Abstract
This study aimed to investigate pathogenesis and novel diagnostic biomarkers of biliary atresia (BA). Serum samples from infants with BA and non-BA neonatal cholestasis (NC) were collected for miRNA microarray analysis, and then differentially expressed miRNAs were screened. Differentially expressed miRNAs were validated by qRT-PCR using an independent serum samples from infants with BA and NC. Diagnostic utility of validated miRNAs was further analyzed using serum samples by receiver-operating characteristic curve analysis. Totally, 13 differentially expressed miRNAs were identified including 11 down-regulated and 2 up-regulated ones. Target genes of hsa-miR-4429 and hsa-miR-4689 were significantly involved in FoxO signaling pathway. Eight differentially expressed miRNAs were chosen for validation by qRT-PCR analysis, and four miRNAs (hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p) were differentially expressed. The area under the curve of hsa-miR-4429 and hsa-miR-4689 was 0.789 (sensitivity = 83.33%, specificity = 80.00%) and 0.722 (sensitivity = 66.67%, specificity = 80.00%), respectively. Differentially expressed miRNAs including hsa-miR-4429 and hsa-miR-4689 might play critical roles in BA by regulating their target genes, and these two miRNAs may have the potential to become diagnostic biomarkers.
Biliary atresia (BA), a rare but serious cholestatic disorder in newborn infants, is caused by obstruction of extrahepatic or intrahepatic bile ducts with an occurrence rate of about 1/12,000 cases in United States and a higher incidence in Asia1,2. If not recognized and treated, BA will develop to progressive biliary cirrhosis and liver failure which could cause death within two years3. Kasai operation can reestablish bile flow in up to two-thirds of BA patients when performed earlier than 60 days of age2. However, a majority of patients will need liver transplant for survival if significant fibrosis and cirrhosis still exist after surgical intervention. Therefore, early identification and timely surgery are crucial for better prognosis. Unfortunately, definitive diagnosis of BA requires invasive and time-consuming diagnostic procedures, such as intra-operative cholangiogram and liver biopsy4. Consequently, it is urgently necessary for identification of noninvasive and convenient diagnostic biomarkers which may be helpful in distinguishing BA from the other neonatal cholestatic diseases.
MicroRNAs (miRNAs) are an abundant class of endogenous small and noncoding RNAs of about 22-nucleotides that post-transcriptionally regulate gene expressions5. Many studies have reported that miRNAs play important roles in experimental BA. The up-regulated expression of miR-29 in liver of murine BA model could lead to dysregulations of Igf1 and Il1RAP which are respectively responsible for cholangiocyte survival and modulation of inflammation6. In addition, miR-133a/b, miR-30b/c, miR-200a, miR-195, miR-365 and miR-320 have regulatory roles in pathogenesis of BA according to the miRNA expression profiles of extrahepatic bile ducts and gallbladder from murine BA model7. Shen et al. have reported that miR-222 was highly expressed in the extrahepatic bile ducts which might be responsible for liver fibrosis in the murine BA model8. The PI3K/Akt signaling pathway is activated in BA by elevated expression of miR-200b through suppressing FOG2, leading to increased growth and migration of human hepatic stallate cells9. Although understanding of BA pathogenesis has been improved, BA still remains to be a significant challenge and needs to be further investigated10. Therefore, it is of great importance to focus on the pathogenesis and diagnosis of BA.
Expression patterns of serum miRNAs can detect various diseases and distinguish similar disorders because serum levels of miRNAs are reproducible, stable and consistent among individuals of the same species11,12. What’s more, circulating miRNAs have been proposed as novel noninvasive biomarkers with encouraging diagnostic utility of BA. Zahm et al. have analyzed the serum miRNA expression profiles of BA patients and controls with indeterminate cholestasis by Low Density Array (TaqMan® Array Human MicroRNA A Cards) and they found miR-200b/429 have promising diagnostic clinical performance for BA13. However, only 134 of 375 miRNAs were detected in the study of Zahm et al. and information about the molecular mechanisms of BA and diagnostic utility of serum miRNAs remains insufficient.
In order to screen more differentially expressed miRNAs, human microRNA microarrays from Agilent Technologies containing probes for 1523 miRNAs were adopted in this current study,. Meanwhile, advanced bioinformatics analysis including prediction of target genes, identification of interaction relationships between target genes and functional enrichment analysis was also conducted for better understanding of the molecular mechanisms of BA. Moreover, the differentially expressed miRNAs were further validated in a larger and independent cohort of 45 infants with BA and 30 controls with non-BA neonatal cholestasis to find potential serum miRNA biomarkers.
Results
Screening of differentially expressed miRNAs and clustering analysis
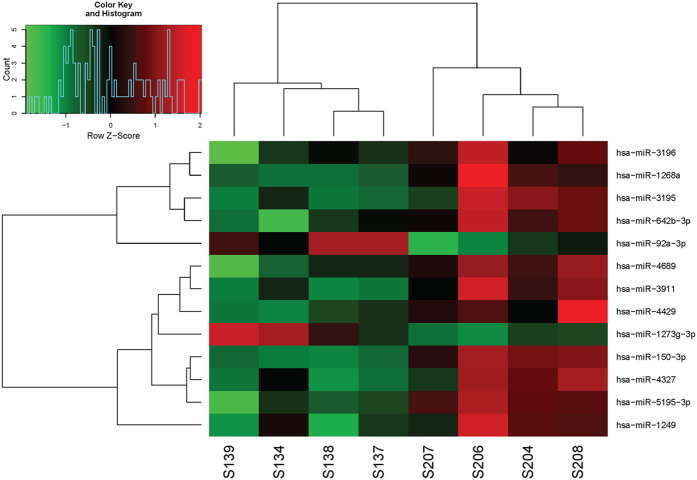
The differentially expressed miRNAs between infants with BA and NC controls were screened using a human miRNA microarray. A total of 13 differentially expressed miRNAs were identified, including 11 down-regulated and 2 up-regulated miRNAs (Table 1). The serum expression levels of hsa-miR-1273 g-3p and hsa-miR-92a-3p were significantly increased in the infants with BA. Meanwhile, infants with BA had significantly lower expression levels of hsa-miR-1268a, hsa-miR-3911, hsa-miR-4689, hsa-miR-3196, hsa-miR-4429, hsa-miR-4327, hsa-miR-150-3p, hsa-miR-642b-3p, hsa-miR-1249, hsa-miR-3195 and hsa-miR-5195-3p. Furthermore, hierarchical clustering analysis indicated the serum samples of infants with BA could be well distinguished from NC controls according to the expression levels of these 13 differentially expressed miRNAs (Fig. 1).
Table 1. The screened differentially expressed miRNAs in the serum samples of infants with biliary atresia.
| MiRNAs | Name | Fold change | P value |
|---|---|---|---|
| Down-regulated | hsa-miR-1268a | −1.52824 | 0.031041 |
| hsa-miR-3911 | −1.64985 | 0.014516 | |
| hsa-miR-4689 | −1.65676 | 0.015478 | |
| hsa-miR-3196 | −1.68217 | 0.043657 | |
| hsa-miR-4429 | −1.72581 | 0.042748 | |
| hsa-miR-4327 | −1.75703 | 0.026081 | |
| hsa-miR-150-3p | −2.03072 | 0.002125 | |
| hsa-miR-642b-3p | −2.10194 | 0.01667 | |
| hsa-miR-1249 | −2.16191 | 0.039503 | |
| hsa-miR-3195 | −2.23659 | 0.034854 | |
| hsa-miR-5195-3p | −2.45733 | 0.005206 | |
| Up-regulated | hsa-miR-92a-3p | 1.528466 | 0.011198 |
| hsa-miR-1273g-3p | 2.342245 | 0.039625 |
Figure 1. Hierarchical clustering analysis for the selected differentially expressed miRNAs.
The horizontal axis represents the serum samples from infants with biliary atresia (BA) (S139, S134, S138 and S137) and non-BA neonatal cholestasis (NC) controls (S207, S206, S204 and S208). The miRNA names are shown on the left vertical axis. Colored bars indicate the range of fold changes.
Functional and pathway enrichment analysis
The number of predicted target genes for hsa-miR-1268a, hsa-miR-3911, hsa-miR-4689, hsa-miR-3196, hsa-miR-4429, hsa-miR-4327, hsa-miR-150-3p, hsa-miR-642b-3p, hsa-miR-1249, hsa-miR-3195, hsa-miR-5195-3p, hsa-miR-92a-3p and hsa-miR-1273 g-3p was 15, 26, 24, 35, 182, 131, 115, 333, 9, 9, 183, 209 and 74, respectively (Supplementary Table 1).
The functional enrichment analysis revealed that target genes of hsa-miR-150-3p (FOXO3 and SMAD5), hsa-miR-642b-3p (WT1, PROX1, NFYB, CBL and RB1), hsa-miR-4327 (CREBBP, SALL1 and SOX2), hsa-miR-5195-3p (SMAD3, SOX9 and CITED2), hsa-miR-4429 (CREB5 and TFAP2B) and hsa-miR-92a-3p (RNF4, SOX4 and HNF1B) were significantly related to the protein binding (FDR = 0.000126268), sequence-specific DNA binding transcription factor activity (FDR = 0.001452774), positive regulation of transcription, DNA-dependent (FDR = 0.004632587) and nucleus (FDR = 0.00852864) (Table 2). The ROCK1 and ANK3 respectively targeted by hsa-miR-4689 and hsa-miR-3911 were also involved with protein binding. Another target gene of hsa-miR-4689 (SGK1) and hsa-miR-3911 (PLCB1) were related to nucleus. The members of frizzled (FZD) family (FZD1, FZD2 and FZD3) and G protein-coupled receptors (such as GPR64 targeted by hsa-miR-1249) were related to G-protein coupled receptor activity (FDR = 0.000125237). Meanwhile, FZD1 and FZD2 which were respectively targeted by hsa-miR-642b-3p and hsa-miR-3196 were also involved with the positive regulation of transcription, DNA-dependent.
Table 2. The enriched Gene Ontology (GO) terms in molecular function (MF), biological process (BP) and cellular component (CC) categories for target genes of all the 13 differentially expressed miRNAs.
| GO_ID | GO_term | Category | Count | Target Genes | FDR |
|---|---|---|---|---|---|
| GO:0005515 | Protein binding | MF | 433 | FZD1, FZD3, IGF1R, CBL, CREBBP, FOXO3, SMAD3, SMAD5, RB1, SALL1, SOX2, SOX4, TFAP2B, WT1, CREB5 | 0.000126268 |
| GO:0004930 | G-protein coupled receptor activity | MF | 11 | FZD2, LPAR4, GPR34, FZD3, FZD1, GPR64, GPR75, GPR126, ELTD1, GPR115, TAS2R20 | 0.000125237 |
| GO:0003700 | Sequence-specific DNA binding transcription factor activity | MF | 100 | CBL, CREBBP, CREM, ELK4, FOXO3, GABPB1, SMAD3, SMAD5, SMAD7, PROX1, RB1, SALL1, SOX2, SOX4,TFAP2B,WT1, CREB5… | 0.001452774 |
| GO:0001105 | RNA polymerase II transcription coactivator activity | MF | 8 | CREBBP, POU3F1, SOX4, SOX11, TFAP2B, CITED2, ANKRD1, HIPK2 | 0.049058312 |
| GO:0045893 | Positive regulation of transcription, DNA-dependent | BP | 61 | FZD1, FZD2, CREBBP, SMAD3, SMAD5, NFYB, PROX1, RB1, RNF4, SALL1, SOX2, SOX4, SOX9, HNF1B, TFAP2B, WT1, CREB5, CITED2 | 0.004632587 |
| GO:0006351 | Transcription, DNA-dependent | BP | 170 | CREM, ELK4, GABPB1, GATA2, HIC1, HOXA5, JUND, SMAD3, SMAD5, SMAD7, MTF1, NFYB, PBX1, RB1, RNF4, SALL1, SOX2 | 0.004014426 |
| GO:0045944 | Positive regulation of transcription from RNA polymerase II promoter | BP | 77 | JUND, SMAD3, SMAD5, SMAD7, MTF1, PBX1, PROX1, RB1, RNF4, SALL1, SOX2, SOX4, SOX9, HNF1B, TFAP2B, | 0.006415167 |
| GO:0007156 | Homophilic cell adhesion | BP | 25 | CDH2, CDH10, DSC2, PCDH7, ROBO2, MPZL2, NPTN, PCDH11X, CDH20, PCDH18 | 0.008450129 |
| GO:0005634 | Nucleus | CC | 396 | CBL, CREBBP, SMAD3, SMAD5, RB1, RNF4, SALL1, SOX2, SOX9, HNF1B, ZEB1, TFAP2B, WT1, CREB5, | 0.00852864 |
| GO:0005576 | Extracellular region | CC | 56 | ADM, ANGPT2, COL1A2, EREG, FBN1, IGF1, IGSF1, KITLG, NOTCH3, YBX1, LRP8, ADAM12, NRP1, CD200R1 | 0.009038887 |
| GO:0005883 | Neurofilament | CC | 6 | NEFM, NEFH, NEFL, NRP1, INA, DLGAP2 | 0.017283848 |
FDR: false discovery rate.
There were 29 significantly enriched pathways for the target genes (Supplementary Table 2), and the top ten pathways are listed in the Table 2. The target genes of hsa-miR-642b-3p (such as PPM1A, PAK2, PTPRR, PPKACB and MAP2K6), hsa-miR-4429 (such as MAPK1, AKT3, CACNG2, PPM1B, RAP1A and RASA1), hsa-miR-92a-3p (such as RAP1B, MAP2K4, NLK, DUSP10 and CACNA1I) and hsa-miR-3196 (such as JUND) were significantly enriched in the MAPK signaling pathway (FDR = 0.016391713, Table 3). Meanwhile, MAPK1, AKT3 and IGF1R regulated by hsa-miR-4429 also participate in the Proteoglycans in cancer (FDR = 0.004268857), Rap1 signaling pathway (FDR = 0.018406673) and Ras signaling pathway (FDR = 0.024181237). Besides, ROCK1 targeted by hsa-miR-4689 and PLCB1 targeted by hsa-miR-3911 were significantly involved in Proteoglycans in cancer, and PLCB1 was Rap1 signaling pathway and Glutamatergic synapse (FDR = 0.022324586). Moreover, the target genes of hsa-miR-4327 (CREBBP and IGF1R), hsa-miR-4689 (SGK1), hsa-miR-150-3p (IGF1, PRKAA2 and FOXO3), hsa-miR-4429 (MAPK1, AKT3, IGF1R and PTEN) and hsa-miR-92a-3p (NLK, KLF2 and PTEN) were revealed to participate in FoxO signaling pathway (FDR = 0.036456906).
Table 3. The top ten enriched pathways for target genes of all the 13 differentially expressed miRNAs.
| Pathway_ID | Name | Count | Target Genes | FDR |
|---|---|---|---|---|
| hsa04740 | Olfactory transduction | 5 | CALM1, ADCY3, PRKX, PRKACB, ADRBK2 | 0.001989049 |
| hsa05205 | Proteoglycans in cancer | 31 | ITGAV, ITGA5, IGF1, FRS2, CAV2, PIK3R3, MAPK1, PLCE1, GAB1, ITPR1, AKT3, ACTB, IGF1R, IQGAP1, FZD3, PRKACB, ROCK1 | 0.004268857 |
| hsa04010 | MAPK signaling pathway | 32 | PPM1A, RAP1B, MAPK1, CACNG2, MAP4K4, AKT3, NLK, DUSP10, PTPRR, RASA1, MAP2K4, PPM1B, RAP1A, PRKACB, CACNA1I, MAP2K6 | 0.016391713 |
| hsa05414 | Dilated cardiomyopathy | 15 | ACTG1, ITGB8, ITGAV, DAG1, ITGA5, IGF1, CACNA1D, ATP2A2, CACNG2, ADCY3, ACTB, PRKX, CACNA2D4, PRKACB, TPM3 | 0.02136154 |
| hsa04015 | Rap1 signaling pathway | 27 | CNR1, MLLT4, FLT1, IGF1, GNAI1, CALM1, RAP1B, ANGPT1, PIK3R3, MAPK1, PLCE1, LPAR4, AKT3, ADCY3, ACTB, IGF1R | 0.018406673 |
| hsa04520 | Adherens junction | 13 | ACTG1, CREBBP, MLLT4, YES1, LMO7, WASL, MAPK1, NLK, ACTB, IGF1R, SMAD3, TGFBR2, IQGAP1 | 0.016409495 |
| hsa05412 | Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 13 | ACTG1, CDH2, ITGB8, ITGAV, DAG1, ITGA5, DSC2, CACNA1D, PKP2, ATP2A2, CACNG2, ACTB, CACNA2D4 | 0.016093657 |
| hsa04724 | Glutamatergic synapse | 17 | GRIK5, GNAI1, CACNA1D, SLC1A2, MAPK1, ITPR1, PPP3CA, ADCY3, SLC17A6, DLGAP1, GNG7, PRKX | 0.022324586 |
| hsa04360 | Axon guidance | 18 | NRAS, DCC, SEMA6D, SRGAP1, SEMA3A, SEMA6A, PAK7, GNAI1, NRP1, MAPK1, ROBO2, PAK2 | 0.02165145 |
| hsa04014 | Ras signaling pathway | 27 | IGF1, CALM1, RAP1B, ANGPT1, PIK3R3, MAPK1, PLCE1, ARF6, GAB1, AKT3, IGF1R, PAK2, FGF23, GNG7 | 0.024181237 |
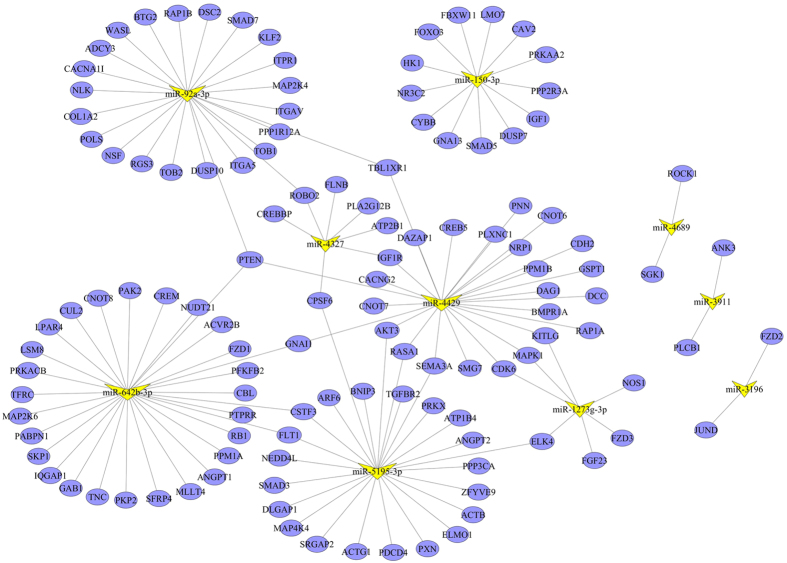
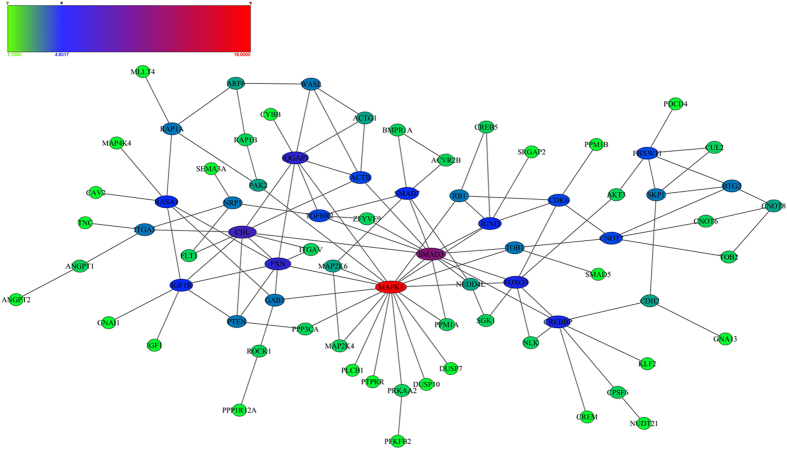
In the constructed regulatory network (Fig. 2), PTEN was simultaneously targeted by hsa-miR-92a-3p, hsa-miR-4429 and hsa-miR-642b-3p. The MAPK1 had the highest degree of 18 in the PPI network, followed by SMAD3 (degree = 12) and CBL (degree = 9) (Fig. 3, Table 4). Moreover, PTEN was interacted with IGF1R, PXN, CBL and PPP3CA in the PPI network.
Figure 2. The regulatory network for differentially expressed miRNAs.
The blue nodes represent the target genes. The yellow triangles indicate differentially expressed miRNAs with the size corresponding to degree. The blue lines show the potential regulatory relationships between miRNAs and genes.
Figure 3. Protein-protein interaction (PPI) network for the predicted target genes of differentially expressed miRNAs.
Colored bars indicate the degree of genes.
Table 4. The target genes with degrees not less than five in the protein-protein interaction network.
| Target genes | Degree |
|---|---|
| MAPK1 | 18 |
| SMAD3 | 12 |
| CBL | 9 |
| PXN | 8 |
| IQGAP1 | 8 |
| FOXO3 | 7 |
| CREBBP | 7 |
| SMAD7 | 6 |
| JUND | 6 |
| RASA1 | 6 |
| IGF1R | 6 |
| CNOT7 | 5 |
| FBXW11 | 5 |
| ACTB | 5 |
| TGFBR2 | 5 |
| CDK6 | 5 |
Validation of important miRNAs and enrichment analysis
Eight differentially expressed miRNAs (hsa-miR-92a-3p, hsa-miR-3911, hsa-miR-4689, hsa-miR-3196, hsa-miR-4429, hsa-miR-150-3p, hsa-miR-642b-3p and hsa-miR-1249) whose target genes were significantly enriched in important functions and pathways were selected as putative biomarkers for further validation. The expression levels of these eight candidate miRNAs were firstly measured by qRT-PCR in an independent cohort of serum samples from 10 infants with BA and 10 NC controls. The qRT-PCR revealed that hsa-miR-92a-3p and hsa-miR-4429 were respectively up-regulated and down-regulated with significant differences, showed the same change patterns as shown in with microarray analysis. However, the expression levels of hsa-miR-4689 (fold change = 2.36) and hsa-miR-150-3p (fold change = 4.08) were significantly increased which were opposite to the microarray analysis (Fig. 4). This discrepancy between first screening and validation might be due to technical limitations of microarray, such as cross-hybridizations, signal saturations and limited dynamic range14. The other four miRNAs were excluded for further analysis since no significant differences were detected.
Figure 4. Validation of selected miRNAs by qRT-PCR.
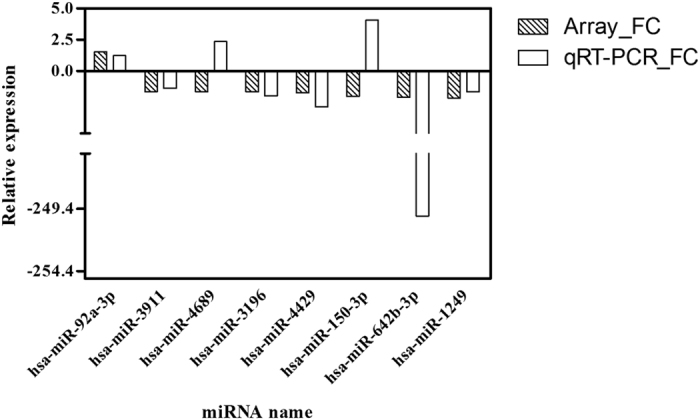
Serum expression levels of hsa-miR-92a-3p, hsa-miR-3911, hsa-miR-4689, hsa-miR-3196, hsa-miR-4429, hsa-miR-150-3p, hsa-miR-642b-3p and hsa-miR-1249 were measured in 10 infants with BA and 10 NC controls.
The functional enrichment analysis indicated that target genes of hsa-miR-150-3p (FOXO3 and SMAD5), hsa-miR-4429 (CREB5 and TFAP2B), hsa-miR-4689 (ROCK1) and hsa-miR-92a-3p (RNF4, SOX4 and HNF1B) were significantly related to the protein binding (FDR = 0.015416) (Table 5). The GNAI1 targeted by hsa-miR-4429 and GPR180 by hsa-miR-92a-3p were significantly related to the function of G-protein coupled receptor signaling pathway (FDR = 4.92E-05). A totoal of 14 significant pathways were enriched for the target genes of hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p (Table 6). There were 12 target genes (AKT3, CPD, FOXO3, IGF1, IGF1R, KLF2, MAPK1, NLK, NRAS, PRKAA2, PTEN and SGK1) that revealed to participate in FoxO signaling pathway (FDR = 0.007496). Meanwhile, target genes of hsa-miR-150-3p (IGF1 and PRKAA2), hsa-miR-4429 (AKT3, MAPK1, PTEN and TSC1), hsa-miR-92a-3p (TSC1 and PTEN) were simultaneously involved in the mTOR signaling pathway (FDR = 0.012814) and PI3K-Akt signaling pathway (FDR = 0.020308).
Table 5. The enriched GO terms for target genes of hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p.
| GO_ID | GO_term | Category | Count | Target Genes | FDR |
|---|---|---|---|---|---|
| GO:0001077 | RNA polymerase II core promoter proximal region sequence-specific DNA binding transcription factor activity involved in positive regulation of transcription | MF | 15 | KLF5, GATA2, MYB, NFIA, PBX1, SOX4, TCF21, TFAP2B, BTG2, KLF4, ONECUT2, CREB5, EHF, RIT1, MYOCD | 0.012047 |
| GO:0005515 | Protein binding | MF | 232 | FOXO3, SMAD5, CREB5, KLF5, RNF4, HNF1B, ROCK1, GATA2, MYB, PBX1, SOX4, TFAP2B, BTG2, KLF4, CREB5, RIT1, MYOCD | 0.015416 |
| GO:0007156 | Homophilic cell adhesion via plasma membrane adhesion molecules | BP | 22 | CDH2, CDH10, DSC2, ROBO2, NPTN, PCDH11X, CDH20, PCDHAC2, PCDHAC1, PCDHA13, PCDHA12, PCDH19, PCDH11Y… | 8.62E-07 |
| GO:0007186 | G-protein coupled receptor signaling pathway | BP | 2 | GNAI1, GPR180 | 4.92E-05 |
| GO:0045944 | Positive regulation of transcription from RNA polymerase II promoter | BP | 50 | ATRX, KLF5, CCNC, CUX1, DDX3X, ESRRG, FOXO3, GATA2, HOXA5, IGF1, SMAD7, PPP1R12A, NFIA, NPAS2, PAX9, RNF4, SOX4, DHX36… | 0.00105 |
| GO:0017148 | Negative regulation of translation | BP | 9 | DDX3X, TSC1, BTG2, FXR1, TOB1, SYNCRIP, IGF2BP3, CPEB3, NANOS1 | 0.002412 |
| GO:0033693 | Neurofilament bundle assembly | BP | 3 | NEFM, NEFH, NEFL | 0.012468 |
| GO:0018107 | Peptidyl-threonine phosphorylation | BP | 7 | DYRK1A, MAPK1, TNKS, OXSR1, HIPK3, NLK, WNK1 | 0.014844 |
| GO:0005883 | Neurofilament | CC | 6 | NEFM, NEFH, NEFL, NRP1, INA, DLGAP2 | 0.000132 |
| GO:0005730 | Nucleolus | CC | 74 | HNF1B, TDG, TEAD1, NR2C2, FXR1, DYRK2, CGGBP1, TNKS, BAZ2A, DUSP10, RTF1, DAZAP1, HBP1, AFF4, GRHL1, ERGIC2, WAC, POLK | 0.010514 |
Table 6. The enriched pathways for target genes of hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p.
| Pathway_ID | Name | Count | Target genes | FDR |
|---|---|---|---|---|
| hsa03018 | RNA degradation | 10 | BTG2, CNOT6, CNOT7, DHX36, PAN3, PAPD5, POLS, TOB1, TOB2, XRN1 | 0.00254 |
| hsa04730 | Long-term depression | 8 | GNA13, GNAI1, GRIA3, IGF1, IGF1R, ITPR1, MAPK1, NRAS | 0.007796 |
| hsa04360 | Axon guidance | 12 | DCC, GNAI1, MAPK1, NRAS, NRP1, PAK7, PLXNC1, RGS3, ROBO2, ROCK1, SEMA3A, SEMA6D | 0.006358 |
| hsa04068 | FoxO signaling pathway | 12 | AKT3, CPD, FOXO3, IGF1, IGF1R, KLF2, MAPK1, NLK, NRAS, PRKAA2, PTEN, SGK1 | 0.007496 |
| hsa05410 | Hypertrophic cardiomyopathy (HCM) | 9 | ATP2A2, CACNA2D4, CACNG2, DAG1, IGF1, ITGA5, ITGAV, PRKAA2, TPM3 | 0.007272 |
| hsa05414 | Dilated cardiomyopathy | 9 | ADCY3, ATP2A2, CACNA2D4, CACNG2, DAG1, IGF1, ITGA5, ITGAV, TPM3 | 0.011524 |
| hsa04150 | mTOR signaling pathway | 7 | AKT3, IGF1, MAPK1, PRKAA2, PTEN, TSC1, ULK1 | 0.012814 |
| hsa04611 | Platelet activation | 11 | ADCY3, AKT3, COL1A2, GNA13, GNAI1, ITPR1, MAPK1, PPP1R12A, RAP1A, RAP1B, ROCK1 | 0.012208 |
| hsa05214 | Glioma | 7 | AKT3, CDK6, IGF1, IGF1R, MAPK1, NRAS, PTEN | 0.016841 |
| hsa04510 | Focal adhesion | 14 | AKT3, CAV2, COL1A2, IGF1, IGF1R, ITGA5, ITGAV, MAPK1, PAK7, PPP1R12A, PTEN, RAP1A, RAP1B, ROCK1 | 0.02147 |
| hsa04151 | PI3K-Akt signaling pathway | 20 | AKT3, CDK6, COL1A2, CREB5, FOXO3, IGF1, IGF1R, ITGA5, ITGAV, KITLG, MAPK1, MCL1, MYB, NRAS, PHLPPL, PPP2R3A, PRKAA2, PTEN, SGK1, TSC1 | 0.020308 |
| hsa05218 | Melanoma | 7 | AKT3, CDK6, IGF1, IGF1R, MAPK1, NRAS, PTEN | 0.022286 |
| hsa03015 | mRNA surveillance pathway | 8 | DAZAP1, GSPT1, HBS1L, MSI2, NXT2, PNN, PPP2R3A, SMG7 | 0.024566 |
| hsa05412 | Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 7 | ATP2A2, CACNA2D4, CACNG2, CDH2, DAG1, ITGA5, ITGAV | 0.024824 |
Diagnostic utility of potential miRNAs
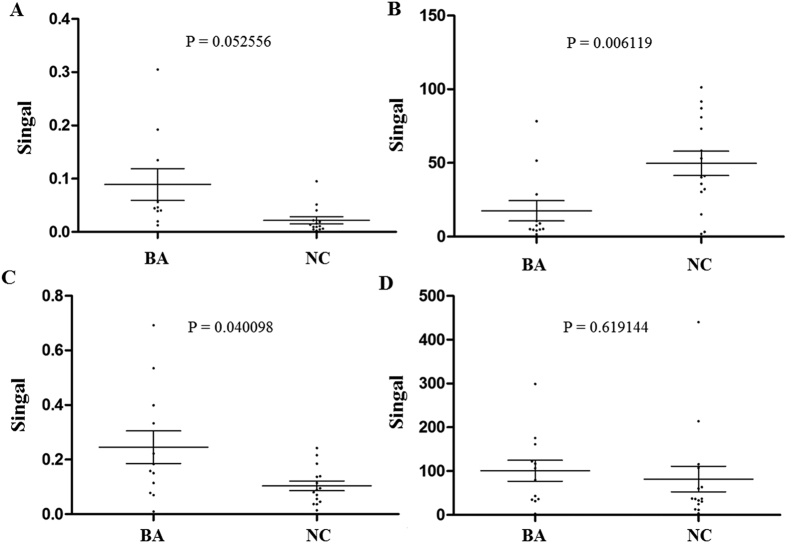
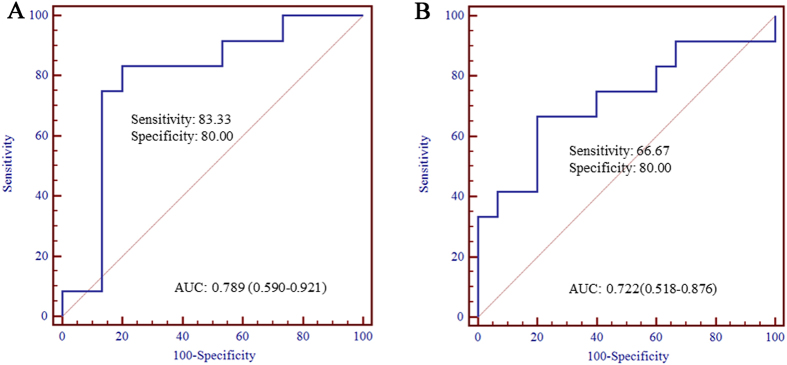
The hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p were selected for subsequent analysis of diagnostic utility. The expression levels of these miRNAs were detected by qRT-PCR using serum samples from another 35 infants with BA and 20 NC controls. Significantly down-regulated expression levels of hsa-miR-4429 were observed in serum samples of infants with BA in comparison to NC controls (P = 0.006119, Fig. 5B). The serum expression levels of hsa-miR-4689 were obviously higher in infants with BA (P = 0.040098, Fig. 5C). However, there was no significant differences in the expression levels of hsa-miR-150-3p (P = 0.052556) and hsa-miR-92a-3p (P = 0.619144) (Fig. 5A,D). After constructing ROC curves, the cutoff value for hsa-miR-4429 was 28.7112, with a sensitivity of 83.33% and a specificity of 80.00%. At the cutoff value of 0.1388 for hsa-miR-4689, the sensitivity was 66.67% and the specificity was 80.00%. The AUC was 0.789 (95% CI, 0.590–0.921) and 0.722 (95% CI, 0.518–0.876) for hsa-miR-4429 and hsa-miR-4689, respectively (Fig. 6).
Figure 5.
The expression levels of hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p in an independent set of serum samples from infants with BA (n = 35) and NC controls (n = 20) (A) hsa-miR-150-3p; (B) hsa-miR-4429; (C) hsa-miR-4689; (D) hsa-miR-92a-3p).
Figure 6.
Receiver operating characteristic curve analysis for BA diagnosis (A) hsa-miR-4429, (B) hsa-miR-4689; AUC: area under the curve).
Discussion
Long-term survival can be achieved without liver transplantation when the Kasai procedure is conducted within the first 30–45 days of life15. Since early diagnosis and convenient screening means of BA haven’t been well established, treatment for BA is inadequate due to delayed diagnosis and poor understanding of the pathogenesis. In this study, 13 differentially expressed miRNAs were identified using the Agilent miRNA microarray expression profiling. Moreover, eight differentially expressed miRNAs were selected for validation by qRT-PCR using an independent serum samples from infants with BA and NC controls. The validation analysis revealed that four miRNAs including hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p were differentially expressed.
In our study, functional enrichment analysis indicated that GNAI1 targeted by hsa-miR-4429 and GPR180 by hsa-miR-92a-3p were significantly related to the function of G-protein coupled receptor signaling pathway. It has been revealed that secretin can contribute to increased ductular choleresis after stimulation of G-protein-coupled receptor and cAMP/protein kinase A (PKA)-dependent signalling pathway16. Moreover, decreased expression of the type III InsP3R in the cholangiocytes of patients with biliary atresia is associated with impaired Ca2+ signaling and messenger molecule interacts with specific G protein-coupled receptors can induce activation of phospholipase C and formation of InsP3 to increase Ca2+ 17,18. Accordingly, hsa-miR-4429 and hsa-miR-92a-3p may be important for BA by targeting GNAI1 and GPR180 to influence the G-protein coupled receptor signaling pathway.
Moreover, target genes of hsa-miR-150-3p (IGF1, PRKAA2 and FOXO3), hsa-miR-4689 (SGK1), hsa-miR-4429 (MAPK1, AKT3, IGF1R and PTEN) and hsa-miR-92a-3p (NLK, KLF2 and PTEN) were significantly enriched in FoxO signaling pathway. BA is manifested by progressive inflammation and fibrosis of extrahepatic and intrahepatic bile ducts which can lead to cirrhosis19. It has been reported the FoxO3/Bim signaling pathway was obviously activated in patients with primary biliary cirrhosis20. Meanwhile, target genes of hsa-miR-4429 (AKT3, MAPK1, PTEN and TSC1) were significantly involved in the mTOR and PI3K-Akt signaling pathway. Increased expression levels of miR-200b in biliary atresia patients can acitvate PI3K/Akt signaling to accelerate migration and proliferation of hepatic stallate cells9. It has been reported that cystic proliferation of cholangiocytes of the polycystic kidney rat is associated with activation of the PI3K/mTOR pathway21. Therefore, it could be speculated thathsa-miR-4429 and hsa-miR-4689 might play important roles in BA by regulating their target genes that participate in these important signaling pathways.
Furthermore, the diagnostic utility of these four miRNAs (hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p) were analyzed in a larger independent sample set from another 35 infants with BA and 20 NC controls. The AUC of hsa-miR-4429 and hsa-miR-4689 was respectively 0.789 (95% CI, 0.590–0.921; sensitivity = 83.33%, specificity = 80.00%) and 0.722 (95% CI, 0.518–0.876; sensitivity = 66.67%, specificity = 80.00%), suggesting hsa-miR-4429 and hsa-miR-4689 might have the potential to be used as diagnostic biomarkers of BA. The human serum samples in the study of Zahm et al. were obtained from the Childhood Liver Disease Research and Education Network’s prospective longitudinal study of cholestasis in infancy, and the results revealed that miR-200b/429 might has promising diagnostic performance with the AUC values greater than 0.8013. However, the expression levels of miR-200b/429 didn’t significantly altered in the serum samples of infants with type III BA aged less than 90 days enrolled from the Children’s Hospital of Fudan University in our study. It could be speculated the different results might be due to that samples obtained from different sources were investigated. However, there are some limitations in our study. Some characteristics of participates, such as ethnic and region, were not considered due to the limited sample size. Besides, the expression levels of import target genes were not studied.
In conclusion, the differentially expressed miRNAs (especially hsa-miR-150-3p, hsa-miR-4429, hsa-miR-4689 and hsa-miR-92a-3p) might play important roles in the pathogenesis of BA by regulating their target genes. Furthermore, hsa-miR-4429 and hsa-miR-4689 might have promising diagnostic performance for BA. In our next study, expression levels of important target genes will be investigated by integrated miRNA and mRNA analysis to find more potential biomarkers for BA.
Methods
Study population and sample collection
In our study, 45 infants with BA and 30 infants with non-BA neonatal cholestasis (NC) similar in age and sex distribution during the same time period were enrolled from the Children’s Hospital of Fudan University (Table 7). These participants were enrolled based on the diagnoses of operative cholangiogram and liver pathology. The inclusion criteria for infants with BA were as follows: aged less than 90 days; type III BA according to the classification of BA phenotype22; serum direct or conjugated hyperbilirubinemia (>20% of total bilirubin and >2 mg/dL). Infants were excluded when they had liver failure, malignancy, ischemic hepatopathy, hypoxia or shock within the preceding 2 weeks; treated with extracorporeal membrane oxygenation–associated cholestasis or prior hepatobiliary surgery. Meanwhile, infants with birth weight less than 1500 g, drug- or total parenteral nutrition-associated cholestasis, bacterial or fungal sepsis, or primary hemolytic disease were also excluded unless they were diagnosed with BA or another cholestatic disease definitively23,24.
Table 7. Distribution of study subjects and liver function tests.
| BA | NC | P value | |
|---|---|---|---|
| Age (days)* | 70.30 ± 15.15 | 64.25 ± 10.33 | 0.32 |
| Male/Female | 25/20 | 18/12 | 0.35 |
| Diagnosis type | III1 | N/A | |
| TB (μmol/L) | 188.52 ± 100.36 | 158.62 ± 50.46 | 0.59 |
| DB (μmol/L) | 150.45 ± 71.25 | 125.32 ± 35.33 | 0.51 |
| DB/TB | 0.76 ± 0.45 | 0.74 ± 0.36 | 0.11 |
| AST (IU/L) | 268.15 ± 220.40 | 225.50 ± 200.67 | 0.15 |
| ALT (IU/L) | 135.10 ± 105.25 | 146.30 ± 135.25 | 0.55 |
| γ-GGT | 766.35 ± 650.67 | 245.15 ± 210.42 | < 0.01 |
Type III biliary atresia refers to the discontinuity of both right and left hepatic ducts to the level of porta hepatis. Unfortunately, type III BA is common, accounting for >90% of cases. *at liver biopsy sample day.
BA: biliary atresia; NC: non-BA neonatal cholestasis infants; ALT: Alanine transaminase; AST: Aspartate transaminase; DB: Direct bilirubin; TB: Total bilirubin; γ-GGT: Gamma glutamyl transpeptidase.
Blood samples were collected from these participants within a few days of enrollment preoperatively when the parents had given voluntary informed consent for their children. Our studies have been reviewed and approved by the ethics committee of the Children’s Hospital of Fudan University and all the experiments were carried out in accordance with relevant guidelines and regulations.
Sample processing and total RNA isolation
All whole blood samples collected from each participant were allowed to stand for about 1 h at room temperature. Then, these whole blood samples were separated into serum by centrifugation at 820 × g for 10 min at 4 °C, followed by further centrifugation at 16,000 × g for 10 min at 4 °C to completely remove cell debris. The supernatant serum was stored at −20 °C until analysis. Total RNA was isolated from 400 μl serum sample by using mirVana miRNA isolation kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. The concentration and quality of total RNA were monitored by NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and Agilent’s 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Serum miRNA expression profiling and microarray analysis
Serum samples of 4 infants with BA and 4 NC controls were used for miRNA microarray analysis. Human miRNA microarrays from Agilent Technologies (8*60 K), containing probes for 1523 human miRNAs from the Sanger miRbase V18.0 database (http://www.sanger.ac.uk/Software/ Rfam/ mirna), were adopted. Total RNA (100 ng) extracted from each serum sample was used as inputs for sample labeling and hybridization preparation in accordance with the manufacturer’s protocol (Agilent Technologies, Santa Clara, CA).
The microarray image information was converted into spot intensity values using Scanner Control Software Rev. 7.0 (Agilent Technologies, Santa Clara, CA). The signal after background subtraction was exported directly into the GeneSpring GX version 12.5 software (Agilent Technologies, Santa Clara, CA) for quartile normalization. Then, differentially expressed miRNAs in serum were identified using the paired t-test with the cut-off criteria of P < 0.05 and |fold change|>1.5. In order to ensure the screened differentially expressed miRNAs were accurately identified, hierarchical clustering analysis of samples was employed using heatmap.2 function of th gplots package in R25 based on the expression values.
The target genes of differentially expressed miRNAs were predicted by at least two databases of the following five usual prediction databases: TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org/microrna/home.do), PicTar (http://pictar.mdc-berlin.de/), MirTarget2 from miRDB (http://mirdb.org/miRDB/download.html) and PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html). Moreover, the Gene Ontology (GO) functional and pathway enrichment analysis were conducted for the target genes using the Database for Annotation, Visualization and Integrated Discovery (DAVID) online tools26 with the cut-off criterion of false discovery rate (FDR) < 0.05. The GO terms were identified in biological process (BP), cellular component (CC) and molecular function (MF) categories. The regulatory relationships for targets genes that simultaneously involved in significantly enriched functions and pathways were selected to constructe miRNA-target gene regulatory network. Protein-protein interactions (PPIs) for these target genes were revealed by the genemania (http://www.genemania.org/). The miRNA-target gene regulatory network and PPI network were both visualized using Cytoscape (Version 3.1.1)27.
Quantitative real-time PCR (qRT-PCR)
The relative quantification of selected differentially expressed miRNAs was performed by qRT-PCR reaction with the miScript SYBR Green PCR Kit (Qiagen) using ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The miRNA specific primers were designed by Primer Express software (Version 2.0, Applied Biosystems) based on the miRNA sequences obtained from miRbase database (http://microrna.sanger.ac.uk/). Primer sequences are listed in the Table 8.
Table 8. Primers used for quantitative real-time PCR (qRT-PCR).
| miRNAs | Primer sequences |
|---|---|
| hsa-miR-92a-3p | UAUUGCACUUGUCCCGGCCUGU |
| hsa-miR-3911 | UGUGUGGAUCCUGGAGGAGGC |
| hsa-miR-4689 | UUGAGGAGACAUGGUGGGGGCC |
| hsa-miR-3196 | CGGGGCGGCAGGGGCCU |
| hsa-miR-4429 | AAAAGCUGGGCUGAGAGGCG |
| hsa-miR-150-3p | CUGGUACAGGCCUGGGGGACAG |
| hsa-miR-642b-3p | AGACACAUUUGGAGAGGGACCC |
| hsa-miR-1249 | ACGCCCUUCCCCCCCUUCUUCA |
| has-miR-1228 | UCACACCUGCCUCGCCCCCC |
Extracted total RNA (60 ng) from serum samples was reverse transcribed into cDNA using miScript Reverse Transcription Kit (Qiagen). Each reaction was performed in a 20 μl volume system containing 1.5 μl cDNA, 2 μl of each primer and 1 × QuantiTect SYBR Green PCR Master Mix (Qiagen). MiR-1228 was used as a stable endogenous control for normalization since it functions as a housekeeping gene according to the study of Hu et al.28. All reactions were carried out in triplicate. The relative expression levels of miRNAs were calculated by the 2−△△Ct method.
Statistics
Receiver-operating characteristic (ROC) curve analysis was performed to determine the specificity and sensitivity of miRNA as a diagnostic biomarker. MedCalc (version 10.4.7.0; MedCalc, Mariakerke, Belgium) software was adopted to perform ROC analysis. Area under the ROC curve (AUC) was calculated as an accuracy index for evaluating the diagnostic performance of selected miRNA. The 95% confidence interval (CI) was used to reflect statistical significance.
Additional Information
How to cite this article: Dong, R. et al. Serum microRNA microarray analysis identifies miR-4429 and miR-4689 are potential diagnostic biomarkers for biliary atresia. Sci. Rep. 6, 21084; doi: 10.1038/srep21084 (2016).
Supplementary Material
Acknowledgments
This study received financial support from National Key Clinical Specialty Construction Programs of China (2014–2016), Shanghai ‘Non Key-in-Key Discipline’ Clinical medical centers (2014–2016), Shanghai Hospital Development Center (SHDC12014106), National Natural Science Foundation of China (no. 81370472, no. 81300517, no. 81401243 and no. 81500394), Shanghai City Health Bureau for Youth Scientific Fund Project (no. 20134y100), Shanghai Rising-Star Program (A type) (no. 15QA1400800) and The Science Foundation of Shanghai (no. 13ZR1451800, no. 14ZR1404000, and no. 14411969860).
Footnotes
Author Contributions R.D. and Z.S. performed the experiments and wrote the paper. C.Z. collected the specimens. G.C. statistically analyzed the data. S.Z. designed the research and wrote the paper.
References
- Mack C. L., Feldman A. G. & Sokol R. J. Clues to the etiology of bile duct injury in biliary atresia. Seminars in liver disease 32, 307–316, doi: 10.1055/s-0032-1329899 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack C. L. What Causes Biliary Atresia? Unique Aspects of the Neonatal Immune System Provide Clues to Disease Pathogenesis. CMGH Cellular and Molecular Gastroenterology and Hepatology (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. et al. Metabonomics Reveals Metabolite Changes in Biliary Atresia Infants. J Proteome Res, doi: 10.1021/acs.jproteome.5b00125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Serum markers may distinguish biliary atresia from other forms of neonatal cholestasis. Journal of pediatric gastroenterology and nutrition 50, 411–416, doi: 10.1097/MPG.0b013e3181cb42ee (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felekkis K., Touvana E., Stefanou C. & Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia 14, 236–240 (2010). [PMC free article] [PubMed] [Google Scholar]
- Hand N. J. et al. MicroRNA profiling identifies miR-29 as a regulator of disease-associated pathways in experimental biliary atresia. Journal of pediatric gastroenterology and nutrition 54, 186–192, doi: 10.1097/MPG.0b013e318244148b (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessho K. et al. Integrative genomics identifies candidate microRNAs for pathogenesis of experimental biliary atresia. BMC systems biology 7, 104, doi: 10.1186/1752-0509-7-104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. J., Dong R., Chen G. & Zheng S. microRNA-222 modulates liver fibrosis in a murine model of biliary atresia. Biochemical and biophysical research communications 446, 155–159, doi: 10.1016/j.bbrc.2014.02.065 (2014). [DOI] [PubMed] [Google Scholar]
- Xiao Y. et al. Up-regulation of miR-200b in biliary atresia patients accelerates proliferation and migration of hepatic stallate cells by activating PI3K/Akt signaling. Cellular signalling 26, 925–932, doi: 10.1016/j.cellsig.2014.01.003 (2014). [DOI] [PubMed] [Google Scholar]
- Zagory J. A., Nguyen M. V. & Wang K. S. Recent advances in the pathogenesis and management of biliary atresia. Current opinion in pediatrics 27, 389–394, doi: 10.1097/MOP.0000000000000214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell research 18, 997–1006, doi: 10.1038/cr.2008.282 (2008). [DOI] [PubMed] [Google Scholar]
- Zahm A. M. et al. Circulating MicroRNA Is a Biomarker of Pediatric Crohn Disease. Journal of pediatric gastroenterology and nutrition 53, 10.1097/MPG.1090b1013e31822200cc, doi: 10.1097/MPG.0b013e31822200cc (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm A. M., Hand N. J., Boateng L. A. & Friedman J. R. Circulating MicroRNA is a Biomarker of Biliary Atresia. Journal of pediatric gastroenterology and nutrition 55, 366–369, doi: 10.1097/MPG.0b013e318264e648 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. Large scale real-time PCR validation on gene expression measurements from two commercial long-oligonucleotide microarrays. BMC genomics 7, 59, doi: 10.1186/1471-2164-7-59 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol R. J. et al. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 46, 566–581, doi: 10.1002/hep.21790 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzabosco M., Spirli C. & Okolicsanyi L. Pathophysiology of the intrahepatic biliary epithelium. Journal of gastroenterology and hepatology 15, 244–253 (2000). [DOI] [PubMed] [Google Scholar]
- Guerra M. T. & Nathanson M. H. Calcium signaling and secretion in cholangiocytes. Pancreatology 15, S44–48, doi: 10.1016/j.pan.2015.05.477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa N., Ehrlich B. E. & Nathanson M. H. Calcium signaling in cholangiocytes. World journal of gastroenterology 12, 3466–3470 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack C. L. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Seminars in liver disease 27, 233–242, doi: 10.1055/s-2007-985068 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopycinska J. et al. Activation of FoxO3a/Bim axis in patients with Primary Biliary Cirrhosis. Liver Int 33, 231–238 (2013). [DOI] [PubMed] [Google Scholar]
- Ren X. S. et al. Activation of the PI3K/mTOR Pathway Is Involved in Cystic Proliferation of Cholangiocytes of the PCK Rat. PloS one 9, e87660, doi: 10.1371/journal.pone.0087660 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby A., Makin E. & Davenport M. Classification of the biliary atresia phenotype. Pediatrics international : official journal of the Japan Pediatric Society 52, 897, doi: 10.1111/j.1442-200X.2010.03275.x (2010). [DOI] [PubMed] [Google Scholar]
- Dong R. et al. Identification of HSP90 as potential biomarker of biliary atresia using two-dimensional electrophoresis and mass spectrometry. PloS one 8, e68602, doi: 10.1371/journal.pone.0068602 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Serum markers may distinguish biliary atresia from other forms of neonatal cholestasis. Journal of pediatric gastroenterology and nutrition 50, 411–416, doi: 10.1097/MPG.0b013e3181cb42ee (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes G. et al. (2011).
- Dennis G. Jr et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4, P3 (2003). [PubMed] [Google Scholar]
- Kohl M., Wiese S. & Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol 696, 291–303, doi: 10.1007/978-1-60761-987-1_18 (2011). [DOI] [PubMed] [Google Scholar]
- Hu J. et al. Human miR-1228 as a stable endogenous control for the quantification of circulating microRNAs in cancer patients. International Journal of Cancer 135, 1187–1194, doi: 10.1002/ijc.28757 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.