reasons. When a physician wants to utilize a clinical tool, the first parameters examined are the sensitivity and specificity. Unfortunately, different groups report inconsistent results4,5 due to small sample sizes, and this leads to varied sensitivity and specificity values which precludes the application of TCS for the diagnosis of PD. Therefore, we sought to perform a comprehensive study to evaluate the diagnostic accuracy of TCS. Our study, containing 1,926 PD patients and 2,460 healthy controls from 13 countries, revealed a high pooled sensitivity and specificity, which strongly indicates that TCS could be applied as a clinical tool for the diagnosis of PD patients from healthy controls. Nevertheless, some technical shortcomings must be acknowledged.
One inevitable problem that a sonographer may confront is transcranial insonability. In European populations, 4–15% of participates were found to have an insufficient temporal window5,9,16,17,24,25. However, the value rises to 15–60% in Asian populations10,11,21,22,28,34. This high recording failure rate in TCS application would mostly affect patients of advanced age with female gender56 or patients with a small temporal window seen in Asian populations. Recently, high-resolution ultrasound systems with standardized settings or with automated segmentation technique were reported to reduce inter-observer and intra-observer variability57, which may help improve TCS image quality and decrease the incidence of insufficient temporal window. Moreover, a novel approach using transcranial B-mode sonography, a 3-D ultrasound platform, was shown to be technically feasible and less dependent on sonographer experience or good bone windows58. These innovations and developments in ultrasound systems may effectively improve the application value and diagnostic accuracy of TCS.
To our knowledge, this is the first systematic review and meta-analysis assessing the overall diagnostic accuracy of TCS in PD. A thorough literature search and careful data extraction were performed to avoid any bias. Nevertheless, limitations still exist in our study. First, although we carefully explored the heterogeneity by meta-regression and sensitivity analyses, notable heterogeneity was still observed, which can be due to random variation between individual studies59. Second, failure to acquire unpublished data or studies not published in English or Chinese for language limitation may affect the validity of our results.
In conclusion, our systematic review and meta-analysis suggest that TCS has high diagnostic accuracy in the diagnosis of PD patients from the healthy population. As a non-invasive, non-radioactive and convenient neuroimaging technique, application of TCS in routine clinical practice is of great value in the diagnosis of PD. However, large cohorts of high-quality prospective studies are still required to further confirm the value of TCS in the diagnosis of PD.
Additional Information
How to cite this article: Li, D.-H. et al. Diagnostic Accuracy of Transcranial Sonography of the Substantia Nigra in Parkinson’s disease: A Systematic Review and Meta-analysis. Sci. Rep. 6, 20863; doi: 10.1038/srep20863 (2016).
Acknowledgments
This work was supported by grants from the National Program of Basic Research (2011CB504104) of China, National Natural Science Fund (81430022, 81371407, 81071024, 81171202, 30870879, and 81471287), Shanghai Shuguang Program (11SG20), and the Fifth National Undergraduate Student Innovating Program (2011015).
Footnotes
Author Contributions D.H.L., J.L. and S.D.C. conceived and designed the experiments. D.H.L. and Y.C.H. performed publication searches and selection. D.H.L. and Y.C.H. analyzed the data. D.H.L. prepared the figures. D.H.L., Y.C.H. and J.L. contributed materials/ analysis tools. D.H.L. wrote the paper. J.L. and S.D.C. revised the paper. All authors reviewed the manuscript.
References
- Hughes A. J., Daniel S. E., Blankson S. & Lees A. J. A clinicopathologic study of 100 cases of Parkinson’s disease. Archives of neurology 50, 140–148 (1993). [DOI] [PubMed] [Google Scholar]
- Bajaj N. P. et al. Accuracy of clinical diagnosis in tremulous parkinsonian patients: a blinded video study. Journal of neurology, neurosurgery, and psychiatry 81, 1223–1228, 10.1136/jnnp.2009.193391 (2010). [DOI] [PubMed] [Google Scholar]
- Becker G., Seufert J., Bogdahn U., Reichmann H. & Reiners K. Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45, 182–184 (1995). [DOI] [PubMed] [Google Scholar]
- Stenc Bradvica I. et al. Transcranial sonography and the pocket smell test in the differential diagnosis between parkinson’s disease and essential tremor. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 10.1007/s10072-015-2152-y(2015) . [DOI] [PubMed] [Google Scholar]
- Sierra M. et al. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology 80, 621–626, 10.1212/WNL.0b013e31828250d6 (2013). [DOI] [PubMed] [Google Scholar]
- Whiting P. F. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine 155, 529–536, 10.7326/0003-4819-155-8-201110180-00009 (2011). [DOI] [PubMed] [Google Scholar]
- Ferreira M. L., Smeets R. J., Kamper S. J., Ferreira P. H. & Machado L. A. Can we explain heterogeneity among randomized clinical trials of exercise for chronic back pain? A meta-regression analysis of randomized controlled trials. Physical therapy 90, 1383–1403, 10.2522/ptj.20090332 (2010). [DOI] [PubMed] [Google Scholar]
- Deeks J. J., Macaskill P. & Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of clinical epidemiology 58, 882–893, 10.1016/j.jclinepi.2005.01.016 (2005). [DOI] [PubMed] [Google Scholar]
- Tunc S. et al. A population-based study on combined markers for early Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society 30, 531–537, 10.1002/mds.26100 (2015). [DOI] [PubMed] [Google Scholar]
- Izawa M. O., Miwa H., Kajimoto Y. & Kondo T. Combination of transcranial sonography, olfactory testing, and MIBG myocardial scintigraphy as a diagnostic indicator for Parkinson’s disease. European journal of neurology: the official journal of the European Federation of Neurological Societies 19, 411–416, 10.1111/j.1468-1331.2011.03533.x (2012). [DOI] [PubMed] [Google Scholar]
- Shin H. Y., Joo E. Y., Kim S. T., Dhong H. J. & Cho J. W. Comparison study of olfactory function and substantia nigra hyperechogenicity in idiopathic REM sleep behavior disorder, Parkinson’s disease and normal control. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 34, 935–940, 10.1007/s10072-012-1164-0(2013) . [DOI] [PubMed] [Google Scholar]
- Bottcher T. et al. Clinical, genetic, and brain sonographic features related to Parkinson’s disease in Gaucher disease. Journal of neurology 260, 2523–2531, 10.1007/s00415-013-7011-2 (2013). [DOI] [PubMed] [Google Scholar]
- Schmidauer C. et al. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Annals of neurology 58, 630–634, 10.1002/ana.20572 (2005). [DOI] [PubMed] [Google Scholar]
- Ressner P., Skoloudik D., Hlustik P. & Kanovsky P. Hyperechogenicity of the substantia nigra in Parkinson’s disease. Journal of neuroimaging: official journal of the American Society of Neuroimaging 17, 164–167, 10.1111/j.1552-6569.2007.00114.x (2007). [DOI] [PubMed] [Google Scholar]
- Stockner H. et al. Midbrain sonography in patients with essential tremor. Movement disorders: official journal of the Movement Disorder Society 22, 414–417, 10.1002/mds.21344 (2007). [DOI] [PubMed] [Google Scholar]
- Fathinia P. et al. Parkinson’s disease-like midbrain hyperechogenicity is frequent in amyotrophic lateral sclerosis. Journal of neurology 260, 454–457, 10.1007/s00415-012-6654-8 (2013). [DOI] [PubMed] [Google Scholar]
- Lauckaite K. et al. Specificity of transcranial sonography in parkinson spectrum disorders in comparison to degenerative cognitive syndromes. BMC neurology 12, 12, 10.1186/1471-2377-12-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor-Seng-Shu E. et al. Substantia nigra echogenicity and imaging of striatal dopamine transporters in Parkinson’s disease: a cross-sectional study. Parkinsonism & related disorders 20, 477–481, 10.1016/j.parkreldis.2014.01.015 (2014). [DOI] [PubMed] [Google Scholar]
- Walter U., Wittstock M., Benecke R. & Dressler D. Substantia nigra echogenicity is normal in non-extrapyramidal cerebral disorders but increased in Parkinson’s disease. Journal of neural transmission (Vienna, Austria: 1996) 109, 191–196 (2002). [DOI] [PubMed] [Google Scholar]
- Mahlknecht P. et al. Substantia nigra hyperechogenicity as a marker for Parkinson’s disease: a population-based study. Neuro-degenerative diseases 12, 212–218, 10.1159/000348595 (2013). [DOI] [PubMed] [Google Scholar]
- Kwon D. Y. et al. Transcranial brain sonography in Parkinson’s disease with restless legs syndrome. Movement disorders: official journal of the Movement Disorder Society 25, 1373–1378, 10.1002/mds.23066 (2010). [DOI] [PubMed] [Google Scholar]
- Huang Y. W., Jeng J. S., Tsai C. F., Chen L. L. & Wu R. M. Transcranial imaging of substantia nigra hyperechogenicity in a Taiwanese cohort of Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society 22, 550–555, 10.1002/mds.21372 (2007). [DOI] [PubMed] [Google Scholar]
- Fernandes Rde C. et al. Transcranial sonography as a diagnostic tool for Parkinson’s disease: a pilot study in the city of Rio de Janeiro, Brazil. Arquivos de neuro-psiquiatria 69, 892–895 (2011). [DOI] [PubMed] [Google Scholar]
- Mehnert S. et al. Transcranial sonography for diagnosis of Parkinson’s disease. BMC neurology 10, 9, 10.1186/1471-2377-10-9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresojevic N. et al. Transcranial sonography in patients with Parkinson’s disease with glucocerebrosidase mutations. Parkinsonism & related disorders 19, 431–435, 10.1016/j.parkreldis.2012.12.006 (2013). [DOI] [PubMed] [Google Scholar]
- Luo W. F., Zhang Y. C., Sheng Y. J., Fang J. C. & Liu C. F. Transcranial sonography on Parkinson’s disease and essential tremor in a Chinese population. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 33, 1005–1009, 10.1007/s10072-011-0876-x(2012). [DOI] [PubMed] [Google Scholar]
- Lauckaite K. et al. Ultrasonographic (TCS) and clinical findings in overlapping phenotype of essential tremor and Parkinson’s disease (ET-PD). BMC neurology 14, 54, 10.1186/1471-2377-14-54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., He W., Zhang H. Q., Zhang H. X. & Cao C. J. Transcranial sonography in diagnosis of Parkinson disease. Chinese Journal of Medical Imaging Technology 29, 1430–1433 (2013). [Google Scholar]
- Sheng Y. J., Zhang Y. C., Fang J. C. & Luo W. F. Predictive value of transcranial sonography in the diagnosis of Parkinson disease. Chinese Journal of Medical Imaging Technology 28, 1069–1071 (2012). [Google Scholar]
- Zhang Y. C., Fang J. C., Sheng Y. J., Luo W. F. & Liu C. F. Application of transcranial ultrasonography in diagnosis of Parkinson disease. Chinese Journal of Medical Imaging Technology 26, 2255–2257 (2010). [Google Scholar]
- Chitsaz A. et al. Transcranial sonography on Parkinson’s disease and essential tremor. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences 18, S28–31 (2013). [PMC free article] [PubMed] [Google Scholar]
- Prestel J., Schweitzer K. J., Hofer A., Gasser T. & Berg D. Predictive value of transcranial sonography in the diagnosis of Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society 21, 1763–1765, 10.1002/mds.21054 (2006). [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Kim S. T., Jeon S. H. & Lee W. Y. Midbrain transcranial sonography in Korean patients with Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society 22, 1922–1926, 10.1002/mds.21628 (2007). [DOI] [PubMed] [Google Scholar]
- Ryu J. H., Lee M. S. & Baik J. S. Sonographic abnormalities in idiopathic restless legs syndrome (RLS) and RLS in Parkinson’s disease. Parkinsonism & related disorders 17, 201–203, 10.1016/j.parkreldis.2010.11.014 (2011). [DOI] [PubMed] [Google Scholar]
- Rong Wang Y., Jiang H. L., Jiangtao X. & Li Z. [Diagnostic value of Transcanial Sonography in Parkinson’s disease]Chinese. China Journal of Modern Medicine 21, 822–825 (2011). [Google Scholar]
- Alonso-Canovas A. et al. Sonography for diagnosis of Parkinson disease-from theory to practice: a study on 300 participants. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine 33, 2069–2074, 10.7863/ultra.33.12.2069 (2014). [DOI] [PubMed] [Google Scholar]
- Ambrosius W. et al. Substantia nigra hyperechogenicity in Polish patients with Parkinson’s disease. Folia morphologica 73, 267–271, 10.5603/fm.2014.0042 (2014). [DOI] [PubMed] [Google Scholar]
- Li D., Wang B., Long H. & Wen F. Diagnostic Accuracy of Calretinin for Malignant Mesothelioma in Serous Effusions: a Meta-analysis. Scientific reports 5, 9507, 10.1038/srep09507 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter U. et al. Transcranial brain sonography findings in discriminating between parkinsonism and idiopathic Parkinson disease. Archives of neurology 64, 1635–1640, 10.1001/archneur.64.11.1635 (2007). [DOI] [PubMed] [Google Scholar]
- Borasio G. D. et al. Dopaminergic deficit in amyotrophic lateral sclerosis assessed with [I-123] IPT single photon emission computed tomography. Journal of neurology, neurosurgery, and psychiatry 65, 263–265 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideyama T., Momose T., Shimizu J., Tsuji S. & Kwak S. A positron emission tomography study on the role of nigral lesions in parkinsonism in patients with amyotrophic lateral sclerosis. Archives of neurology 63, 1719–1722, 10.1001/archneur.63.12.1719 (2006). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. Combination of olfactory test and substantia nigra transcranial sonopraphy in the differential diagnosis of Parkinson’s disease: a pilot study from China. Translational neurodegeneration 1, 25, 10.1186/2047-9158-1-25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. et al. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Archives of neurology 59, 999–1005 (2002). [DOI] [PubMed] [Google Scholar]
- Zecca L. et al. In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Movement disorders: official journal of the Movement Disorder Society 20, 1278–1285, 10.1002/mds.20550 (2005). [DOI] [PubMed] [Google Scholar]
- Behnke S., Berg D., Naumann M. & Becker G. Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. Journal of neurology, neurosurgery, and psychiatry 76, 423–425, 10.1136/jnnp.2004.049221 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez R. et al. Plasma ceruloplasmin ferroxidase activity correlates with the nigral sonographic area in Parkinson’s disease patients: a pilot study. Neurochemical research 36, 2111–2115, 10.1007/s11064-011-0535-x (2011). [DOI] [PubMed] [Google Scholar]
- Walter U., Witt R., Wolters A., Wittstock M. & Benecke R. Substantia nigra echogenicity in Parkinson’s disease: relation to serum iron and C-reactive protein. Journal of neural transmission (Vienna, Austria: 1996) 119, 53–57, 10.1007/s00702-011-0664-z (2012). [DOI] [PubMed] [Google Scholar]
- Berg D., Godau J., Riederer P., Gerlach M. & Arzberger T. Microglia activation is related to substantia nigra echogenicity. Journal of neural transmission (Vienna, Austria: 1996) 117, 1287–1292, 10.1007/s00702-010-0504-6 (2010). [DOI] [PubMed] [Google Scholar]
- Sadowski K., Szlachta K., Serafin-Krol M., Galazka-Friedman J. & Friedman A. Brain tissue echogenicity–implications for substantia nigra studies in parkinsonian patients. Journal of neural transmission (Vienna, Austria: 1996) 119, 363–367, 10.1007/s00702-011-0707-5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I., Bubacco L. & Greggio E. LRRK2 and neuroinflammation: partners in crime in Parkinson’s disease? Journal of neuroinflammation 11, 52, 10.1186/1742-2094-11-52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapansky J., Nardozzi J. D. & LaVoie M. J. The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson’s disease. Neuroscience, 10.1016/j.neuroscience.2014.09.049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenah J. M. et al. Transcranial sonography findings in a large family with homozygous and heterozygous PINK1 mutations. Journal of neurology, neurosurgery, and psychiatry 79, 1071–1074, 10.1136/jnnp.2007.142174 (2008). [DOI] [PubMed] [Google Scholar]
- Barrett M. J. et al. Transcranial sonography and functional imaging in glucocerebrosidase mutation Parkinson disease. Parkinsonism & related disorders 19, 186–191, 10.1016/j.parkreldis.2012.09.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. H. et al. Transcranial sonography of the substantia nigra and its correlation with DAT-SPECT in the diagnosis of Parkinson’s disease. Parkinsonism & related disorders 21, 923–928, 10.1016/j.parkreldis.2015.05.024 (2015). [DOI] [PubMed] [Google Scholar]
- Spiegel J. et al. Transcranial sonography and [123I]FP-CIT SPECT disclose complementary aspects of Parkinson’s disease. Brain: a journal of neurology 129, 1188–1193, 10.1093/brain/awl042 (2006). [DOI] [PubMed] [Google Scholar]
- Okawa M. et al. Transcranial sonography of the substantia nigra in Japanese patients with Parkinson’s disease or atypical parkinsonism: clinical potential and limitations. Internal medicine (Tokyo, Japan) 46, 1527–1531 (2007). [DOI] [PubMed] [Google Scholar]
- Sakalauskas A., Lukosevicius A., Lauckaite K., Jegelevicius D. & Rutkauskas S. Automated segmentation of transcranial sonographic images in the diagnostics of Parkinson’s disease. Ultrasonics 53, 111–121, 10.1016/j.ultras.2012.04.005 (2013). [DOI] [PubMed] [Google Scholar]
- Plate A. et al. Three-dimensional sonographic examination of the midbrain for computer-aided diagnosis of movement disorders. Ultrasound in medicine & biology 38, 2041–2050, 10.1016/j.ultrasmedbio.2012.07.017 (2012). [DOI] [PubMed] [Google Scholar]
- Halligan S. et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 237, 893–904, 10.1148/radiol.2373050176 (2005). [DOI] [PubMed] [Google Scholar]