Abstract
Excessive and unregulated inflammation contributes to multiorgan failure and death in sepsis. Triggering receptor expressed on myeloid cells type 1(TREM-1) is expressed on neutrophils and monocytes and is upregulated in the presence of bacterial pathogens. Engagement of TREM-1 results in increased expression of proinflammatory chemokines and cytokines and amplifies the inflammatory response. In this article, we will review the structure and signaling pathway of TREM-1 and review the role of TREM-1 and soluble TREM-1 in the inflammatory response during sepsis. Based on these studies, modulation of the TREM-1 signaling pathway has been suggested as a potential therapeutic strategy for the treatment of sepsis, to dampen the inflammatory response without interrupting the ability of the host to clear pathogens. This basic science research may someday lead to other treatments for sepsis and other diseases.
Keywords: Sepsis, Toll-like receptor sepsis, TREM-1, Triggering receptor expressed on myeloid cells type 1
Sepsis is a leading cause of mortality and morbidity and is associated with high hospital costs.1 Excessive inflammation leading to multiple organ failure and hemodynamic collapse contributes to this lethality. A family of cell surface receptors known as triggering receptor expressed on myeloid cells (TREM) has garnered interest recently as modulators of the inflammatory response to bacterial pathogens in sepsis. The first identified member of this family, TREM-1, is widely expressed on myeloid cells and acts as an inflammatory trigger and amplifier after exposure to extracellular fungal and bacterial pathogens.2–4 One must remember that this research may lead to new treatment modalities for sepsis and other diseases.
Currently, the treatment for sepsis is aimed toward rapid diagnosis and identification of the causative organism, followed by tailored antibiotic use and aggressive supportive care until resolution of symptoms.5 Regulation of neutrophils and monocytes is important under inflammatory conditions because the release of cytotoxic substances can cause collateral tissue damage. Enhancement of TREM-1 expression promotes excessive inflammation and increases circulating cytokines and chemokines. Therefore, therapeutics aimed at modulating TREM-1 expression may protect septic patients from death while maintaining effective bacterial clearance. This review focuses on what is currently known about the structure, regulation, and function of TREM-1 and its potential therapeutic role in sepsis.
STRUCTURE AND SIGNALING OF TREM-1
The TREM family of protein receptors consists of TREM-1, TREM-2, TREM-3 (mouse), TREM-like transcript (TLT)-1, and TLT-2. The TREM gene cluster is located on human chromosome 6p21 and mouse chromosome 17C3.6 All are members of the immunoglobulin superfamily. Neutrophils and monocytes express TREM-1,2 and its function is well studied as reviewed below. The roles of TREM-2, TREM-3, TLT-1, and TLT-2 are less well known. It is possible that TREM-2 is involved in inhibiting inflammation in macrophages7; TLT-1 has been characterized as an inhibitory receptor expressed on platelets.8 The expression and function of TREM-3 and TLT-2 are not known.
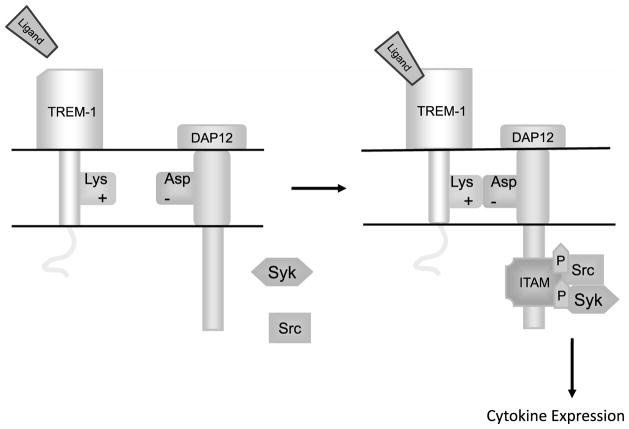
Triggering receptor expressed on myeloid cells type 1 is a 30-kDa glycoprotein transmembrane receptor with a 194-amino-acid ectodomain, a 29-amino-acid trans-membrane region, and a 5-amino-acid short cytoplasmic tail lacking known signaling motifs (Figure).9 Crystallographic studies of TREM-1 have revealed conflicting structural information.2,10,11 In one report, investigators demonstrated a head-to-tail dimer with 2 predicted ligand binding surfaces,11 whereas in another study, investigators described the TREM-1 structure as a monomer in solution with 1 ligand binding site.2,10 The natural ligand for TREM-1 is unknown; however, there is evidence for the presence of a ligand for TREM-1 on human platelets.12 Confirmation of the structure of TREM-1 may aid in the search for the unknown extracellular ligand and facilitate the development of therapeutic reagents.
Figure.
Overview of triggering receptor expressed on myeloid cells type 1 (TREM-1) signaling pathway. Engagement of TREM-1 by its unidentified ligand leads to its association with DNAX activation protein-12 (DAP-12) in the transmembrane region of the cell. This association causes a salt bride formation between a positively charged lysine and negatively charged aspartic acid that leads to the formation of an immunoreceptor-tyrosine–based activation motif (ITAM) on the cytoplasmic domain. The tyrosine residues in the DAP12 ITAM then undergo Src kinase–induced phosphorylation, which forms a docking site for tyrosine kinase Syk. Syk phosphorylation activates a signaling cascade, which leads to increased expression of proinflammatory cytokines and chemokines.
TREM-1 SIGNALING, EXPRESSION, AND REGULATION
Although the cytoplasmic domain of TREM-1 lacks intrinsic signaling capacity, it associates with a transmembrane adaptor protein, DNAX activation protein-12 (DAP-12), to transmit a signal into the cytoplasmic region of myeloid cells (Figure).2,13 The TREM-1 transmembrane region contains positively charged lysine, which forms a salt bridge with negatively charged aspartic acid in the transmembrane region of DAP-12.13,14 The association between TREM-1 and DAP-12 causes the immunoreceptor-tyrosine–based activation motif on the cytoplasmic domain of DAP-12 to form.15–19 This association leads to Src kinase–induced phosphorylation of tyrosine residues in the DAP-12 immunoreceptor-tyrosine–based activation motif, forming a docking site for the protein tyrosine kinase Syk. Syk phosphorylation leads to the activation of several signaling molecules, including PI3 kinase, phospholipase-C-γ, and mitogen-activated protein kinases, which leads to increased expression of proinflammatory cytokines and chemokines such as interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and IL-1β2,15,18,20,21; mobilization of intracellular calcium2; and changes in actin cytoskeleton in myeloid cells.15,16,22 In the presence of lipopolysaccharide (LPS) and bacteria such as Pseudomonas aeruginosa and Staphylococcus aureus, TREM-1 expression is upregulated on human neutrophils and monocytes.2–4,23 In vitro studies demonstrate that cross-linking of TREM-1 with an anti-TREM-1 monoclonal antibody stimulates the secretion of IL-8 and myeloperoxidase from neutrophils and the secretion of IL-8, monocytic chemotactic protein-1, IL-1β, and TNF-α from monocytes.2,3 In addition, LPS-induced endotoxemia in human subjects resulted in increased surface expression of TREM-1 and plasma soluble form of TREM-1 (sTREM-1) levels on monocytes.4
TREM-1 AND TOLL-LIKE RECEPTOR RELATIONSHIP
Toll-like receptors (TLRs) are transmembrane proteins that recognize microbial products termed pathogen-associated molecular patterns.24 Expressed on many cell types, including monocytes, macrophages, and dendritic cells, TLRs recognize pathogens and induce inflammation via intra-cellular downstream signaling, resulting in the release of inflammatory mediators.24,25 Evidence suggests that a synergistic relationship exists between TLRs and TREM-1, resulting in an enhanced upregulation of proinflammatory cytokines and downregulation of anti-inflammatory cytokines.2,3,9,26 Binding of an activating monoclonal antibody to TREM-1 on neutrophils and monocytes in combination with either TLR2, TLR3, or TLR4 synergistically amplified the cellular inflammatory response.2,3,9,26
Silencing TREM-1 expression by small interfering RNA (siRNA) in LPS-treated macrophages decreases the transcription of important adaptor proteins in the TLR4 signaling pathway such as CD14 and myeloid differentiation factor 88 (MyD88).27–29 Wild-type and MyD88-null mice treated with LPS induced similar expression of TREM-1 and sTREM-1, indicating that the upregulation of TREM-1 in a model of sepsis is independent of MyD88. However, Tlr4-null mice treated with LPS had decreased expression of TREM-1 compared with wild-type mice. Induction of inflammatory cytokines by TLR4 signaling requires Toll-interleukin 1 receptor-domain-containing adapter-inducing interferon-β (TRIF). The siRNA silencing of TRIF expression in the presence of LPS inhibited the surface expression of TREM-1, demonstrating the necessity of an intact TLR4-TRIF signaling pathway to produce an exuberant inflammatory response.30 Taken together, these results suggest that the upregulation of TREM-1 in sepsis requires an intact TLR4 signaling pathway that is independent of MyD88 in macrophages.30
SOLUBLE TREM-1
A soluble form of TREM-1 (sTREM-1) is detectable in biological fluids and tissues in response to infection.4,31,32 The secreted, soluble form of TREM-1 is unable to transmit a signal but, upon release into the blood, competes with TREM-1 for endogenous ligands, acting to down-regulate the TREM-1 pathway and blunting inflammation. Two mechanisms have been proposed to explain the origins of sTREM-1. The first mechanism involves translation of an alternative mRNA splice variant that encodes a protein that lacks the transmembrane region and cytoplasmic tail and is directly secreted into the extra-cellular environment from within the cell.33
A second proposed mechanism is that sTREM-1 is generated by proteolytic cleavage of membrane-bound TREM-1. This is supported by a study in which increasing sTREM-1 levels correlated with decreasing cell surface TREM-1 expression on monocytes and neutrophils 24 hours after LPS stimulation.34 In the presence of a broad-acting metalloprotein inhibitor, LPS-stimulated monocytes and neutrophils had stable TREM-1 cell expression and reduced sTREM-1 levels, supporting the hypothesis that matrix metalloproteinases, a disintegrin and metalloprotease, or other metalloproteinases may shed the TREM-1 ectodomain via cleavage of a single peptide bound within the membrane.34
It is possible that both mechanisms are involved in producing sTREM-1 in vivo. Strategies to enhance the generation of soluble TREM-1 may dampen the inflammatory response. For example, therapeutics to increase the cell surface expression of TREM-1 by promoting cleavage of the TREM-1 ectodomain and releasing it into the circulation may create competition for TREM-1 ligands, resulting in a less exuberant inflammatory response.
sTREM-1 AS A DIAGNOSTIC TOOL
Initially, sTREM-1 levels demonstrated promise as a diagnostic predictor of infection in septic critically ill patients. The positive association between sTREM-1 and infection prompted investigators to determine whether sTREM-1 levels in bronchoalveolar lavage could distinguish infection from inflammation. In early studies, Gibot and colleagues35 and Gibot and Cravoisy36 demonstrated high specificity and sensitivity of bronchoalveolar lavage sTREM-1 levels for predicting ventilator-associated pneumonia. However, subsequent studies have shown much lower sensitivity and specificity for sTREM-1 levels, despite standardized methods to detect sTREM-1.37,38
Recent studies have shown that that sTREM-1 is also upregulated in noninfectious causes of inflammation, making its use as a predictor of infection questionable.39–42 For example, serum sTREM-1 levels are increased in patients diagnosed with ulcerative colitis and Crohn disease in the absence of culture-positive infection.42 In another study, patients with acute pancreatitis, regardless of the presence of infection, had increased serum sTREM-1 levels.41 In a rat model of severe acute pancreatitis, serum and ascites sTREM-1 levels were increased 6 hours after sodium deoxycholate-induced pancreatitis when compared with sham-treated rats.39 In addition, TREM-1 expression was significantly increased in the organs (pancreas, liver, and kidneys) of rats with deoxycholate pancreatitis.39
Another potential use of monitoring sTREM-1 levels may be to guide antibiotic therapy. Gibot et al31 and Determann et al43 reported declines in sTREM-1 levels upon initiation of antibiotic therapy, but it was not an investigational endpoint. The use of serial sTREM-1 levels to guide the duration of antibiotic therapy is an intriguing concept that needs further investigation.
TREM-1 AS A THERAPEUTIC TARGET IN SEPSIS
Inflammation is important for the resolution of infection, but overstimulation of inflammatory pathways can harm the host. The balance between appropriate containment of infection and control of the inflammatory response is illustrated in a study examining different levels of TREM-1 knockdown in a peritonitis model of septicemia in mice. Different siRNA sequences to silence the expression of TREM-1 led to differences in downregulation of TREM-1.44 Partial silencing (30%) of TREM-1 improved survival, possibly through blunting of the systemic inflammatory response, whereas more complete (80%) silencing of TREM-1 expression resulted in 100% lethality. However, in a model of endotoxemia (in the absence of exogenous bacteria), silencing of TREM-1 protects against lethality. More complete silencing impaired bacterial clearance by decreasing neutrophil degranulation and oxidative burst activities.44,45 Improved outcomes in endotoxemia were associated with decreased circulating levels of proinflammatory (TNF-α, IL-1β, and IL-6) and anti-inflammatory (IL-10) cytokines.44
The administration of a synthetic peptide (LP-17) based on the ectodomain of TREM-1 conferred protection in endotoxemic mice.46 This soluble form of TREM-1 lacks a transmembrane and cytoplasmic region; thus, it does not initiate cell activation and subsequent cytokine release but competes with the TREM-1 protein for endogenous ligands. Pretreatment of mice with LP17 resulted in partial blockade of the TREM-1 signaling pathway, improving survival without decreasing the effectiveness of bacterial clearance.46 In contrast, the administration of an activating anti-TREM-1 monoclonal antibody in a mouse model of LPS-induced septic shock increased mortality from 50% to 100%.31 In a cecalligation and puncture model of septic shock, the administration of an LP17 reduced inflammation and hyper-responsiveness, resulting in lower mortality.31 A survival benefit was also demonstrated in mice that received a fusion protein (mTREM-1/immunoglobulin 1) 1 hour after LPS injection.3 Modulation of the TREM-1 pathway, using a synthetic peptide or a fusion protein, may be a therapeutic tool in the treatment of sepsis.
CONCLUSION
The expression of TREM-1 is an important component of the inflammatory response and interacts with TLRs to amplify the inflammatory response in sepsis. Increased activation of TREM-1 signaling may have harmful effects on the host. Although initial studies using sTREM-1 as a biomarker of infection were promising, follow-up studies have not borne out the utility. However, sTREM-1 levels may be a useful indicator of successful antibiotic therapy. Upregulation of TREM-1 expression may result in an exuberant inflammatory response, whereas complete blockade severely blunts the ability of the host to clear the pathogens. Therefore, the goal for a clinically useful therapeutic agent is to provide partial blockade without affecting bacterial clearance. This basic science can lead to potential treatments for many disease processes, including sepsis.
Acknowledgments
This work is supported in part by grants from the National Institute of Health (K24 HL068796 to L.M.S. and F31 NR011390-01 to S.J.P.) and Achievement Rewards for College Scientists Fellowship, Chisholm Foundation (S.J.P.).
Footnotes
This work is basic science.
The authors have disclosed that they have no significant relationship with, or financial interest in, any commercial companies pertaining to this article.
Contributor Information
Steven J. Palazzo, Recent graduate from the University of Washington School of Nursing, Seattle. His research is focused on the utility of biomarkers for diagnosing ventilator-associated pneumonia. He is an assistant clinical professor at the College of Nursing, Seattle University and has 8 years’ experience as a medical/cardiac critical care nurse at Harborview Medical Center.
Terri Simpson, Associate professor of nursing in the Department of Biobehavioral Nursing and Health Systems at the University of Washington, Seattle.
Lynn M. Schnapp, Professor in the Division of Pulmonary and Critical Care Medicine at the University of Washington, Seattle.
References
- 1.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 2.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164(10):4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 3.Bouchon A, Facchetti F, Weigand M, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 4.Knapp S, Gibot S, de Vos A, Versteeg H, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173(12):7131–7134. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allcock R, Barrow A, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003;33(2):567–577. doi: 10.1002/immu.200310033. [DOI] [PubMed] [Google Scholar]
- 7.Turnbull I, Gilfillan S, Cella M, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177(6):3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 8.Washington A, Quigley L, McVicar D. Initial characterization of TREM-like transcript (TLT)-1: a putative inhibitory receptor within the TREM cluster. Blood. 2002;100(10):3822–3824. doi: 10.1182/blood-2002-02-0523. [DOI] [PubMed] [Google Scholar]
- 9.Bleharski J, Kiessler V, Buonsanti C, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170(7):3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 10.Kelker M, Foss T, Peti W, et al. Crystal structure of human triggering receptor expressed on myeloid cells 1 (TREM-1) at 1.47 A. J Mol Biol. 2004;342(4):1237–1248. doi: 10.1016/j.jmb.2004.07.089. [DOI] [PubMed] [Google Scholar]
- 11.Radaev S, Kattah M, Rostro B, Colonna M, Sun P. Crystal structure of the human myeloid cell activating receptor TREM-1. Structure. 2003;11(12):1527–1535. doi: 10.1016/j.str.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110(3):1029–1035. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 13.Aoki N, Kimura S, Xing Z. Role of DAP12 in innate and adaptive immune responses. Curr Pharm Des. 2003;9(1):7–10. doi: 10.2174/1381612033392503. [DOI] [PubMed] [Google Scholar]
- 14.Olcese L, Cambiaggi A, Semenzato G, Bottino C, Moretta A, Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J Immunol. 1997;158(11):5083–5086. [PubMed] [Google Scholar]
- 15.Vivier E, Nunès J, Vély F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 16.Lanier L. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15(3):308–314. doi: 10.1016/s0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 17.Campbell K, Colonna M. DAP12: a key accessory protein for relaying signals by natural killer cell receptors. Int J Biochem Cell Biol. 1999;31(6):631–636. doi: 10.1016/s1357-2725(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 18.Lanier L, Corliss B, Wu J, Leong C, Phillips J. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391(6668):703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 19.Lanier L, Bakker A. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today. 2000;21(12):611–614. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 20.Smith K, Wu J, Bakker A, Phillips J, Lanier L. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161(1):7–10. [PubMed] [Google Scholar]
- 21.Tomasello E, Cant C, Bühring H, et al. Association of signal-regulatory proteins beta with KARAP/DAP-12. Eur J Immunol. 2000;30(8):2147–2156. doi: 10.1002/1521-4141(2000)30:8<2147::AID-IMMU2147>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.McVicar DW, Burshtyn DN. Intracellular signaling by the killer immunoglobulin-like receptors and Ly49. Sci STKE. 2001;2001(75):re1. doi: 10.1126/stke.2001.75.re1. [DOI] [PubMed] [Google Scholar]
- 23.Bouchon A, Hernández-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194(8):1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 25.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 26.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187(suppl 2):S397–S401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 27.Ornatowska M, Azim AC, Wang X, et al. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1377–L1384. doi: 10.1152/ajplung.00140.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413(6851):78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 29.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117(5):979–987. doi: 10.1016/j.jaci.2006.02.023. quiz 988. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Heiderscheidt CA, Joo M, et al. MYD88-dependent and -independent activation of TREM-1 via specific TLR ligands. Eur J Immunol. 2010;40(1):162–171. doi: 10.1002/eji.200839156. [DOI] [PubMed] [Google Scholar]
- 31.Gibot S, Kolopp-Sarda M, Béné M, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200(11):1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahdy A, Lowes D, Galley H, Bruce J, Webster N. Production of soluble triggering receptor expressed on myeloid cells by lipopolysaccharide-stimulated human neutrophils involves de novo protein synthesis. Clin Vaccine Immunol. 2006;13(4):492–495. doi: 10.1128/CVI.13.4.492-495.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gingras M, Lapillonne H, Margolin J. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol Immunol. 2002;38(11):817–824. doi: 10.1016/s0161-5890(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 34.Gómez-Piña V, Soares-Schanoski A, Rodríguez-Rojas A, et al. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179(6):4065–4073. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 35.Gibot S, Cravoisy A, Levy B, Bene M, Faure G, Bollaert P. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350(5):451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 36.Gibot S, Cravoisy A. Soluble form of the triggering receptor expressed on myeloid cells-1 as a marker of microbial infection. Clin Med Res. 2004;2(3):181–187. doi: 10.3121/cmr.2.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huh J, Lim C, Koh Y, et al. Diagnostic utility of the soluble triggering receptor expressed on myeloid cells-1 in bronchoalveolar lavage fluid from patients with bilateral lung infiltrates. Crit Care. 2008;12(1):R6. doi: 10.1186/cc6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudhuis G, Beuving J, Bergmans D, et al. Soluble triggering receptor expressed on myeloid cells-1 in bronchoalveolar lavage fluid is not predictive for ventilator-associated pneumonia. Intensive Care Med. 2009;35(7):1265–1270. doi: 10.1007/s00134-009-1463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamei K, Yasuda T, Ueda T, Qiang F, Takeyama Y, Shiozaki H. Role of triggering receptor expressed on myeloid cells-1 in experimental severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17(3):305–312. doi: 10.1007/s00534-009-0191-6. [DOI] [PubMed] [Google Scholar]
- 40.Ferat-Osorio E, Esquivel-Callejas N, Wong-Baeza I, et al. The increased expression of TREM-1 on monocytes is associated with infectious and noninfectious inflammatory processes. J Surg Res. 2008;150(1):110–117. doi: 10.1016/j.jss.2007.12.805. [DOI] [PubMed] [Google Scholar]
- 41.Ferat-Osorio E, Wong-Baeza I, Esquivel-Callejas N, et al. Triggering receptor expressed on myeloid cells-1 expression on monocytes is associated with inflammation but not with infection in acute pancreatitis. Crit Care. 2009;13(3):R69. doi: 10.1186/cc7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol. 2006;12(21):3416–3419. doi: 10.3748/wjg.v12.i21.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Determann R, Millo J, Gibot S, et al. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med. 2005;31(11):1495–1500. doi: 10.1007/s00134-005-2818-7. [DOI] [PubMed] [Google Scholar]
- 44.Gibot S, Massin F, Marcou M, et al. TREM-1 promotes survival during septic shock in mice. Eur J Immunol. 2007;37(2):456–466. doi: 10.1002/eji.200636387. [DOI] [PubMed] [Google Scholar]
- 45.Radsak M, Salih H, Rammensee H, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol. 2004;172(8):4956–4963. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 46.Gibot S, Buonsanti C, Massin F, et al. Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock. Infect Immun. 2006;74(5):2823–2830. doi: 10.1128/IAI.74.5.2823-2830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]