Abstract
In Alzheimer’s disease (AD), there is a loss in cholinergic innervation targets of basal forebrain which has been implicated in substantial cognitive decline. Amyloid beta peptide (Aβ1–42) accumulates in AD that is highly toxic for basal forebrain cholinergic (BFC) neurons. Although the gonadal steroid estradiol is neuroprotective, the administration is associated with risk of off-target effects. Previous findings suggested that non-classical estradiol action on intracellular signaling pathways has ameliorative potential without estrogenic side effects. After Aβ1–42 injection into mouse basal forebrain, a single dose of 4-estren-3α, 17β-diol (estren), the non-classical estradiol pathway activator, restored loss of cholinergic cortical projections and also attenuated the Aβ1–42-induced learning deficits. Estren rapidly and directly phosphorylates c-AMP-response–element-binding-protein and extracellular-signal-regulated-kinase-1/2 in BFC neurons and restores the cholinergic fibers via estrogen receptor-α. These findings indicated that selective activation of non-classical intracellular estrogen signaling has a potential to treat the damage of cholinergic neurons in AD.
Alzheimer’s disease (AD) is the most common form of dementia and is a progressive neurodegenerative disorder of insidious onset that causes gradual loss of memory and cognitive function1. The loss of memory and cognitive function is underpinned by widespread death of neurons in the hippocampus and cortex1,2,3. The basal forebrain cholinergic (BFC) system provides a substantial projection to the hippocampus and cortex to promote cortical arousal, attention and cognitive function and is particularly vulnerable in AD4,5,6. Besides of the pathological aggregation of tau protein7 the other major neuropathological hallmark of AD is the accumulation of extracellular neurotoxic amyloid-β (Aβ) peptides such as Aβ1–42 in the brain8,9. Current strategies to combat AD have so far largely been ineffective resulting in an inability to treat the profound cell loss that occurs in AD1,10,11. Among many different factors controlling the vulnerability of cholinergic neurons to AD, estradiol is an essential contributor. Despite the fact that chronic administration of estrogens can improve the synaptic connectivity in the cerebral cortex following neuronal cell death12,13,14 and estradiol was found to be neuroprotective in in vitro and in vivo models of the disease there is a controversy about estrogen’s neuroprotective actions in AD15,16,17,18,19,20. However, the most common method of chronic administration of estrogens is also associated with detrimental effects, such as an increased risk of stroke and breast cancer21,22,23. One strategy to overcome this shortcoming has been to use synthetic compounds with selective properties of estradiol that do not exhibit negative side-effects during prolonged treatment21,24. Besides its classical genomic action, estradiol also exerts rapid effects on cells by altering cytoplasmic signal transduction pathways (non-genomic or non-classical for estrogen action)25,26,27 and it is these non-classical actions that are important for mediating the ameliorative effects of estradiol15,28,29,30,31,32. However, estradiol activates both the classical and non-classical pathways and so a selective non-classical estradiol pathway activator is required to induce ameliorative effects without inducing the classical pathway with the risk of unwanted side effects. “Activators of Non-Genomic Estrogen Like Signaling” (ANGELS) such as estren (4-estren-3α, 17β-diol) mimic estradiol’s rapid induction of cell signaling pathways in bone cells, successfully maintaining bone health in gonadectomized mice33, while exerting no action on reproductive tissues via classical nuclear receptors24. To elucidate the ameliorative effect of ANGELS on the neurodegenerative process we examined the effect of estren on Aβ1–42-induced cholinergic damage in BFC in vivo.
Results
Aβ1–42 induces BFC cell body and fiber loss in vivo
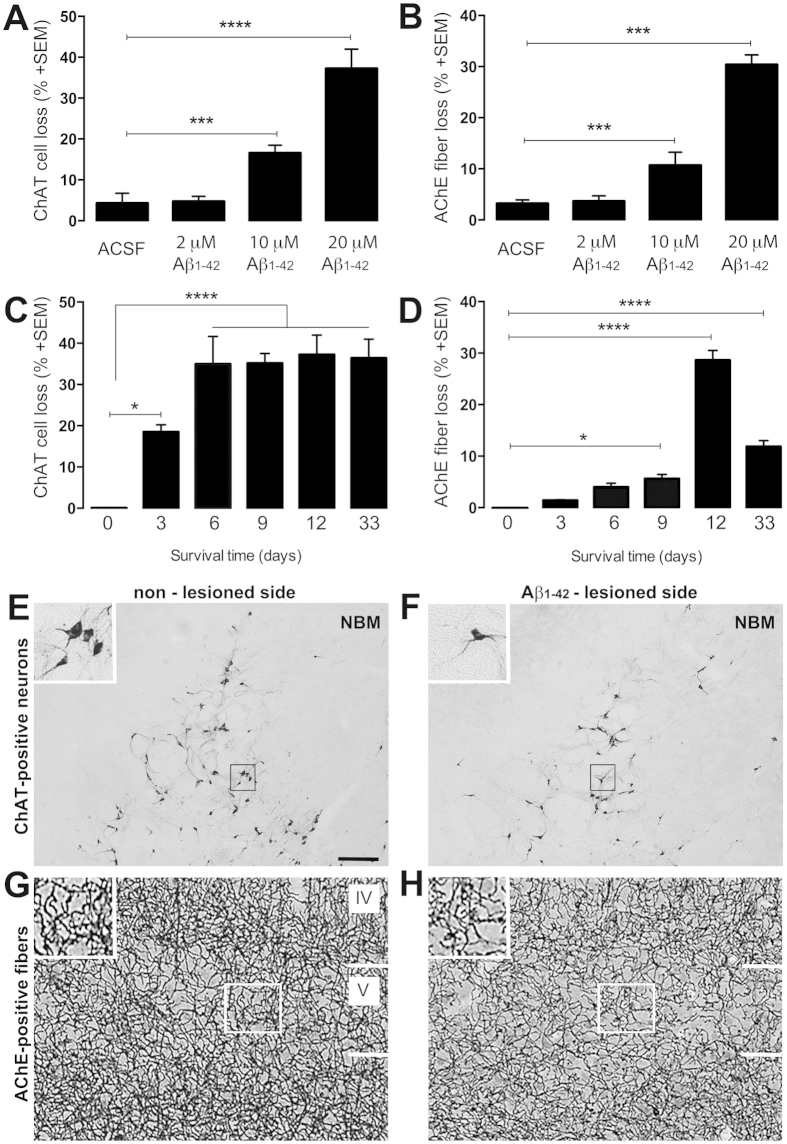
First, we evaluated the most effective dose of Aβ1–42, and survival time of neurons after Aβ1–42 application into nucleus basalis magnocellularis (NBM) in ovariectomized (OVX) mice. To facilitate oligomerization and the neurotoxic effect we aged Aβ1–42 for 2 days. The most neurotoxic concentration of Aβ1–42 was 20 μM (Fig. 1A,B,E,F), and after 12 days (Fig. 1C,D,G,H) the microinjection of Aβ1–42 into the NBM complex caused the most profound damage, eliminating 37% of cholinergic cells in NBM and 30% of cholinergic fibers from the somatosensory cortex. Interestingly, Aβ1–42-induced cortical cholinergic fiber loss is attenuated by day 33 following Aβ1–42 microinjection (Fig. 1D) suggesting an endogenous restoration capacity of remaining cholinergic fibers.
Figure 1. Effect of administering Aβ1–42 to NBM on loss of cholinergic cells and fibers.
Histogram shows the percentage of ChAT- and AChE-positive cell and fiber loss 12d after administration of different Aβ1–42 concentrations (A,B) and at various survival times after a fixed concentration (20 μM) of Aβ1–42 (C,D). ChAT immunolabeled cell bodies in the NBM (E,F) and AchE-positive fibers in layer IV and V of the somatosensory cortex (G,H) at the non-treated (E,G) and Aβ1–42-treated brain side (F,H). Scale bars, 50 μm; inserts 25 μm. Histograms show mean ± SEM (n = 4–9). ***P < 0.001 (one way ANOVA with post hoc Bonferroni test).
Single dose of estren treatment restores cholinergic fiber density in the somatosensory cortex after Aβ1–42 lesion
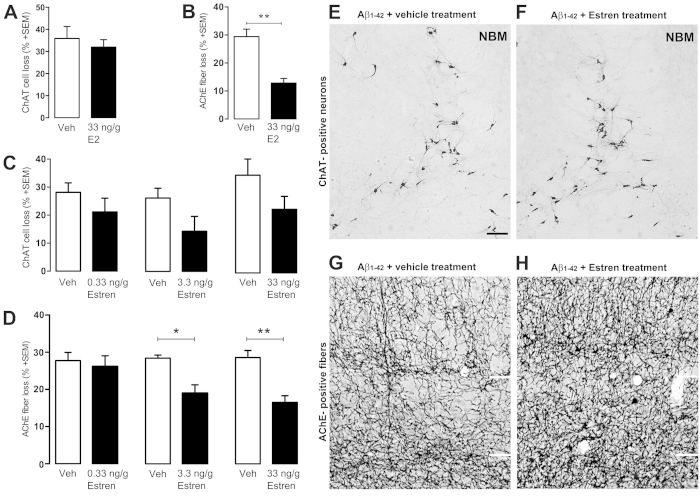
Next, we tested whether estren has an ameliorative action on Aβ1–42-induced cholinergic cell death and fiber loss using different concentrations of estren that do not have uterotrophic effects24. We treated the animals with a single dose of estren after Aβ1–42 injection. Administration of estren evoked a clear concentration-dependent decrease in cholinergic fiber loss in the lesioned ipsilateral somatosensory cortex with the most significant decrease at 33 ng/g estren treatment (Fig. 2D,G,H) (p < 0.01). In contrast, estren treatment did not have an effect on cholinergic cell loss in the NBM (Fig. 2C,E,F). As a positive control, application of single uterotropic dose of estradiol demonstrated a similar restorative action on BFC fibers but did not have an effect on cholinergic cell loss (Fig. 2A,B).
Figure 2. Estren attenuates Aβ1–42–induced lesions of cortical projections.
Aβ1–42 -induced ChAT cell and fiber loss in estradiol (A,B), estren (C–F) treated mice compared to the vehicle treated group. Photomicrographs demonstrate ChAT positive cell bodies in the NBM and AChE-stained fibers in layer IV and V of the somatosensory cortex at the contralateral nonlesioned (E,G) brain side and ipsilateral lesioned (F,H) side after 12 d of Aβ1–42 injection and estren administration. Scale bar, 50 μm. Histograms show mean ± SEM (n = 4–9). *P < 0.05; **P < 0.01; ***P < 0.001 (t-test (A,B) or ANOVA with post hoc Tukey test).
Estren ameliorates the learning deficits resulting from Aβ1–42 administration
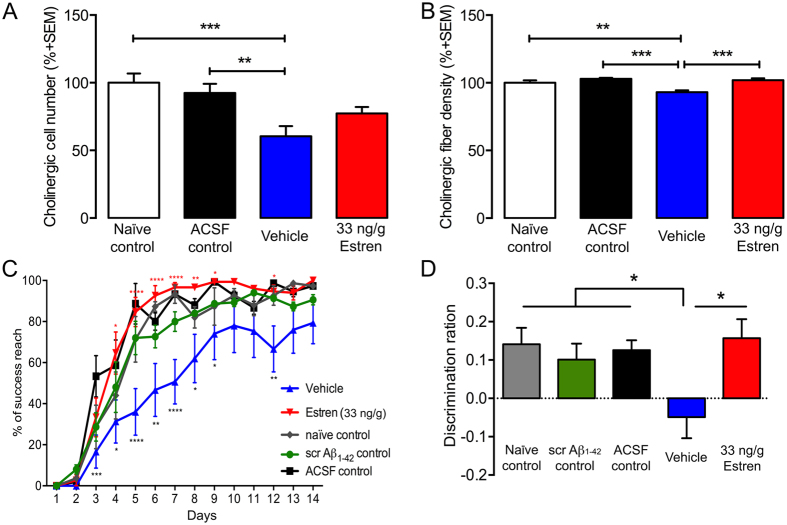
In the following experiments we examined whether the estren-induced ameliorative effect has a behavioural manifestation. Previous studies have demonstrated that lesions of BFC system are associated with a striking deficit in motor learning and recognition memory detected by pallet retrieval and novel object recognition task, respectively34. Accordingly, OVX mice were treated in the same manner as detailed above, with the exception that Aβ1–42 or scrambled Aβ1–42 was injected bilaterally into the NBM, and then the pellet retrieval and novel object recognition tests were performed 12 d following Aβ1–42 or scrambled Aβ1–42 application and estren treatment (Fig.3).
Figure 3. Effect of estren treatment on Aβ1–42 -induced behavioural deficits.
Aβ1–42 -induced bilateral ChAT neuronal (A) and fiber (B) loss in estren treated mice compared to control groups. Estren treatment successfully rescued reaching performance on the single pellet retrieval task (C) and novel object recognition (D) in Aβ1–42 –lesioned mice. Histograms show mean ± SEM (n = 10). *P < 0.05; **P < 0.01; ***P < 0.001 (ANOVA (A) and repeated-measures ANOVA (B) with post hoc Bonferroni test).
Estren significantly attenuated the deficits in successful pellet retrieval, and in the discrimination index in novel object recognition following Aβ1–42 administration (Fig. 3C,D). At the end of the behavioral experiments the brains of animals were examined for cholinergic cell and fiber loss (Fig. 3A,B). The behavioural observations in these experiments were supported by the morphological data since estren treatment significantly (p < 0.001) increased the cholinergic fiber density in somatosensory cortex after bilateral Aβ1–42-induced cholinotoxicity in NBM (Fig. 3B).
Estren rapidly and directly activates intracellular signaling system in BFC neuron
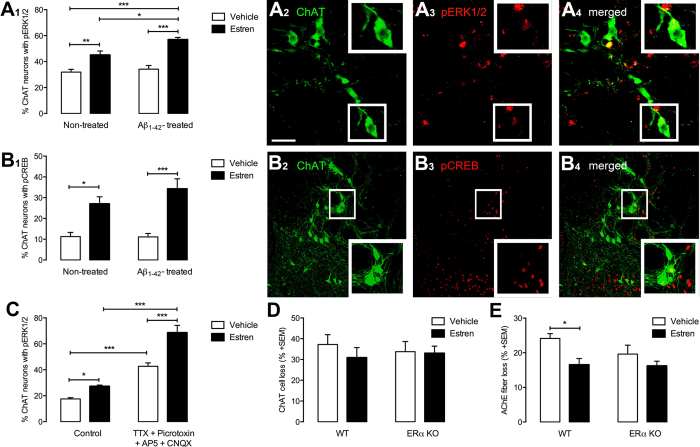
Previous in vitro study exhibit clearly how estren attenuates Aβ1–42 toxicity in cortical neurons in tissue culture via activation of the mitogen-activated-protein-kinase (MAPK) signaling pathway35. We have shown that the non-classical estradiol intracellular signaling pathway such as MAPK/cAMP response element binding protein (CREB) pathway plays a critical role in estradiol-induce ameliorative actions on BFC neurons in vivo32. To assess whether estren can activate the non-classical pathway in cholinergic neurons in NBM extracellular-signal-regulated-kinase-1/2 (ERK1/2) and CREB phosphorylation was examined in cholinergic neurons in NBM following Aβ1–42 and estren administration. While Aβ1–42 alone did not change phosphorylation estren increased the ERK1/2 and CREB phosphorylation in cholinergic neurons alone and in Aβ1–42 injected animals within 15 minutes (Fig. 4A1). In addition estren-induced ERK1/2 phosphorylation is more prominent in the presence of Aβ1–42 (Fig. 4A1). This rapid action of estren on CREB and ERK1/2 phosphorylation suggests a non-classical mechanism that does not require de novo gene transcription. Previous studies have revealed that inhibition of either protein synthesis or transcription is ineffective in modulating CREB phosphorylation in such a restricted time frame36. We also examined the possible role of afferent inputs to cholinergic neurons in NBM in the rapid estren response by incubating acute brain slices in a cocktail containing TTX and amino acid receptor blockers that effectively isolate cholinergic neurons from synaptic inputs in vitro. Our data showed that estren phosphorylated ERK1/2 in the presence of the blocking cocktail, suggesting a GABAA/NMDA/AMPA/kainate receptor independent direct estren-induced non-classical action on BFC neurons (Fig. 4C). In these experiments the application of the blocking cocktail alone induced significant increase of ERK1/2 in BFC neurons that might be explained by the pivotal role of amino acid receptors in the regulation of basal ERK1/2 phosphorylation in these neurons.
Figure 4. Estren rapidly phosphorylates ERK1/2 and CREB in cholinergic neurons in the NBM.
Graphs show the percentage of ERK1/2 (A1) and CREB (B1) phosphorylation in ChAT neurons within the NBM 30 min after estren administration. Photomicrographs demonstrate dual-label immunofluorescence of pERK1/2 (A2–A4) or pCREB (B2–B4) immunoreactivity (red) in ChAT neurons (green). Bar graphs showing the percentage of ChAT neurons expressing pERK1/2 immunoreactivity in the NBM 30 min after incubation in vitro in ACSF containing vehicle (open bars) or estren (closed bars) with or without 0.5 μM TTX and amino acid receptor blocker cocktail (10 μM CNQX, 20 μM AP5, 50 μM picrotoxin) (C). The effect of estren treatment on cholinergic cells and fibers after Aβ1–42 lesion in neuron-specific ERα knock out mice (D). Scale bars, 20 μm (A2-A4) and 40 μm (B2–B4). Histograms show mean ± SEM (n = 5–6). *P < 0.05; **P < 0.01; ***P < 0.001 (ANOVA with post hoc Bonferroni test).
Estren restores the cholinergic fiber density via neuronal estrogen receptor α
BFC neurons predominantly express classical estrogen receptor α (ERα)37 and our recent data demonstrated the restorative effects of estradiol on BFC neurons are indeed mediated by ERα32. We used a neuron-specific ERα KO mouse to examine the role of ERα in estren-induced action on BFC neurons following Aβ1–42 toxicity. Our results showed a single dose of estren does not affect Aβ1–42-induced cell loss (Fig. 4D) in NBM in the absence of this receptor, but needs ERα to exert a restorative action on cholinergic fiber density (Fig. 4E) suggesting a critical role of neuronal ERα in estren-induced restorative action on BFC neurons in NBM.
Discussion
We report here that a single dose of estren treatment restores cholinergic fiber density in the somatosensory cortex and it effectively reduces the motor learning and recognition memory deficits after Aβ1–42-induced loss of subcortical cholinergic input. This restorative action was absent in neuronal ERα KO mice. Furthermore in vitro and in vivo experiments demonstrated that BFC neurons in the mouse respond to estren in a rapid and direct manner through an MAPK/CREB signaling pathway.
A feature of the pathogenesis of AD is the increased concentration of toxic soluble oligomers of Aβ peptides38. Several laboratories including our own have shown that soluble Aβ oligomer injection into the NBM causes cell loss in NBM, unilateral cortical cholinergic fiber loss in the somatosensory cortex in vivo and concomitant memory deficits39,40,41. The available transgenic animal models of AD do not effectively model the sporadic forms of AD and the cholinergic deficit accompanying the disease42,43,44. Based on the established use of soluble Aβ oligomers, we applied the Aβ1–42 by intracerebral injection in our experiments and our result demonstrated the Aβ1–42 was able to damage one third of the BFC neurons in NBM and impairs learning memory.
Estrogen-induced sprouting effect that is thought to contribute to the neural benefits of estrogen treatment depends upon activation of intracellular signal transduction pathways, including MAPK and CREB12,45,46,47,48. It is worth noting that the estren treatment we have used here does not exert ERα mediated classical genomic action on uterus24. In contrast, estren restores BFC neurons via ERα that does not necessarily involve genomic processes32,35,36. Indeed, estren rapidly increases the number of BFC neurons with phosphorylated MAPK and CREB suggesting that non-classical actions may be involved in estren-induced restorative mechanisms in BFC neurons. Our experimental data also provide evidence that estren directly phosphorylates MAPK and CREB in BFC neurons and restores the cholinergic fibers via ERα. Previous reports, including our own studies, also demonstrate that ERα is highly involved in the estrogen-mediated neurotrophic and sprouting mechanisms in the basal forebrain and in other brain regions32,49,50,51.
Estrogen-induced responses in different neuronal cell types range from enhancement of survival, to prevention of cell death and to facilitation of neurite outgrowth12. The origin of the estren or estradiol-induced cholinergic fiber restoration is not clear, it is likely that estren or estradiol may enhance the endogenous capacity of the surviving neurons to replace the cortical projections that are lost when BFC neurons begin to die following Aβ1–42-induced neurotoxicity32. This possibility is supported by the fact that more than 50% of cholinergic neurons survived in NBM (Fig. 1A) and endogenous cholinergic fiber restoration was observed following Aβ1–42 –induced neurotoxicity (Fig. 1D).
Decreasing levels of estradiol with menopause are associated with decreased cognitive function and progression of neurodegenerative disorders. Estren is a promising treatment alternative for hormone replacement therapy with beneficial effects for bone, vascular health and neurodegenerative conditions like Alzheimer’s disease without unwanted estrogenic side effects23,24,28,29,30,45,52. Our findings described in this study increase the understanding and translational value of esten treatment, particularly for neurodegenerative conditions. AD is the most common neurodegenerative disease. Neuroprotective therapies in late stages of AD are ineffective due to massive neuronal death, which precedes symptoms of dementia. However, new diagnostic tools are being developed and with early diagnosis of neurodegenerative conditions estren or other ANGELS might offer promising therapeutic options for treatment of AD patients with earlier, mild stages of the disease.
Here we demonstrated in vivo that the non-classical estradiol signaling pathway activator estren can effectively ameliorate the Aβ1–42-induced morphological and behavioral deficits in the brain. Our results clearly imply that estren directly acts on cholinergic MAPK/CREB intracellular signaling system via ERα to restore the BFC neurons against Aβ1–42 neurotoxicity. Further studies are required to comprehensively characterize the action of the estren, or find a more effective activator than estren of the non-classical estradiol signaling pathway; this may provide a basis for a future therapeutic approach to alleviate cholinergic loss in AD.
Methods
Aβ1–42 preparation
The Aβ1–42 is routinely produced as a recombinant protein fused with maltose binding protein (MBP) with a proteolytic cleavage site for Factor X protease between the two segments (Wilson C. MSc Thesis, University of Otago, 2007). This strategy utilises the solubilizing character of the maltose binding protein (product of the MalE gene) to ensure expression of soluble protein at high concentration in Escherichia coli. After expression of this recombinant fusion protein in bacteria, the product was purified on an amylose column to which the MBP segment of the protein binds. Following binding to amylose resin, the pure fusion protein was eluted from the resin with maltose, and concentrated by ammonium sulphate precipitation. The carrier MBP was then cleaved off the fusion protein by Factor X protease, and the released Aβ1–42 isolated and further purified by hydrophobic chromatography with 0–50% v/v acetonitrile/0.1% v/v TFA, using FPLC. The fractions containing pure Aβ1–42 were detected immunologically with an antibody against residues 17–24 of Aβ1–42 and lyophilized to remove solvent. Mass spectrometry was used to confirm the expected molecular ion for the desired product. Before the intra-cerebral microinjection of this product, we dissolved the prepared monomer in artificial cerebrospinal fluid (ACSF: 147 mM Na+, 3.5 mM K+, 2 mM Ca2+, 1 mM Mg2+, pH 7.3) and ‘aged’ the solution at RT for 48 h to facilitate the formation of toxic soluble aggregates, as documented by SDS/PAGE. The optimal incubation time for our preparations of Aβ1–42 to produce the highly toxic oligomers is 48–120 h.
Animals
All experiments were approved and performed in accordance with the regulations of the ANZCCART and the University of Otago Animal Ethics Committee. All mice were bred and housed at the University of Otago, Hercus-Taieri Resource Unit. The animals were maintained under conditions of 12-h light/dark cycle (lights on at 0700 h) with food and water available ad libitum except for the behavioural experiments. All experiments were performed on adult (10–12 weeks old) female mice. Four mouse lines were used: C57BL/6J wild type control; ERαloxP/loxP control (ERαloxP/loxP, data not shown); neuron- specific ERαlox/loxpknockout mice CamkIICre; ERαloxP/loxP (nERαKO) and their wild type siblings (WT)53,54.
In vivo experiments
Aβ1–42 and estrogenic injections
Mice of all mouse lines were anesthetized with Avertin (0.1 ml/10 g body weight) and bilaterally ovariectomized (OVX). Two weeks after the OVX they were anesthetized with isoflurane and they were mounted in a stereotaxic apparatus, and given 1 μl of aged Aβ1–42 diluted in ACSF slowly (0.1 μl/min) into the NBM of the right hemisphere or bilaterally for the behavioural studies. Aβ1–42 was injected at the stereotaxic coordinates relative to bregma at anteroposterior (−0.7 mm), mediolateral (−2 mm), and dorsoventral (−3.75 and −4.75 mm, 0.5 μl at both coordinates) from dura. Control injections were performed using 1 μl ACSF or 1 μl 300 μM scrambled Aβ1–42 (AnaSpec). Based on the results the 20 μM Aβ1–42 dose and the 12 d survival time were selected for all subsequent experiments, except for the signaling experiments, where the animals were sacrificed 30 min after estren treatment. Estren or E2 was administered subcutaneously with different concentrations (estren: 0.3, 3.3 33 ng/g (Straloids); E2: 33 ng/g (Sigma)) 1 h after intracerebral injection of Aβ1–42.
In vitro experiments
The acute brain slice preparation for assessing ERK1/2 and CREB phosphorylation in vitro has been described previously55,56. Briefly, female C57BL/6 J wild type mice were decapitated 2 weeks following OVX, their brains rapidly removed and placed in oxygenated ACSF. Coronal 300 μm thick slices were cut on a vibratome and the slices pre-incubated at 30 °C for 30 min in oxygenated ACSF. Slices were transferred into ACSF containing 33.3 ng/g estren or vehicle (<0.01% ethyl alcohol) with or without 0.5 μM TTX and amino acid receptor blocker cocktail (10 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX), 20 μM l-2-amino-5-phosphonopentanoic acid (AP5), 50 μM picrotoxin and 2 μM strychnine) for 30 min. The slices were fixed then in 4% PFA, and 30 μm-thick coronal sections cut on a freezing microtome. Dual-labeling fluorescence immunohistochemistry and analysis for ERK1/2, pERK1/2, CREB and pCREB were performed as described below.
Immunohistochemistry
Free-floating peroxidase-based immunohistochemistry for choline acetyltransferase (ChAT) was undertaken as described previously32. Briefly, brain sections were incubated with primary antibodies recognizing ChAT (1:2000; Chemicon). This was followed by biotinylated donkey anti-goat IgGs (1:200; Jackson) and the avidin-biotin-HRP complex (1:200; Vector Elite ABC kit, Vector Laboratories) incubations. Labeling was then visualized with nickel-diaminobenzidine tetrahydrochloride (DAB) using glucose oxidase that resulted in a black precipitate within the labeled cells.
Free-floating dual-label fluorescence immunohistochemistry was performed to detect pERK1/2, ERK1/2, pCREB, CREB within ChAT neurons as described previously56,57,58 and Aβ1–42 in the NBM (Suppl Fig. 1). Briefly, brain sections were incubated with one of the primary antibodies recognizing pERK1/2, ERK1/2, pCREB or CREB (pERK1/2, 1:500, ERK1/2, 1:500, pCREB, 1:100, CREB, 1:1000; Cell Signaling Technologies; Aβ1–42, 1:500, Thermo Fisher Scientific) followed by incubation with chicken anti-rabbit Alexa Fluor 647 secondary antibody (1:500; Life Technologies). The sections were then processed further for ChAT immunolabeling (1:2000; Chemicon). This was followed by biotinylated donkey anti-goat Alexa Fluor 488 secondary antibody (1:200; Life technologies, USA) incubation. Sections were mounted on slides, air dried, and then coverslipped with VectaShield mounting medium (Vector Laboratories Inc).
Specificities of the primary antibodies have been tested and reported previously37,57,59,60,61. The omission of the primary antibodies resulted in complete absence of the immunoreactivity.
Acetylcholine esterase (AChE) histochemistry
AChE histochemistry with silver nitrate intensification was performed to label and visualize cholinergic fibers in the cortex32,62. Brain sections were incubated in sodium acetate buffered (0.1 M; pH 6) acetylthiocoline-iodide (0.05%), sodium citrate (0.1 M), copper sulfate (0.03 M), and potassium ferricyanide (5 mM) solution. This was followed with ammonium sulfide (1%) and then silver nitrate (1%) incubation.
Analysis of histological data
All measurements were performed by an investigator blind to the experimental groupings. Cholinergic cell body and fiber loss was analyzed as described with slight modification32,63,64. ChAT-positive cell bodies were counted in the NBM (plate 34 –35) on both brain sides according to the Paxinos and Franklin (2001) brain atlas65. Three sections starting from the bregma −1.2, with 120 μm inter-sectional distance from each animal was selected and analyzed for ChAT cell counting and 10 cortical sections for the AChE fiber density measurements (plate 28–40). Effects of Aβ1–42 lesion are expressed by forming percentage ratios of cell numbers and fiber density in the ipsi- and contralateral hemispheres except the behavioural studies where bilateral injections were delivered and the values are normalized to the naïve control group. Sections with ChAT and AChE labeling were examined under an Olympus BX51 microscope. Using a Cell-P Image Analysis software (Olympus, Japan) after background subtraction and gray scale threshold determination the surface area density of cortical AChE-positive fibers was measured.
Using the same anatomical areas as described above, three sections from NBM were selected from each animal, and the numbers of single ChAT-positive and double-labeled (ChAT + ERK1/2 or ChAT + pERK1/2; ChAT + CREB or ChAT + pCREB) neurons were determined using a Zeiss LSM 710 upright confocal laser-scanning microscope55. ERK1/2, pERK1/2, CREB and pCREB in ChAT-immunoreactive neurons are presented as the percentage of total number of ChAT-immunoreactive neurons in NBM. Note, using immunohistochemistry these proteins were successfully detected in the NBM by several research groups, including our own14,57,58.
Behavioural experiments and analysis
After 12 days (D12) post bilateral 33 ng/g Aβ1–42 injection the following behavioural tests were carried out (started at 09.00 h).
For the single pellet skilled reaching task (performed on d12-d27) a three lane Plexiglas reaching apparatus (30 cm deep, 10 cm wide, and 30 cm high for each lane) was constructed to allow simultaneous recording of three animals34. Mice were fasted to 90% of their body weight and maintained at this level for the full 2-week testing period. Animals were habituated during the first day by placing them into the lanes for 15 min. Next day the sugar pellets (20 mg, Bio-Serv) were freely available on the lane floor within tongue reach as well as just outside the slot opening. Pellets were gradually removed from the floor until only the pellets just outside of slot remained. Pellets were gradually moved further away from the slot (approximately 1 cm maximal distance) to force the mice to use their paw and not their tongue to reach for pellets. All mice were weighed daily and fed approximately 2 g of food after each training period to maintain their body weight at 90%. From day 2 pellets were presented one at a time and reaches were recorded with a video-recorder. Each animal was presented with a total of 15 pellets during each 15 min test period for 14 d. The reach was scored if the mouse successfully brought the pellet back to its mouth and consumed it34. The single pellet skilled reaching task was performed at the middle of the dark cycle when the animal’s motivation to eat is very high.
The novel object recognition paradigm (d30-d31) was used to evaluate recognition memory. The test was performed at the start of the dark cycle (3 h into the dark cycle) when the activity levels of animals are high. Animals were allowed to explore a set of two identical objects for a 5 min period, afterwards the mice were returned to their cages. The next day (24 h later) the animals were presented with a similar set of objects in the same environment, where one object was novel to them; they were allowed to freely explore the objects again for a 5 min period. A discrimination preference, for a novel over a familiar object was calculated as follows: time near a new object less the time near the old object, divided by time near the new object plus the time near the old object66. The total distance travelled in the arena during the habituation period was used as a measure of exploration. No significant differences were found in the travelled distance between the different experimental groups. Animals were monitored and the videos analyzed with the TopScan (CleverSys. Inc., USA) system.
Statistical analysis
Data in all experiments were expressed as mean ± SEM, except the survival curve data. Data are analysed by one-way ANOVA followed by Bonferroni’s or Tukey’s post hoc test (estren time and dose dependence study) with a value of p < 0.05 considered significant. Repeated measure and/or two-way ANOVA was used to analyze the behavioural data, the in vitro signaling and ERα KO data. P values of post hoc tests were adjusted using the Bonferroni test with a nominal significance level of 0.05. All statistical analysis were performed using Statistica 7.0 (StatSoft) and Prism (version 6; GraphPad Software).
Additional Information
How to cite this article: Kwakowsky, A. et al. Treatment of beta amyloid 1–42 (Aβ1–42)-induced basal forebrain cholinergic damage by a non-classical estrogen signaling activator in vivo. Sci. Rep. 6, 21101; doi: 10.1038/srep21101 (2016).
Supplementary Material
Acknowledgments
We acknowledge the excellent work of the members of the Hercus Taieri Resource Unit, University of Otago. This work was supported by the Health Research Council (IMA & WT), Otago Medical School and The Department of Physiology, University of Otago, Kispál Gyula grant, Medical School, University of Pécs, Hungarian Brain Research Program (KTIA_NAP_13-2014-0001), OTKA (112807) (IMA) and the Aotearoa Foundation, Centre for Brain Research and University of Auckland (AK).
Footnotes
Author Contributions I.M.A. and A.K. designed research; A.K., S.K., K.P., K.P. and W.P.T. performed research; A.K. and I.M.A. wrote the paper.
References
- Huang Y. & Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley K. K. & Chan D. The hippocampus in neurodegenerative disease. Frontiers of neurology and neuroscience 34, 95–108 (2014). [DOI] [PubMed] [Google Scholar]
- Brun A. & Englund E. Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopathological grading. Histopathology 5, 549–564 (1981). [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Struble R. G., Clark A. W. & Price D. L. Alzheimer disease: plaques, tangles, and the basal forebrain. Annals of neurology 12, 494 (1982). [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. The Journal of comparative neurology 521, 4124–4144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni R. B., Munoz J. P. & Barbeito L. The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Archives of medical research 32, 367–381 (2001). [DOI] [PubMed] [Google Scholar]
- Kruger L. & Mandelkow E. M. Tau neurotoxicity and rescue in animal models of human Tauopathies. Curr Opin Neurobiol 36, 52–58 (2015). [DOI] [PubMed] [Google Scholar]
- Lesne S. E. et al. Brain amyloid-beta oligomers in ageing and Alzheimer’s disease. Brain : a journal of neurology 136, 1383–1398 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Paula V. J., Radanovic M., Diniz B. S. & Forlenza O. V. Alzheimer’s disease. Sub-cellular biochemistry 65, 329–352 (2012). [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer’s disease: genes, proteins, and therapy. Physiological reviews 81, 741–766 (2001). [DOI] [PubMed] [Google Scholar]
- Wollen K. A. Alzheimer’s disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Alternative medicine review : a journal of clinical therapeutic 15, 223–244 (2010). [PubMed] [Google Scholar]
- Lee S. J. & McEwen B. S. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annual review of pharmacology and toxicology 41, 569–591 (2001). [DOI] [PubMed] [Google Scholar]
- Aggarwal P. & Gibbs R. B. Estrogen replacement does not prevent the loss of choline acetyltransferase-positive cells in the basal forebrain following either neurochemical or mechanical lesions. Brain research 882, 75–85 (2000). [DOI] [PubMed] [Google Scholar]
- Horvath K. M. et al. 17beta-estradiol enhances cortical cholinergic innervation and preserves synaptic density following excitotoxic lesions to the rat nucleus basalis magnocellularis. Neuroscience 110, 489–504 (2002). [DOI] [PubMed] [Google Scholar]
- Abraham I. M., Koszegi Z., Tolod-Kemp E. & Szego E. M. Action of estrogen on survival of basal forebrain cholinergic neurons: promoting amelioration. Psychoneuroendocrinology 34 Suppl 1, S104–112 (2009). [DOI] [PubMed] [Google Scholar]
- Henderson V. W. Oestrogens and dementia. Novartis Found Symp 230, 254–265; discussion 265–273 (2000). [DOI] [PubMed] [Google Scholar]
- Wang C. N., Chi C. W., Lin Y. L., Chen C. F. & Shiao Y. J. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem 276, 5287–5295 (2001). [DOI] [PubMed] [Google Scholar]
- Behl C. Oestrogen as a neuroprotective hormone. Nature reviews Neuroscience 3, 433–442 (2002). [DOI] [PubMed] [Google Scholar]
- Singh M., Sumien N., Kyser C. & Simpkins J. W. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci 13, 1083–1089 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani A. R., Pluchino N., Luisi S. & Luisi M. Estrogen, cognition and female ageing. Hum Reprod Update 13, 175–187 (2007). [DOI] [PubMed] [Google Scholar]
- Luine V. N. Estradiol and cognitive function: past, present and future. Hormones and behavior 66, 602–618 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. P., Nachtigall M. J., Nachtigall L. E. & Naftolin F. The Women’s Health Initiative trial and related studies: 10 years later: a clinician’s view. The Journal of steroid biochemistry and molecular biology 142, 4–11 (2014). [DOI] [PubMed] [Google Scholar]
- Manson J. E. Current recommendations: what is the clinician to do? Fertility and sterility 101, 916–921 (2014). [DOI] [PubMed] [Google Scholar]
- Kwakowsky A., Koszegi Z., Cheong R. Y. & Abraham I. M. Neuroprotective effects of non-classical estrogen-like signaling activators: from mechanism to potential implications. CNS & neurological disorders drug targets 12, 1219–1225 (2013). [PubMed] [Google Scholar]
- Nilsson S. et al. Mechanisms of estrogen action. Physiological reviews 81, 1535–1565 (2001). [DOI] [PubMed] [Google Scholar]
- Vasudevan N. & Pfaff D. W. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Frontiers in neuroendocrinology 29, 238–257 (2008). [DOI] [PubMed] [Google Scholar]
- Micevych P. & Dominguez R. Membrane estradiol signaling in the brain. Frontiers in neuroendocrinology 30, 315–327 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Chen S., Ming Wang J. & Brinton R. D. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience 132, 299–311 (2005). [DOI] [PubMed] [Google Scholar]
- Dominguez R., Jalali C. & de Lacalle S. Morphological effects of estrogen on cholinergic neurons in vitro involves activation of extracellular signal-regulated kinases. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 982–990 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J. et al. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience 124, 549–560 (2004). [DOI] [PubMed] [Google Scholar]
- Marin R. et al. Estradiol prevents amyloid-beta peptide-induced cell death in a cholinergic cell line via modulation of a classical estrogen receptor. Neuroscience 121, 917–926 (2003). [DOI] [PubMed] [Google Scholar]
- Koszegi Z., Szego E. M., Cheong R. Y., Tolod-Kemp E. & Abraham I. M. Postlesion estradiol treatment increases cortical cholinergic innervations via estrogen receptor-alpha dependent nonclassical estrogen signaling in vivo. Endocrinology 152, 3471–3482 (2011). [DOI] [PubMed] [Google Scholar]
- Kousteni S. et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298, 843–846 (2002). [DOI] [PubMed] [Google Scholar]
- Conner J. M., Culberson A., Packowski C., Chiba A. A. & Tuszynski M. H. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron 38, 819–829 (2003). [DOI] [PubMed] [Google Scholar]
- Cordey M., Gundimeda U., Gopalakrishna R. & Pike C. J. The synthetic estrogen 4-estren-3 alpha,17 beta-diol (estren) induces estrogen-like neuroprotection. Neurobiology of disease 19, 331–339 (2005). [DOI] [PubMed] [Google Scholar]
- Falkenstein E., Tillmann H. C., Christ M., Feuring M. & Wehling M. Multiple actions of steroid hormones–a focus on rapid, nongenomic effects. Pharmacological reviews 52, 513–556 (2000). [PubMed] [Google Scholar]
- Kalesnykas G., Roschier U., Puolivali J., Wang J. & Miettinen R. The effect of aging on the subcellular distribution of estrogen receptor-alpha in the cholinergic neurons of transgenic and wild-type mice. The European journal of neuroscience 21, 1437–1442 (2005). [DOI] [PubMed] [Google Scholar]
- Forny-Germano L. et al. Alzheimer’s disease-like pathology induced by amyloid-beta oligomers in nonhuman primates. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 13629–13643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli L., Casamenti F., Scali C., Bartolini L. & Pepeu G. Differential effects of amyloid peptides beta-(1–40) and beta-(25–35) injections into the rat nucleus basalis. Neuroscience 66, 781–792 (1995). [DOI] [PubMed] [Google Scholar]
- Harkany T. et al. Beta-amyloid(Phe(SO3H)24)25–35 in rat nucleus basalis induces behavioral dysfunctions, impairs learning and memory and disrupts cortical cholinergic innervation. Behavioural brain research 90, 133–145 (1998). [DOI] [PubMed] [Google Scholar]
- Abraham I. et al. Chronic corticosterone administration dose-dependently modulates Abeta(1–42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. Journal of neuroendocrinology 12, 486–494 (2000). [DOI] [PubMed] [Google Scholar]
- Gotz J. & Ittner L. M. Animal models of Alzheimer’s disease and frontotemporal dementia. Nature reviews Neuroscience 9, 532–544 (2008). [DOI] [PubMed] [Google Scholar]
- Wirths O. & Bayer T. A. Neuron loss in transgenic mouse models of Alzheimer’s disease. International journal of Alzheimer’s disease 2010, 1–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O., Dins A. & Bayer T. A. AbetaPP accumulation and/or intraneuronal amyloid-beta accumulation? The 3xTg-AD mouse model revisited. Journal of Alzheimer’s disease : JAD 28, 897–904 (2012). [DOI] [PubMed] [Google Scholar]
- Singer C. A., Figueroa-Masot X. A., Batchelor R. H. & Dorsa D. M. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 19, 2455–2463 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S. et al. Enhancement of rat hippocampal long-term potentiation by 17 beta-estradiol involves mitogen-activated protein kinase-dependent and -independent components. Neuroscience letters 332, 65–69 (2002). [DOI] [PubMed] [Google Scholar]
- Guerra B., Diaz M., Alonso R. & Marin R. Plasma membrane oestrogen receptor mediates neuroprotection against beta-amyloid toxicity through activation of Raf-1/MEK/ERK cascade in septal-derived cholinergic SN56 cells. Journal of neurochemistry 91, 99–109 (2004). [DOI] [PubMed] [Google Scholar]
- Carlstrom L., Ke Z. J., Unnerstall J. R., Cohen R. S. & Pandey S. C. Estrogen modulation of the cyclic AMP response element-binding protein pathway. Effects of long-term and acute treatments. Neuroendocrinology 74, 227–243 (2001). [DOI] [PubMed] [Google Scholar]
- Bora S. H., Liu Z., Kecojevic A., Merchenthaler I. & Koliatsos V. E. Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Experimental neurology 194, 506–522 (2005). [DOI] [PubMed] [Google Scholar]
- Dubal D. B. et al. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proceedings of the National Academy of Sciences of the United States of America 98, 1952–1957 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne M. R., Haug C. A., Abraham I. M. & Kwakowsky A. Estradiol modulation of neurotrophin receptor expression in female mouse basal forebrain cholinergic neurons in vivo. Endocrinology 156, 613–626 (2015). [DOI] [PubMed] [Google Scholar]
- Gibbs R. B. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocrine reviews 31, 224–253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermantel T. M. et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52, 271–280 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova E. et al. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis 31, 37–42 (2001). [DOI] [PubMed] [Google Scholar]
- Cheong R. Y. et al. Estradiol acts directly and indirectly on multiple signaling pathways to phosphorylate cAMP-response element binding protein in GnRH neurons. Endocrinology 153, 3792–3803 (2012). [DOI] [PubMed] [Google Scholar]
- Abraham I. M., Han S. K., Todman M. G., Korach K. S. & Herbison A. E. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 5771–5777 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas K. et al. Sex differences in oestrogen-induced p44/42 MAPK phosphorylation in the mouse brain in vivo. Journal of neuroendocrinology 18, 621–628 (2006). [DOI] [PubMed] [Google Scholar]
- Szego E. M. et al. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 4104–4110 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo T. T. et al. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. The Journal of neuroscience : the official journal of the Society for Neuroscience 17, 7594–7605 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty S., Schurov I. L., Sloper P. J. & Hastings M. H. Stimuli which entrain the circadian clock of the neonatal Syrian hamster in vivo regulate the phosphorylation of the transcription factor CREB in the suprachiasmatic nucleus in vitro. The European journal of neuroscience 10, 1063–1072 (1998). [DOI] [PubMed] [Google Scholar]
- von Gall C. et al. CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. The Journal of neuroscience : the official journal of the Society for Neuroscience 18, 10389–10397 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen J. C., Bacon S. J. & Price D. L. A modified histochemical technique to visualize acetylcholinesterase-containing axons. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 33, 134–140 (1985). [DOI] [PubMed] [Google Scholar]
- Horvath K. M. et al. Postnatal treatment with ACTH-(4–9) analog ORG 2766 attenuates N-methyl-D-aspartate-induced excitotoxicity in rat nucleus basalis in adulthood. European journal of pharmacology 405, 33–42 (2000). [DOI] [PubMed] [Google Scholar]
- Harkany T. et al. Short-term consequences of N-methyl-D-aspartate excitotoxicity in rat magnocellular nucleus basalis: effects on in vivo labelling of cholinergic neurons. Neuroscience 108, 611–627 (2001). [DOI] [PubMed] [Google Scholar]
- Paxinos G. & Franklin K. The mouse brain in stereotaxic coordinates . 2nd ed. (San Diego: Academic Press (2000). [Google Scholar]
- Dix S. L. & Aggleton J. P. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behavioural brain research 99, 191–200 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.