Abstract
Background
Trebananib, an investigational peptibody, binds to angiopoietin–1/–2, thereby blocking their interaction with Tie2.
Patients and Methods
This open–label phase 1 study examined trebananib 3 mg/kg or 10 mg/kg IV QW plus sorafenib 400 mg BID or sunitinib 50 mg QD in advanced renal cell carcinoma (RCC). Primary endpoints were adverse event incidence and pharmacokinetics.
Results
Thirty–seven patients were enrolled. During trebananib plus sorafenib administration (n = 17), the most common treatment-related adverse events (TRAEs) included rash (71%), diarrhea (71%), hypertension (65%), and fatigue (65%); grade ≥ 3 TRAEs (41%); and 14% of patients had peripheral edema. During trebananib plus sunitinib administration (n = 19), the most common TRAEs included diarrhea (74%), fatigue (68%), hypertension (58%), and decreased appetite (58%); grade ≥ 3 TRAEs (68%); and 42% of patients had peripheral edema. Trebananib did not appear to alter the pharmacokinetics of sorafenib or sunitinib. No patient developed anti-trebananib antibodies. Objective response rates were 29% (trebananib plus sorafenib) and 53% (trebananib plus sunitinib).
Conclusions
The toxicities of trebananib IV 3 mg/kg or 10 mg/kg plus sorafenib or sunitinib in RCC were similar to those of sorafenib or sunitinib monotherapy, with peripheral edema being likely specific to the combinations. Antitumor activity was observed.
Keywords: Angiogenesis, Angiopoietins, Targeted therapies, Tie2 receptor, Vascular growth factor receptor
Introduction
Simultaneous administration of multiple angiogenic inhibitors targeting the vascular endothelial growth factor (VEGF) pathway in patients with metastatic renal cell carcinoma (RCC) has not produced the expected additive therapeutic effects and has been associated with increased toxicity relative to therapies with single VEGF pathway inhibitors.1–3 One treatment approach that may circumvent some of those limitations involves the combination of inhibitors targeting separate angiogenic pathways.
The angiopoietin pathway is a critically important contributor to angiogenesis.4–6 The pathway ligands angiopoietin 1 and 2 (Ang1 and Ang2) bind to Tie2, a receptor tyrosine kinase primarily expressed in the vascular endothelium. Ang1 and Ang2 promote tumor angiogenesis through vessel remodeling and stabilization. Moreover, Ang2 elevations have been associated with various cancer types and worse clinical outcome.7–10 Trebananib, an investigational peptide Fc–fusion protein (“peptibody”), suppresses tumor angiogenesis by binding to Ang1 and Ang2 and blocking their interaction with Tie2.11 In tumor xenograft models, dual inhibition of Ang1 and Ang2 was associated with greater suppression of angiogenesis and tumor growth when compared with single inhibition of either ligand.11 A first–in–human dose escalation study of patients with advanced solid tumors receiving trebananib monotherapy demonstrated antitumor activity and a specific and manageable toxicity profile.12 In a phase 2 estimation study of patients with recurrent ovarian cancer, the combination of trebananib and paclitaxel was associated with a prolongation of progression–free survival (PFS).13
The objectives of the current study were to evaluate the tolerability, pharmacokinetic (PK) and biomarker profiles, and antitumor activity of trebananib in combination with the VEGF pathway inhibitors sorafenib and sunitinib in patients with advanced RCC.
Patients and Methods
This open–label, phase 1b study evaluated trebananib combined with the VEGF pathway inhibitors sorafenib (Nexavar®; Bayer HealthCare Pharmaceuticals Corporation, Montville, NJ, USA; Onyx Pharmaceuticals, Emeryville, CA, USA), sunitinib (Sutent®; Pfizer, New York, NY, USA), bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA, USA), or motesanib. All patients receiving trebananib plus sorafenib or sunitinib had diagnoses of advanced RCC. Patients in the trebananib plus bevacizumab or motesanib treatment arms had diagnoses of advanced solid tumors across various tumor types. Because comparisons between the two patient groups (ie, patients with RCC and patients with solid tumors across various tumor types) would not be valid, the current report focuses only on the subset of patients with advanced RCC. The results related to patients with advanced solid tumors receiving trebananib plus bevacizumab or motesanib will be reported elsewhere. The study protocol was approved by independent institutional review boards, and the study was conducted in accordance with the Declaration of Helsinki. Patients (≥ 18 years) had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2; and adequate hematological, renal, and hepatic function. Patients with head and neck cancer were later excluded based on safety findings from one of the cohorts receiving trebananib and bevacizumab. Patients with a history of gastrointestinal disease were excluded as the disease may limit absorption of an oral agent. Patients with cardiovascular events during the year prior to enrollment were also excluded; those events included myocardial infarction, unstable or severe angina, coronary or peripheral artery bypass graft, unstable cardiac arrhythmia requiring medication, symptomatic congestive heart failure, and cerebrovascular accident or transient ischemic attack. Additional exclusion criteria included central nervous system metastasis; a recent anticancer therapy; therapies involving rifampin, phenobarbitol, HIV protease or strong cytochrome P450 3A (CYP3A) suppressors, immune modulators, St. John's wort, or coumarin anticoagulants ≥ 2 mg/day; previous treatment with radiation therapy, trebananib, bevacizumab, sorafenib (for the sorafenib cohorts), and sunitinib (for the sunitinib cohorts).
Study Design and Treatment
Sorafenib and sunitinib are orally administered multikinase antagonists to the VEGF and platelet–derived growth factor receptors14,15 and indicated for the treatment of advanced RCC.16,17 Primary endpoints were the incidence of adverse events (AEs) and PK profiles. Secondary endpoints included tumor response and changes in biomarkers.
The following cohorts were enrolled: trebananib 3 mg/kg plus sorafenib 400 mg, trebananib 10 mg/kg plus sorafenib 400 mg, trebananib 3 mg/kg plus sunitinib 50 mg, and trebananib 10 mg/kg plus sunitinib 50 mg. Trebananib was administered intravenously (IV) once weekly (QW). Patients self–administered sorafenib orally twice daily (BID) and sunitinib (4 weeks on/2 weeks off) orally once daily (QD). Sorafenib and sunitinib dosing started on day 1 of week 1 followed by trebananib on day 1 of week 2. Trebananib doses were selected based on a previous monotherapy study in patients with advanced solid tumors; the half–life of trebananib at 3 mg/kg and 10 mg/kg in that study was approximately 4 and 6 days, respectively.12 Recommended doses as indicated in the labeling approved by the Food and Drug Administration as of December 2005 and February 2007 were selected for sorafenib and sunitinib, respectively.
A dose–limiting toxicity (DLT) was any grade 3 or greater toxicity, except for the following modifications: transient grade 3 infusion reactions lasting more than 2 hours; grade 3 fatigue or grade 4 neutropenia for more than 7 days; grade 3 or 4 nausea, diarrhea, vomiting, or neutropenia with fever above 38.5°C; grade 4 aspart ate or alanine aminotransferase greater than 10 times the upper limit of normal; and grade 4 thrombocytopenia, anemia, or hypertension. The DLT had to occur during the initial 28 or 42 treatment days for the trebananib plus sorafenib or trebananib plus sunitinib cohorts, respectively.
Initially, three patients entered the trebananib 3 mg/kg plus sorafenib or sunitinib dose cohorts. If no DLT occurred in either cohort, up to six patients were enrolled in the trebananib 10 mg/kg plus sorafenib or sunitinib dose cohorts; three additional patients could also be enrolled at the investigator's discretion in the trebananib 3 mg/kg cohorts. If two or more patients out of the initial three patients in the trebananib 3 mg/kg cohorts had a DLT, no additional patients were enrolled; if one patient experienced a DLT, up to three additional patients were enrolled in that cohort. In this expanded cohort, if fewer than three patients experienced a DLT, up to six patients were enrolled in the trebananib 10 mg/kg cohorts. Finally, for one dose cohort of each treatment combination, an additional ten patients could be enrolled. A new patient was added for any patient with a trebananib dosing delay or sorafenib or sunitinib dose reduction for non–DLT–related toxicities during the DLT period. Patients who had a DLT during week 1 or withdrew during the first study month were replaced and excluded from the analyses.
Adverse Events
Treatment–related AEs (ie, AEs possibly related to trebananib, sorafenib, or sunitinib administration) per clinical investigator assessment were recorded and graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (NCI–CTCAE 3.0). Serum samples for evaluation of anti–trebananib antibodies were collected prior to dosing during weeks 1, 3, 5, and 8, and every 4 weeks thereafter. Anti–trebananib binding antibodies were detected with a validated electrochemiluminescence (ECL) assay and then further analyzed for neutralizing effects in an ECL receptor–binding neutralizing antibody assay.18
Pharmacokinetics
Serum trebananib concentrations were measured using a validated enzyme–linked immunosorbent assay (MDS Pharma Services, Montreal, Canada; Tandem Labs, Trenton, NJ, USA).12 Plasma concentrations of sorafenib, sunitinib, and sunitinib's active metabolite were analyzed with a validated liquid chromatography/mass spectrometry (LC–MS/MS) method at Covance Laboratories (Madison, WI, USA) for sorafenib, and at BASi Northwest Laboratory (McMinnville, OR, USA) for sunitinib. All PK parameters were estimated by implementing noncompartmental methods with WinNonlin Professional (version 4.1e; Pharsight, Mountain View, CA, USA). The schedule of PK assessments is detailed in the Supplementary Materials (Appendix S1).
Biomarkers
A detailed description of the methodology for measurement of biomarkers has been described previously.19 Serum samples were analyzed for placental growth factor (PLGF), soluble VEGF receptor 1 (sVEGFR–1 or sFlt–1) and 2 (sVEGFR–2), VEGF, soluble c–Kit (sKit), soluble intercellular adhesion molecule 1 (sICAM–1), and vascular cell adhesion molecule 1 (sVCAM–1). Plasma samples were analyzed for hepatocyte growth factor (HGF). Samples were collected according to the schedule described in the Supplementary Materials (Appendix S2).
Tumor Response Evaluations
Tumor assessments were conducted using computer tomography or magnetic resonance imaging within 4 weeks before the treatment start and every 8 weeks thereafter. Tumor response was determined based on the Response Evaluation Criteria in Solid Tumors (RECIST 1.0).20 The denominator for calculating objective response rates (ORRs) was based on all patients receiving at least one dose of trebananib (n = 36). Time to progression (TTP) per investigators' review was calculated as the number of weeks from the first administered dose of trebananib to disease progression; patients who completed the study without developing disease progression were censored. Because the study was not designed to follow patients until death, progression–free survival (PFS) was not estimated.
Statistical Analysis
Responses in biomarkers were evaluated with a regression using an F–test comparing log–transformed analyte relative to baseline values. Descriptive statistics are provided for tolerability, toxicity, PK, and tumor response analyses.
Results
Between September 2006 and April 2010, 36 of 37 enrolled patients received at least one dose of trebananib. Demographic and clinical characteristics are summarized in Table 1; duration of study participation is depicted in Supplementary Figure 1.
Table 1. Demographic and Clinical Characteristicsa.
| Trebananib 3 mg/kg + Sorafenib (n = 3) | Trebananib 10 mg/kg + Sorafenib (n = 14) | Trebananib 3 mg/kg + Sunitinib (n = 5) | Trebananib 10 mg/kg + Sunitinib (n = 14) | All Cohorts Combined (n = 36) | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Female | 2 (67) | 7 (50) | 1 (20) | 4 (29) | 14 (39) |
| Male | 1 (33) | 7 (50) | 4 (80) | 10 (71) | 22 (61) |
| Race/Ethnicity, n (%) | |||||
| Caucasian | 2 (67) | 14 (100) | 5 (100) | 9 (64) | 30 (83) |
| African American | 1 (33) | 0 (0) | 0 (0) | 1 (7) | 2 (6) |
| Hispanic | 0 (0) | 0 (0) | 0 (0) | 4 (29) | 4 (11) |
| Asian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Age, median (range), years ECOG Score, n (%) | 64 (61 – 70) | 59 (47 – 72) | 49 (44 – 65) | 63 (42 – 76) | 61 (42 – 76) |
| 0 | 2 (67) | 8 (57) | 2 (40) | 11 (79) | 23 (64) |
| 1 | 1 (33) | 6 (43) | 3 (60) | 3 (21) | 13 (36) |
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Disease Stage at Enrollment, n (%) | |||||
| II | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| III | 0 (0) | 1 (7) | 1 (20) | 2 (14) | 4 (11) |
| IV | 3 (100) | 13 (93) | 4 (80) | 12 (86) | 32 (89) |
| Lines of Prior Chemotherapy, n (%) | |||||
| 0 | 0 (0) | 2 (14) | 1 (20) | 8 (57) | 11 (31) |
| 1 | 3 (100) | 5 (36) | 1 (20) | 5 (36) | 14 (39) |
| 2 | 0 (0) | 1 (7) | 1 (20) | 0 (0) | 2 (6) |
| ≥ 3 | 0 (0) | 6 (43) | 2 (40) | 1 (7) | 9 (25) |
| Any Prior Radiotherapy, n (%) | |||||
| Yes | 0 (0) | 2 (14) | 2 (40) | 1 (7) | 5 (14) |
| No | 3 (100) | 12 (86) | 3 (60) | 13 (93) | 31 (86) |
| Any Prior Cancer-Related Surgery, n (%) | |||||
| Yes | 3 (100) | 14 (100) | 5 (100) | 14 (100) | 36 (100) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Reason for Study Discontinuation, n (%)b | |||||
| Disease progression | 1 (25) | 10 (71) | 4 (80) | 4 (29) | 19 (53) |
| Withdrawal of consent | 1 (25) | 2 (14) | 0 (0) | 1 (7) | 4 (11) |
| Adverse events | 2 (50) | 1 (7) | 0 (0) | 1 (7) | 4 (11) |
| Death | 0 (0) | 0 (0) | 0 (0) | 3 (21)c | 3 (8) |
| Noncompliance | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 1 (3) |
| Requirement for alternative therapy | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (3) |
| Other | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 1 (3) |
Abbreviation: ECOG = Eastern Cooperative Oncology Group
Demographic and clinical characteristics are presented for all patients who received ≥ 1 dose of trebananib.
Reasons for study discontinuation are presented for all patients who provided informed consent.
Four patients died in the trebananib 10 mg/kg plus sunitinib cohort. For one of those patients, the reason for study discontinuation was categorized as an adverse event. The adverse event was sepsis, which led to the patient's death.
Tolerability
No patient in the trebananib 3 mg/kg plus sorafenib and sunitinib cohorts (n = 3 and 5, respectively) experienced a DLT. Consequently, six patients were enrolled in both trebananib 10 mg/kg cohorts (sorafenib and sunitinib), which were later expanded to include fourteen patients in each cohort. Two patients in the trebananib 10 mg/kg plus sorafenib cohort experienced a DLT (grade 3 decreased blood phosphorus and grade 3 diarrhea); one patient in the trebananib 10 mg/kg plus sunitinib cohort developed a DLT (grade 3 hypertension).
Unless otherwise noted, the AEs summarized here represent treatment–related AEs (ie, AEs possibly related to trebananib, sorafenib, or sunitinib administration) per clinical investigator assessment. Across both trebananib plus sorafenib cohorts, the most common treatment–related AEs were rash (71%), diarrhea (71%), hypertension (65%), and fatigue (65%; Table 2). Two patients (14%) in the 10 mg/kg cohort experienced peripheral edema. Two patients (67%) in the 3 mg/kg cohort and 5 patients (36%) in the 10 mg/kg cohort had grade ≥ 3 treatment–related AEs. Two grade 4 AEs (12%; pulmonary embolism, n = 1; hyperuricemia, n = 1) occurred on study, but they were not considered to be related to treatment. No patients died or developed anti–trebananib antibodies.
Table 2. Patient Incidence of Treatment–Related Adverse Eventsa in the Trebananib Plus Sorafenib Cohortsb.
| Trebananib 3 mg/kg + Sorafenib (n = 3) | Trebananib 10 mg/kg + Sorafenib (n = 14) | Trebananib + Sorafenib Cohorts Combined (n = 17) | ||||
|---|---|---|---|---|---|---|
| Patients With Any Treatment–Related Adverse Event, n (%) | 3 (100) | 14 (100) | 17 (100) | |||
| Grade ≥ 3 | 2 (67) | 5 (36) | 7 (41) | |||
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | |||
| Grade 5 | 0 (0) | 0 (0) | 0 (0) | |||
|
| ||||||
| Treatment–Related Adverse Events Occurring in > 10% of Patients in one or More Treatment Arms, n (%) | All grades | Grade ≥ 3 | All grades | Grade ≥ 3 | All grades | Grade ≥ 3 |
| Rash | 2 (67) | 0 (0) | 10 (71) | 1 (7) | 12 (71) | 1 (6) |
| Diarrhea | 2 (67) | 0 (0) | 10 (71) | 1 (7) | 12 (71) | 1 (6) |
| Hypertension | 3 (100) | 1 (33) | 8 (57) | 0 (0) | 11 (65) | 1 (6) |
| Fatigue | 3 (100) | 0 (0) | 8 (57) | 0 (0) | 11 (65) | 0 (0) |
| Decreased appetite | 2 (67) | 0 (0) | 6 (43) | 0 (0) | 8 (47) | 0 (0) |
| Nausea | 1 (33) | 0 (0) | 7 (50) | 0 (0) | 8 (47) | 0 (0) |
| Alopecia | 2 (67) | 0 (0) | 5 (36) | 0 (0) | 7 (41) | 0 (0) |
| Palmar–plantar erythrodysesthesia syndrome | 0 (0) | 0 (0) | 6 (43) | 0 (0) | 6 (35) | 0 (0) |
| Decreased weight | 2 (67) | 1 (33) | 3 (21) | 0 (0) | 5 (29) | 1 (6) |
| Dysgeusia | 1 (33) | 0 (0) | 3 (21) | 0 (0) | 4 (24) | 0 (0) |
| Headache | 1 (33) | 0 (0) | 3 (21) | 0 (0) | 4 (24) | 0 (0) |
| Vomiting | 1 (33) | 0 (0) | 3 (21) | 0 (0) | 4 (24) | 0 (0) |
| Oral pain | 0 (0) | 0 (0) | 3 (21) | 0 (0) | 3 (18) | 0 (0) |
| Increased aspartate aminotransferase | 1 (33) | 0 (0) | 2 (14) | 0 (0) | 3 (18) | 0 (0) |
| Peripheral neuropathy | 2 (67) | 0 (0) | 1 (7) | 0 (0) | 3 (18) | 0 (0) |
| Dysphonia | 1 (33) | 0 (0) | 2 (14) | 0 (0) | 3 (18) | 0 (0) |
| Erythema | 0 (0) | 0 (0) | 3 (21) | 0 (0) | 3 (18) | 0 (0) |
| Skin exfoliation | 0 (0) | 0 (0) | 3 (21) | 0 (0) | 3 (18) | 0 (0) |
| Hypophosphatemia | 1 (33) | 0 (0) | 1 (7) | 1 (7) | 2 (12) | 1 (6) |
| Dry mouth | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (12) | 0 (0) |
| Peripheral edema | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (12) | 0 (0) |
| Mucosal inflammation | 1 (33) | 0 (0) | 1 (7) | 0 (0) | 2 (12) | 0 (0) |
| Arthralgia | 1 (33) | 0 (0) | 1 (7) | 0 (0) | 2 (12) | 0 (0) |
| Pain in extremity | 1 (33) | 0 (0) | 1 (7) | 0 (0) | 2 (12) | 0 (0) |
| Proteinuria | 2 (67) | 0 (0) | 0 (0) | 0 (0) | 2 (12) | 0 (0) |
| Skin fissures | 1 (33) | 0 (0) | 1 (7) | 0 (0) | 2 (12) | 0 (0) |
Treatment–related adverse events include all adverse events that were considered to be possibly related to any of the study treatments per clinical investigator assessment.
The analysis set for the evaluation of treatment–related adverse events includes all patients who received ≥ 1 dose of trebananib.
In the trebananib plus sunitinib cohorts, the most frequent treatment–related AEs included diarrhea (74%), fatigue (68%), hypertension (58%), and decreased appetite (58%; Table 3). Two patients (40%) in the 3 mg/kg cohort and six patients (43%) in the 10 mg/kg cohort had peripheral edema. Two patients (40%) in the 3 mg/kg cohort and eleven patients (79%) in the 10 mg/kg cohort experienced grade ≥ 3 treatment–related AEs. One patient (7%) in the 10 mg/kg cohort had two grade 4 AEs (congestive cardiac failure and myocardial infarction); four patients (29%) in the 10 mg/kg cohort died (metastatic RCC, renal failure, cerebral ischemia, and sepsis; all n = 1). None of the grade 4 AEs or deaths were considered to be related to treatment. One patient in each of the cohorts had pre–existing anti–trebananib binding antibodies. However, no patient developed anti–trebananib antibodies on study.
Table 3. Patient Incidence of Treatment-Related Adverse Eventsa in the Trebananib Plus Sunitinib Cohortsb.
| Trebananib 3 mg/kg + Sunitinib (n = 5) | Trebananib 10 mg/kg + Sunitinib (n = 14) | Trebananib + Sunitinib Cohorts Combined (n = 19) | ||||
|---|---|---|---|---|---|---|
| Patients With Any Treatment-Related Adverse Event, n (%) | 5 (100) | 14 (100) | 19 (100) | |||
| Grade ≥ 3 | 2 (40) | 11 (79) | 13 (68) | |||
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | |||
| Grade 5 | 0 (0) | 0 (0) | 0 (0) | |||
|
| ||||||
| Treatment-Related Adverse Events Occurring in > 10% of Patients in 1 or More Treatment Arms, n (%) | All grades | Grade ≥ 3 | All grades | Grade ≥ 3 | All grades | Grade ≥ 3 |
| Diarrhea | 4 (80) | 0 (0) | 10 (71) | 1 (7) | 14 (74) | 1 (5) |
| Fatigue | 3 (60) | 1 (20) | 10 (71) | 2 (14) | 13 (68) | 3 (16) |
| Hypertension | 4 (80) | 0 (0) | 7 (50) | 2 (14) | 11 (58) | 2 (11) |
| Decreased appetite | 2 (40) | 0 (0) | 9 (64) | 0 (0) | 11 (58) | 0 (0) |
| Thrombocytopenia | 2 (40) | 1 (20) | 6 (43) | 1 (7) | 8 (42) | 2 (11) |
| Peripheral edema | 2 (40) | 0 (0) | 6 (43) | 0 (0) | 8 (42) | 0 (0) |
| Dysgeusia | 2 (40) | 0 (0) | 6 (43) | 0 (0) | 8 (42) | 0 (0) |
| Dry skin | 3 (60) | 0 (0) | 5 (36) | 0 (0) | 8 (42) | 0 (0) |
| Hypothyroidism | 3 (60) | 0 (0) | 4 (29) | 0 (0) | 7 (37) | 0 (0) |
| Periorbital edema | 1 (20) | 0 (0) | 6 (43) | 0 (0) | 7 (37) | 0 (0) |
| Mucosal inflammation | 3 (60) | 0 (0) | 4 (29) | 0 (0) | 7 (37) | 0 (0) |
| Stomatitis | 1 (20) | 0 (0) | 5 (36) | 2 (14) | 6 (32) | 2 (11) |
| Nausea | 2 (40) | 1 (20) | 4 (29) | 0 (0) | 6 (32) | 1 (5) |
| Neutropenia | 1 (20) | 1 (20) | 4 (29) | 4 (29) | 5 (26) | 5 (26) |
| Increased blood creatinine | 1 (20) | 0 (0) | 5 (36) | 0 (0) | 6 (32) | 0 (0) |
| Increased alanine aminotransferase | 0 (0) | 0 (0) | 4 (29) | 1 (7) | 4 (21) | 1 (5) |
| Increased aspartate aminotransferase | 0 (0) | 0 (0) | 4 (29) | 1 (7) | 4 (21) | 1 (5) |
| Oral pain | 1 (20) | 0 (0) | 3 (21) | 0 (0) | 4 (21) | 0 (0) |
| Dehydration | 2 (40) | 0 (0) | 2 (14) | 0 (0) | 4 (21) | 0 (0) |
| Arthralgia | 2 (40) | 0 (0) | 2 (14) | 0 (0) | 4 (21) | 0 (0) |
| Pain in extremity | 1 (20) | 0 (0) | 3 (21) | 0 (0) | 4 (21) | 0 (0) |
| Rash | 1 (20) | 0 (0) | 3 (21) | 0 (0) | 4 (21) | 0 (0) |
| Vomiting | 2 (40) | 1 (20) | 1 (7) | 0 (0) | 3 (16) | 1 (5) |
| Increased lacrimation | 1 (20) | 0 (0) | 2 (14) | 0 (0) | 3 (16) | 0 (0) |
| Abdominal pain | 1 (20) | 0 (0) | 2 (14) | 0 (0) | 3 (16) | 0 (0) |
| Face edema | 1 (20) | 0 (0) | 2 (14) | 0 (0) | 3 (16) | 0 (0) |
| Dysphonia | 2 (40) | 0 (0) | 1 (7) | 0 (0) | 3 (16) | 0 (0) |
| Palmar-plantar erythrodysesthesia syndrome | 2 (40) | 0 (0) | 1 (7) | 0 (0) | 3 (16) | 0 (0) |
| Skin fissures | 1 (20) | 0 (0) | 2 (14) | 0 (0) | 3 (16) | 0 (0) |
| Anemia | 0 (0) | 0 (0) | 2 (14) | 1 (7) | 2 (11) | 1 (5) |
| Asthenia | 0 (0) | 0 (0) | 2 (14) | 1 (7) | 2 (11) | 1 (5) |
| Peripheral neuropathy | 0 (0) | 0 (0) | 2 (14) | 1 (7) | 2 (11) | 1 (5) |
| Erythema | 1 (20) | 1 (20) | 1 (7) | 0 (0) | 2 (11) | 1 (5) |
| Leukopenia | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (11) | 0 (0) |
| Abdominal distension | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Constipation | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (11) | 0 (0) |
| Flatulence | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (11) | 0 (0) |
| Gastroesophageal reflux disease | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Glossodynia | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Generalized edema | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 0 (0) |
| Temperature intolerance | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 0 (0) |
| Hyperbilirubinemia | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (11) | 0 (0) |
| Decreased ejection fraction | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (11) | 0 (0) |
| Myalgia | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Headache | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (11) | 0 (0) |
| Proteinuria | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 2 (11) | 0 (0) |
| Genital rash | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 0 (0) |
| Epistaxis | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Nasal dryness | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Hyperkeratosis | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Pruritus | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Skin exfoliation | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
| Blister | 1 (20) | 0 (0) | 1 (7) | 0 (0) | 2 (11) | 0 (0) |
Treatment-related adverse events include all adverse events that were considered to be possibly related to any of the study treatments per clinical investigator assessment.
The analysis set for the evaluation of treatment-related adverse events includes all patients who received ≥ 1 dose of trebananib.
Pharmacokinetics
The mean serum concentration–time profiles of trebananib plus sorafenib and sunitinib at week 4 were similar to those reported in an earlier phase 1 monotherapy study of trebananib (Table 4, Figure 1A).12 Coadministration of trebananib did not appear to alter the PK of sorafenib or sunitinib (Table 5; Figures 1B, C, & D).
Table 4. Pharmacokinetics of Trebananib.
| Descriptive Statistics | Tmax (hr)a | Cmax (μg/mL) | AUC0-T (hr·μg/mL) | CL (μg/mL) |
|---|---|---|---|---|
| Trebananib 3 mg/kg + Sorafenib 400 mg | ||||
|
| ||||
| n | 2 | 2 | 2 | 2 |
| Mean | 0.64 | 102 | 4400 | 0.552 |
| %CV | NA | NA | NA | NA |
|
| ||||
| Trebananib 10 mg/kg + Sorafenib 400 mg | ||||
|
| ||||
| n | 9 | 9 | 8 | 7 |
| Mean | 38 | 343 | 15900 | 0.566 |
| %CV | 190 | 42.3 | 30.4 | 29.3 |
|
| ||||
| Trebananib 3 mg/kg + Sunitinib 50 mg | ||||
|
| ||||
| n | 4 | 4 | 4 | 4 |
| Mean | 0.57 | 96.6 | 3540 | 0.799 |
| %CV | 13 | 14.8 | 29.4 | 33.1 |
|
| ||||
| Trebananib 10 mg/kg + Sunitinib 50 mg | ||||
|
| ||||
| n | 11 | 11 | 11 | 11 |
| Mean | 0.52 | 214 | 9490 | 0.927 |
| %CV | 160 | 25.4 | 31.0 | 39.6 |
Abbreviations: AUC0-T = Area under the concentration-time curve from time zero to the time of the dosing interval; Cmax = Maximum observed concentration after IV infusion of trebananib; CL = Clearance; CV = coefficient of variation; NA = Not applicable; Tmax = Time of maximum concentration after IV infusion of trebananib.
Tmax is reported as median and range.
Figure 1.
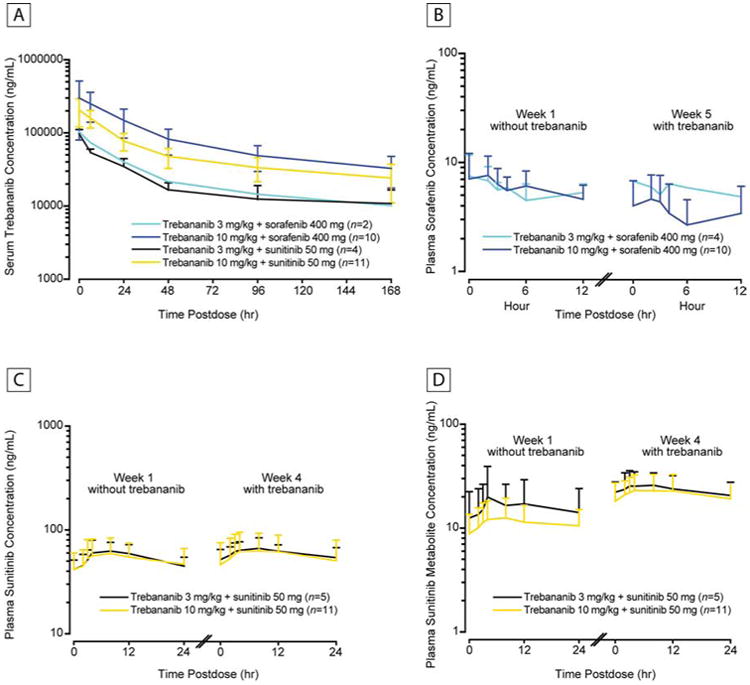
Pharmacokinetic Concentration–Time Profiles. (A) Mean (± SD) Serum Concentration–Time Profiles of Trebananib at Week 4 or 5 Following Weekly IV Infusions of Trebananib in Combination With Sorafenib or Sunitinib. (B) Mean (+ SD) Plasma Concentration–Time Profiles of Sorafenib at Week 1 Without Trebananib and at Week 5 With IV Infusions of Trebananib 3 mg/kg or 10 mg/kg. (C) Mean (+ SD) Plasma Concentration–Time Profiles of Sunitinib at Week 1 Without Trebananib and at Week 4 With IV Infusions of Trebananib 3 mg/kg or 10 mg/kg. (D) Mean (+ SD) Plasma Concentration–Time Profiles of Sunitinib Metabolite at Week 1 Without Trebananib and at Week 4 With IV Infusions of Trebananib 3 mg/kg or 10 mg/kg.
Abbreviations: SD = standard deviation.
Table 5. Pharmacokinetic Parameters of Sorafenib, Sunitinib, and Sunitinib Metabolite With and Without Trebananib.
| Sorafenib | Sorafenib | Sunitinib | Sunitinib | Sunitinib Metabolite | Sunitinib Metabolite | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Trebananib Dose | Without | 3 mg/kg | Without | 10 mg/kg | Without | 3 mg/kg | Without | 10 mg/kg | Without | 3 mg/kg | Without | 10 mg/kg |
| Tmax (hr)a | ||||||||||||
| Mean | 1.1 | 1.0 | 1.0 | 1.0 | 8.0 | 8.0 | 8.1 | 8.3 | 8.1 | 8.0 | 8.0 | 8.0 |
| %CV | 100 | 200 | 37 | NA | 24 | 46 | 35 | 54 | 34 | 39 | 64 | 49 |
| Cmax (μg/mL) | ||||||||||||
| Mean | 0.466 | 0.507 | 0.559 | 0.541 | 0.0642 | 0.0703 | 0.0618 | 0.0676 | 0.0212 | 0.0274 | 0.0133 | 0.025 |
| %CV | 75.9 | 40.6 | 53.2 | NA | 25.9 | 20.2 | 42.9 | 47.0 | 88.1 | 36.5 | 51.6 | 37.7 |
| AUCa (hr·μg/mL) | ||||||||||||
| Mean | 2.58 | 3.18 | 3.13 | 3.92 | 1.30 | 1.337 | 1.25 | 1.41 | 0.448 | 0.566 | 0.268 | 0.486 |
| %CV | 68.1 | 89.7 | 12.2 | NA | 23.4 | 24.5 | 40.4 | 49.6 | 64.0 | 34.1 | 48.1 | 39.2 |
| CL (μg/mL) | ||||||||||||
| Mean | 20.9 | 173 | 14.2 | 78.7 | 44.8 | 54.3 | 46.6 | 50.7 | 14.1 | 20.7 | 10.5 | 19.2 |
| %CV | 79.6 | 159 | 50.7 | NA | 21.7 | 24.6 | 42.1 | 57.6 | 70.4 | 34.4 | 44.1 | 44.4 |
Abbreviations: AUC = Area under the concentration-time curve; Cmax = Maximum observed concentration after IV infusion of trebananib; CL = Clearance; CV = coefficient of variation; NA = Not applicable; Tmax = Time of maximum concentration after IV infusion of trebananib.
Sorafenib AUC0-12; sunitinib AUC0-24.
Biomarkers
In the current report, only the analyses pertaining to PLGF, sVEGFR–2, VEGF, and sKit are presented. No pharmacodynamic effect was observed for sVEGFR–1 or sFlt–1, slCAM–1, sVCAM–1, and HGF. Relative to baseline, PLGF significantly increased and sVEGFR–2 significantly decreased by day 1 of week 4 in both cohorts of trebananib plus sunitinib (Supplementary Figure 2A & B). A significant increase in PLGF (P < 0.01) was also observed in the trebananib 10 mg/kg plus sorafenib cohort. Serum VEGF concentrations significantly increased in the trebananib 10 mg/kg plus sunitinib cohort after day 1 of week 2 (P < 0.01; Supplementary Figure 2C). Steady decreases in sKit levels were significant at the end of study for the trebananib 3 mg/kg plus sorafenib and both trebananib plus sunitinib cohorts (all P < 0.01; Supplementary Figure 2D).
Tumor Response
Tumor response was evaluated in 32 patients (Figure 2). Three patients did not have postbaseline tumor measurements (treatment was terminated for two patients after they developed AEs during the first month; technical problems prevented tumor response measurement for another patient). In the trebananib plus sorafenib cohorts, the ORR was 29%. No patient achieved a complete response. Five patients (29%; 3 mg/kg cohort, n = 1; 10 mg/kg cohort, n = 4) had a partial response with a median time to response of 28.6 weeks. Eight patients (47%; 3 mg/kg cohort, n = 2; 10 mg/kg cohort, n = 6) had stable disease as their best response, four of whom (24%) had stable disease > 6 months. Four patients with a partial response or stable disease did not develop progressive disease before the data analysis cutoff date. Up until treatment week 8, tumor size decreased a median of 16.58% and 14.46% in the 3 mg/kg and 10 mg/kg cohorts, respectively. The median TTP (95% CI) was 40.3 weeks (22.9 weeks – 127.4 weeks) for patients across both trebananib plus sorafenib dose cohorts (n = 17).
Figure 2.
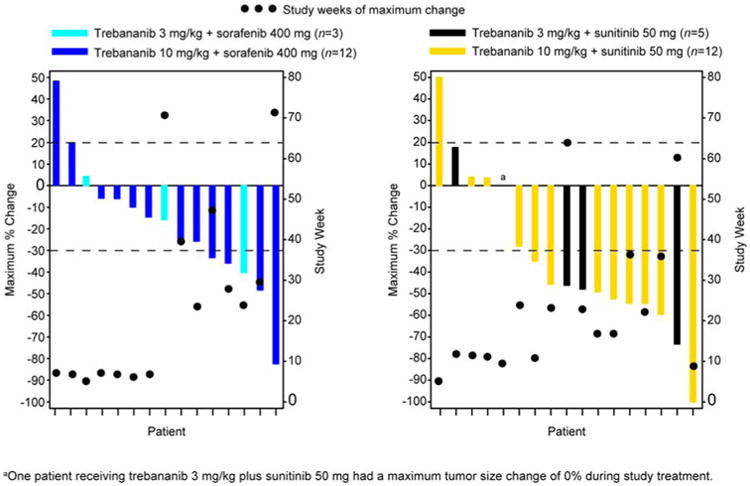
Antitumor Effect of Trebananib (3 mg/kg and 10 mg/kg) Plus Sorafenib or Sunitinib for Each Patient. Best Response Measured by Using Modified RECIST 1.0.20
Abbreviations: RECIST = Response Evaluation Criteria in Solid Tumors.
In the trebananib plus sunitinib cohorts, the ORR was 53%. One patient (5%) in the 10 mg/kg cohort had a complete response. Nine patients (47%; 3 mg/kg cohort, n = 3; 10 mg/kg cohort, n = 6) had a partial response. The median time to complete or partial response was 22.6 weeks. Five patients (26%; 3 mg/kg cohort, n = 2; 10 mg/kg cohort, n = 3) had stable disease. One patient (5%) had stable disease > 6 months. The patient with the complete response and five patients with a partial response or stable disease did not progress by the data cutoff date. Up until treatment week 12, tumor size decreased a median of 18.70% and 23.81% in the 3 mg/kg and 10 mg/kg cohorts, respectively. Across the trebananib plus sunitinib dose cohorts (n = 19), the median TTP (95% CI) was 48.0 weeks (30.6 weeks – 98.3 weeks).
Discussion
In this study, we showed that trebananib IV QW at 3 mg/kg or 10 mg/kg could be combined with sorafenib or sunitinib at full–recommended doses per package insert of these two VEGFR tyrosine kinase inhibitors (TKIs). Toxicities were largely similar in nature, severity, and frequency to those observed with sorafenib or sunitinib as single agents (eg, grade 3 hypertension). Peripheral edema has been previously identified as an AE specific to trebananib treatment.12,13 In this study, peripheral edema (all grade ≤ 2) occurred more frequently in patients receiving trebananib plus sunitinib compared with patients receiving trebananib plus sorafenib. From a tolerability standpoint, the combinations of trebananib plus sorafenib or sunitinib are suitable for further study in the phase 2 and 3 setting. The PK results also were consistent with this conclusion; no drug–drug interactions were observed when trebananib was coadministered with sorafenib or sunitinib.
While trebananib dose levels up to 10 mg/kg weekly combined with sorafenib 400 mg or sunitinib 50 mg were found to be tolerable in this study, the potential effects of higher doses of trebananib on toxicity and antitumor activity are unknown. In an open–label phase 2 study of patients with metastatic RCC, results indicated that trebananib at 10 mg/kg or 15 mg/kg plus sunitinib 50 mg was tolerable and associated with ORRs of 58% and 59%, respectively, similar to the current response rate of 53%.21 A randomized phase 2 study of trebananib at 3 mg/kg or 10 mg/kg plus sorafenib in patients with metastatic RCC found that the addition of trebananib did not prolong PFS.22 The present study was not designed to assess PFS. TTP appeared to suggest efficacy of the treatment regimens in this study, possibly indicating a benefit of trebananib treatment combinations at higher doses up to 15 mg/kg without additional toxicity. A randomized phase 2 study of trebananib at 3 mg/kg and 10 mg/kg or placebo plus weekly paclitaxel in patients with advanced ovarian cancer found a trend toward improved PFS and response rate with increasing doses of trebananib.13 PK analysis uncovered a trend toward improved PFS with increasing exposure to trebananib, and further modeling suggested that doses greater than 10 mg/kg may have an additional impact on PFS.23 Taken together, the findings of recent studies and the current results suggest that the relationship between trebananib exposure and clinical response in metastatic RCC requires more study.
Metastatic RCC eventually escapes VEGF pathway inhibitors, but the mechanisms leading to this resistance are complex and poorly understood.24 Because the vasculature is a target of antiangiogenic treatment, resistance may involve adaptive changes to the host rather than exclusive changes in tumor cells that have been seen in response to treatment with TKIs in other tumors.25 Some data suggest a role of the angiopoietin pathway in resistance development to VEGF pathway inhibition. Studies with a murine neuroendocrine tumor model found that Ang1 is upregulated in response to VEGFR antibody blockade.26 Both the VEGF and angiopoietin pathways induce endothelial cell proliferation, and it is possible that combined treatments with trebananib plus sorafenib or sunitinib provide a more efficient blockade. Overexpression of Ang1 in an Ewing's sarcoma xenograft model prevented tumor regression in response to treatment with a soluble VEGFR (VEGF–Trap, aflibercept).27 In another report, Ang1 was shown to mediate the transient pericyte recruitment occurring during the initial response of tumor vasculature to VEGF blockade.28 Plasma levels of Ang2 were proportional to tumor burden in a cohort of patients with metastatic RCC, and sunitinib treatment decreased levels of Ang2 and soluble Tie2.29 A case–control study found that plasma levels of Ang2 were increased in patients with metastatic RCC compared with matched controls and patients with stage I RCC; Ang2 levels in the patients with metastatic disease decreased by 80% with sunitinib treatment and increased again when resistance developed.10 These observations suggest a functional interaction between the Ang/Tie2 and VEGF signaling pathways.
Efforts are underway to identify circulating biomarkers with prognostic and predictive value in patients receiving antiangiogenic therapies for RCC. Treatment of patients with advanced RCC with sunitinib has been associated with increased levels of VEGF and PLGF and decreased soluble VEGFR–2.30,31 In the current study, we observed a similar pattern: increased levels of plasma VEGF and PLGF and decreased levels of sVEGFR–2 with the combination treatment of trebananib and sunitinib or sorafenib, suggesting a pharmacodynamic effect on these markers. Kit is a tyrosine kinase transmembrane receptor for stem cell factor (SCF) that is inhibited by both sorafenib and sunitinib. Kit is proteolytically cleaved in vivo to produce sKit, which binds and neutralizes SCF.32 Plasma levels of sKit are abnormally elevated in patients with gastrointestinal stromal tumor (GIST), and levels decrease with treatment using both imatinib and sunitinib, whereas they rise in patients treated with placebo.33,34 To assess sKit as a pharmacodynamic biomarker in this study, we measured levels of sKit in serum at baseline and on day 1 of week 4. We observed a decline in sKit with treatment using trebananib plus sunitinib or sorafenib, in concordance with the imatinib and sunitinib studies. In this study, the baseline sKit levels did not appear prognostic for TTP. The study design prevented evaluating whether sKit is a predictive marker for trebananib. In the study of sunitinib in GIST, a correlation between the degree of decline in sKit and increased PFS was observed.34 The biological roles and prognostic significance of sKit in metastatic RCC is not well understood, nor is the predictive value of declining sKit levels with trebananib plus sorafenib or sunitinib treatment. A recent pathologic study found a correlation between Kit overexpression on RCC tumors and response of metastatic disease to sorafenib.35 Further investigation to define the role of Kit and sKit in metastatic RCC is needed. Preclinical models provide evidence that PLGF may play a role in tumor escape from antiangiogenic therapies targeting the VEGF axis.36 The study of blood biomarkers in RCC may lend insight into mechanisms of resistance against antiangiogenic treatments.
In sum, weekly IV infusion of trebananib at 3 mg/kg or 10 mg/kg combined with sorafenib or sunitinib had an acceptable toxicity profile in patients with metastatic RCC. This finding is consistent with other studies of trebananib combined with sunitinib or sorafenib in metastatic RCC.21,22 Data from other combination studies suggest that higher doses of trebananib may be more effective,13,23 an approach that may merit further investigation of trebananib in combination with sorafenib or sunitinib. The role of the angiopoietin pathway in resistance to VEGF pathway inhibition requires further preclinical and clinical study. Plasma and tissue biomarker studies will likely make an important contribution to these investigations.
Conclusion
The study was prompted by the idea that in patients with RCC, coadministration of two angiogenic inhibitors may provide improved efficacy when compared with treatments relying on a single angiogenic inhibitor. Current evidence suggests that this approach may not be very successful when combining inhibitors targeting the same pathway, partially as a result of unacceptable toxicities. Furthermore, efficacy gains have been modest, which has been attributed to the development of resistance mechanisms. Our results show that adding trebenanib to sorafenib or sunitinib does not appear to exacerbate toxicities typically observed with sorafenib or sunitinib monotherapies. Results also suggest evidence of antitumor activity. Those results are important in view of studies showing only moderate efficacy, but increased toxicity, of combination therapies relying on agents all targeting the same angiogenic pathway compared with single angiogenic inhibitors. Yet, many patients' disease progresses while receiving treatment with only a single angiogenic inhibitor. Thus, innovative treatment strategies are needed. We believe that the results from the current study will encourage physicians to more closely investigate the combination of antiangiogenic inhibitors targeting separate angiogenic pathways in patients with RCC.
Supplementary Material
Supplementary Figure 1: Duration (Weeks) of Participation in the Study From Enrollment to Treatment Termination For Each Patient.
Supplementary Figure 2: Changes in Concentrations of Circulating Biomarkers During Treatment With Trebananib Plus Sorafenib or Sunitinib Relative to Baseline. Mean (± SE) Fold Change in PLGF (A), VEGFR-2 (B), VEGF (C), and sKit (D) From Baseline as a Function of Treatment Time Across all Treatment Cohorts.
aIndicates significant change from baseline (P < 0.01). Abbreviations: PLGF = placental growth factor; SE = standard error; sKit = soluble c-Kit; VEGF = vascular endothelial growth factor; VEGFR-2 = vascular endothelial growth factor receptor 2.
Clinical Practice Points.
Although antiangiogenic monotherapies targeting the VEGF pathway have demonstrated efficacy in RCC, many patients will experience disease progression. Attempts to slow progression have focused on simultaneous administration of multiple agents targeting the VEGF pathway. Results from those studies, however, suggest that efficacy gains are only moderate and toxicities are difficult to tolerate. In this phase 1b study of patients with advanced RCC, we evaluated the combination of two agents targeting distinct angiogenic pathways. Trebananib suppresses tumor angiogenesis by binding to Ang1 and Ang2, thereby preventing their interaction with the Tie2 receptor. Trebananib 3 mg/kg and 10 mg/kg was combined with the VEGF pathway inhibitors sorafenib or sunitinib to investigate AEs, PK, biomarkers, and tumor response. The results suggest that combining trebananib with sorafenib or sunitinib does not exacerbate toxicities typically associated with monotherapies involving VEGF pathway inhibitors. The data also provide evidence of antitumor activity. The current study may provide oncologists further impetus for investigating antiangiogenic combination therapies targeting separate angiogenic pathways for the treatment of advanced RCC.
Acknowledgments
The authors would like to thank Terrance J. Williams, PhD (Amgen Inc.), for medical writing support, and NgocDiep Le and Chris M. Storgard for their contribution to study oversight.
Funding: This work was supported by Amgen Inc.
Footnotes
Previous presentation: The results of this study have not been previously published or submitted for publication elsewhere. The results were presented in part at the European Society for Medical Oncology (ESMO) Congresses in Stockholm, Sweden, September 12–16, 2008, and Milan, Italy, October 8–12, 2010.
Trial registry and registration identification number: NCT00861419
Trial registration date: March 12, 2009
Conflict of Interest Statement: David Hong has research funding and Speakers Bureau support from Amgen. Razelle Kurzrock has research/grant funding from Amgen. Nicholas Vogelzang participates in a data safety and monitoring board for Amgen. Erik Rasmussen, Benjamin Wu, Michael Bass, Zhandong Zhong, and Gregory Friberg are employees of Amgen, and hold stocks or stock options in Amgen. Michael Gordon, Wolfram Samlowski, Nizar Tannir, David Friedland, David Mendelson, and Leonard Appleman have no conflicts to declare.
Manufacturer Name: The following companies manufactured products that were implemented in this study: Amgen Inc. (trebananib); Bayer HealthCare Pharmaceuticals Corporation and Onyx Pharmaceuticals (sorafenib [Nexavar®]); Pfizer (sunitinib [Sutent®]; and Pharsight (WinNonlin Professional, version 4.1e).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medioni J, Banu E, Helley D, et al. Salvage therapy with bevacizumab–sunitinib combination after failure of sunitinib alone for metastatic renal cell carcinoma: a case series. Eur Urol. 2009;56:207–11. doi: 10.1016/j.eururo.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–9. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115:2368–75. doi: 10.1002/cncr.24234. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 5.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin–2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 6.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin–1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 7.Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. 1999;155:1967–76. doi: 10.1016/S0002-9440(10)65515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etoh T, Inoue H, Tanaka S, et al. Angiopoietin–2 is related to tumor angiogenesis in gastric carcinoma. Cancer Res. 2001;61:2145–53. [PubMed] [Google Scholar]
- 9.Sfiligoi C, de Luca A, Cascone I, et al. Angiopoietin–2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003;103:466–74. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 10.Bullock AJ, Zhang L, O'Neill AM, et al. Plasma angiopoietin–2 (ANG2) as an angiogenic biomarker in renal cell carcinoma (RCC) J Clin Oncol. 2010;28 Abstract 4630. [Google Scholar]
- 11.Coxon A, Bready J, Min H, et al. Context–dependent role of angiopoietin–1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin–1/2–neutralizing peptibody. Mol Cancer Ther. 2010;9:2641–51. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst RS, Hong D, Chap L, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–65. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 13.Karlan BY, Oza AM, Richardson GE, et al. Randomized, double–blind, placebo–controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012;30:362–71. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 15.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–96. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear–cell renal–cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong ZD, Dinnogen S, Hokom M, et al. Identification and inhibition of drug target interference in immunogenicity assays. J Immunol Methods. 2010;355:21–8. doi: 10.1016/j.jim.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Bass MB, Sherman SI, Schlumberger MJ, et al. Biomarkers as predictors of response to treatment with motesanib in patients with progressive advanced thyroid cancer. J Clin Endocrinol Metab. 2010;95:5018–27. doi: 10.1210/jc.2010-0947. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Atkins MB, Ravaud A, Gravis G, et al. Safety and efficacy of AMG 386 in combination with sunitinib in patients with metastatic renal cell carcinoma (mRCC) in an open–label multicenter phase II study. J Clin Oncol. 2012;30(Suppl) doi: 10.1200/JCO.2014.60.6012. Abstract 4606. [DOI] [PubMed] [Google Scholar]
- 22.Rini B, Szczylik C, Tannir NM, et al. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: A randomized, double–blind, placebo–controlled, phase 2 study. Cancer. 2012;118:6152–61. doi: 10.1002/cncr.27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu JF, Rasmussen E, Karlan BY, et al. Exposure–response relationship of AMG 386 in combination with weekly paclitaxel in recurrent ovarian cancer and its implication for dose selection. Cancer Chemother Pharmacol. 2012;69:1135–44. doi: 10.1007/s00280-011-1787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rini BI, Atkins MB. Resistance to targeted therapy in renal–cell carcinoma. Lancet Oncol. 2009;10:992–1000. doi: 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 25.Ebos JM, Lee CR, Kerbel RS. Tumor and host–mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin Cancer Res. 2009;15:5020–5. doi: 10.1158/1078-0432.CCR-09-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late–stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Bae JO, Tsai JP, et al. Angiopoietin–1/Tie–2 activation contributes to vascular survival and tumor growth during VEGF blockade. Int J Oncol. 2009;34:79–87. [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin–1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 29.van der Veldt AA, Vroling L, de Haas RR, et al. Sunitinib–induced changes in circulating endothelial cell–related proteins in patients with metastatic renal cell cancer. Int J Cancer. 2012;131:E484–93. doi: 10.1002/ijc.26456. [DOI] [PubMed] [Google Scholar]
- 30.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF–related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab–refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743–8. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 32.Dahlen DD, Lin NL, Liu YC, et al. Soluble Kit receptor blocks stem cell factor bioactivity in vitro. Leuk Res. 2001;25:413–21. doi: 10.1016/s0145-2126(00)00122-3. [DOI] [PubMed] [Google Scholar]
- 33.Bono P, Krause A, von Mehren M, et al. Serum KIT and KIT ligand levels in patients with gastrointestinal stromal tumors treated with imatinib. Blood. 2004;103:2929–35. doi: 10.1182/blood-2003-10-3443. [DOI] [PubMed] [Google Scholar]
- 34.Deprimo SE, Huang X, Blackstein ME, et al. Circulating levels of soluble KIT serve as a biomarker for clinical outcome in gastrointestinal stromal tumor patients receiving sunitinib following imatinib failure. Clin Cancer Res. 2009;15:5869–77. doi: 10.1158/1078-0432.CCR-08-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang HL, Zhu Y, Qin XJ, et al. c–KIT: potential predictive factor for the efficacy of sorafenib in metastatic renal cell carcinoma with sarcomatoid feature. Clin Genitourin Cancer. 2013;11:134–40. doi: 10.1016/j.clgc.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Adini A, Kornaga T, Firoozbakht F, et al. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–52. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Duration (Weeks) of Participation in the Study From Enrollment to Treatment Termination For Each Patient.
Supplementary Figure 2: Changes in Concentrations of Circulating Biomarkers During Treatment With Trebananib Plus Sorafenib or Sunitinib Relative to Baseline. Mean (± SE) Fold Change in PLGF (A), VEGFR-2 (B), VEGF (C), and sKit (D) From Baseline as a Function of Treatment Time Across all Treatment Cohorts.
aIndicates significant change from baseline (P < 0.01). Abbreviations: PLGF = placental growth factor; SE = standard error; sKit = soluble c-Kit; VEGF = vascular endothelial growth factor; VEGFR-2 = vascular endothelial growth factor receptor 2.


