Abstract
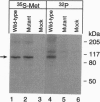
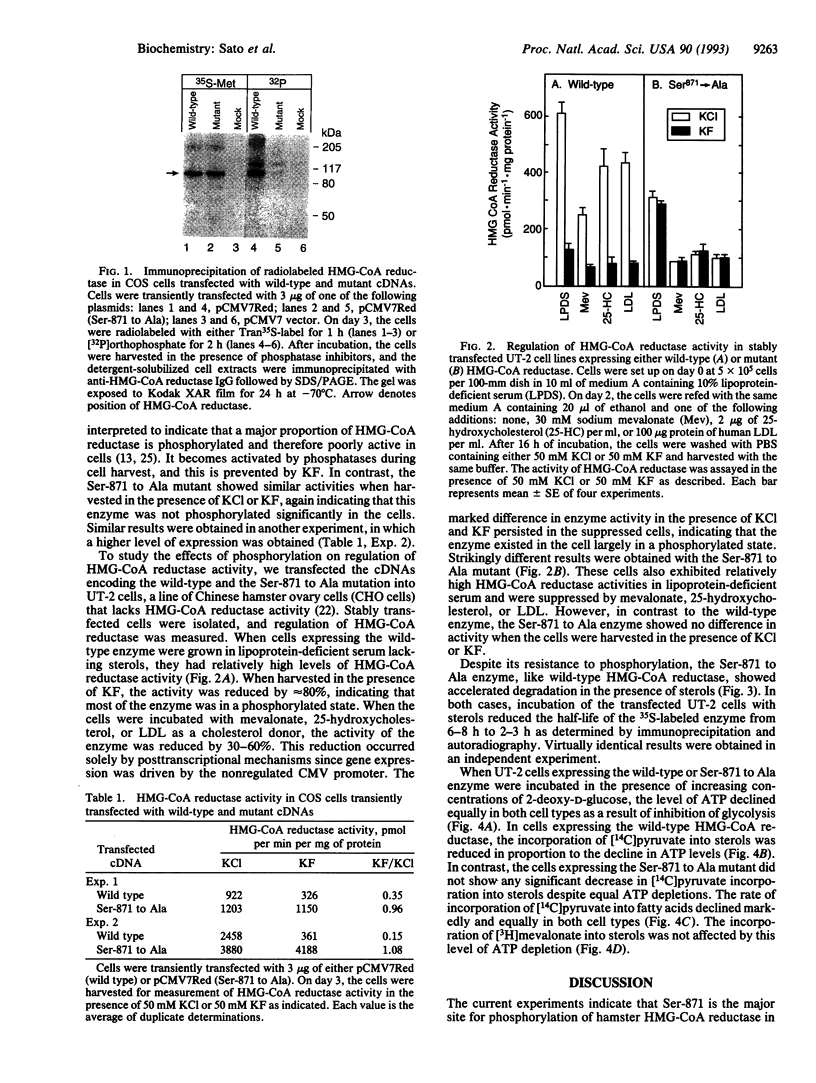
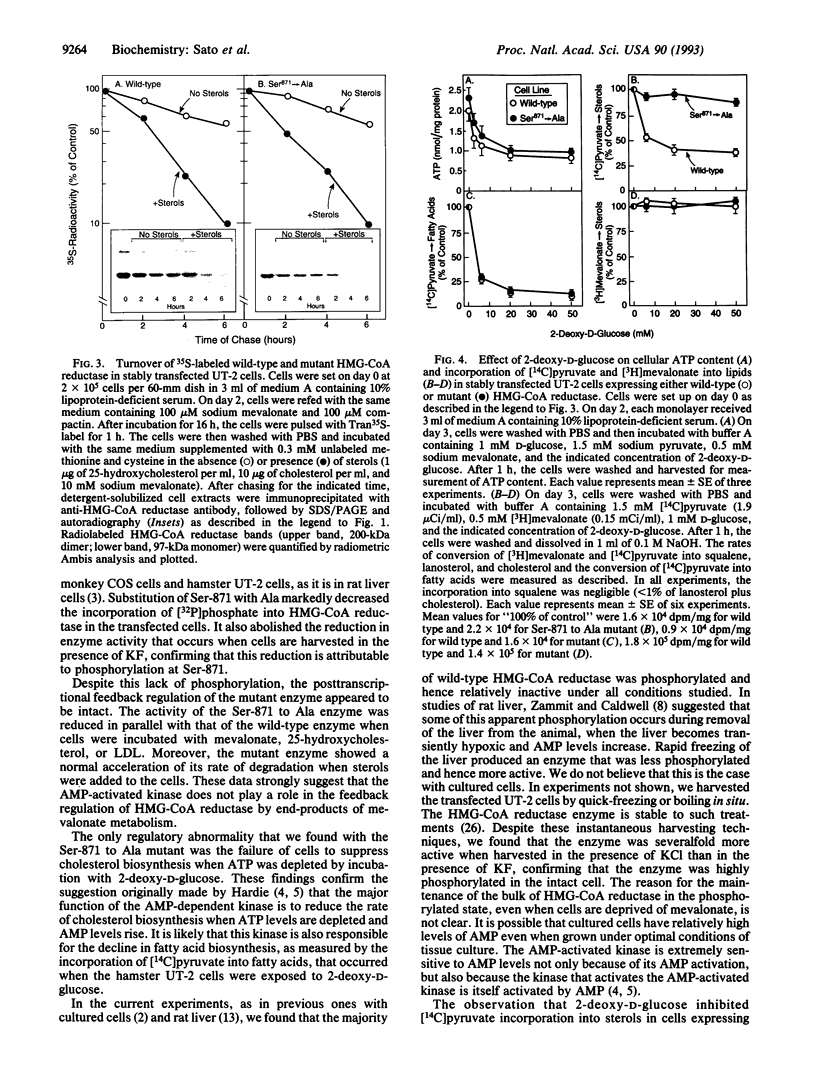
An AMP-activated protein kinase has been reported to phosphorylate rodent 3-hydroxy-3-methylglutaryl-coenzyme A reductase [HMG-CoA reductase; (S)-mevalonate:-NAD+ oxidoreductase (CoA-acylating), EC 1.1.1.88] at Ser-871, thereby lowering its catalytic activity [Clarke, P. R. & Hardie, D. G. (1990) EMBO J. 9, 2439-2446]. To explore the physiologic role of this reaction, we prepared a cDNA encoding a mutant form of hamster HMG-CoA reductase with alanine substituted for serine at residue 871. When overexpressed in transfected cells, the wild-type enzyme, but not the Ser-871 to Ala mutant, was labeled with [32P]phosphate, confirming Ser-871 as the site of phosphorylation. The wild-type enzyme, but not the mutant enzyme, showed reduced activity when the cells were harvested with the phosphatase inhibitor KF, confirming phosphorylation as a mechanism for inactivation within the cell. Despite the lack of phosphorylation, the posttranscriptional feedback regulation of the mutant enzyme was normal, as indicated by reduced activity when cells were incubated with mevalonate, 25-hydroxycholesterol, or low density lipoprotein. Moreover, the mutant enzyme showed a normal acceleration of degradation when the transfected cells were incubated with sterols. Cells expressing the wild-type enzyme showed a decreased incorporation of [14C]pyruvate into sterols when ATP was depleted by incubation with 2-deoxy-D-glucose. No such reduction was seen in cells expressing the Ser-871 to Ala mutant enzyme. We conclude that the AMP-activated protein kinase does not play a role in end-product feedback regulation of HMG-CoA reductase, but rather it comes into play when cellular ATP levels are depleted, thereby lowering the rate of cholesterol synthesis and preserving the energy stores of the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Beg Z. H., Brewer H. B., Jr Regulation of liver 3-hydroxy-3-methylglutaryl-CoA reductase. Curr Top Cell Regul. 1981;20:139–184. doi: 10.1016/b978-0-12-152820-1.50008-0. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Dietschy J. M., Siperstein M. D. 3-Hydroxy-3-methylglutaryl coenzyme A reductase. Solubilization and purification of a cold-sensitive microsomal enzyme. J Biol Chem. 1973 Jul 10;248(13):4731–4738. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Dietschy J. M. Active and inactive forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the liver of the rat. Comparison with the rate of cholesterol synthesis in different physiological states. J Biol Chem. 1979 Jun 25;254(12):5144–5149. [PubMed] [Google Scholar]
- Chin D. J., Gil G., Russell D. W., Liscum L., Luskey K. L., Basu S. K., Okayama H., Berg P., Goldstein J. L., Brown M. S. Nucleotide sequence of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, a glycoprotein of endoplasmic reticulum. Nature. 1984 Apr 12;308(5960):613–617. doi: 10.1038/308613a0. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Hardie D. G. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990 Aug;9(8):2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom R. A., Zammit V. A. Diurnal changes in the fraction of 3-hydroxy-3-methylglutaryl-CoA reductase in the active form in rat liver microsomal fractions. Biochem J. 1984 Jun 15;220(3):739–745. doi: 10.1042/bj2200739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. G., Hardie D. G. Phosphorylation and inactivation of HMG-CoA reductase at the AMP-activated protein kinase site in response to fructose treatment of isolated rat hepatocytes. FEBS Lett. 1992 Jul 13;306(1):59–62. doi: 10.1016/0014-5793(92)80837-7. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hardie D. G., MacKintosh R. W. AMP-activated protein kinase--an archetypal protein kinase cascade? Bioessays. 1992 Oct;14(10):699–704. doi: 10.1002/bies.950141011. [DOI] [PubMed] [Google Scholar]
- Hardie D. G. Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta. 1992 Feb 12;1123(3):231–238. doi: 10.1016/0005-2760(92)90001-c. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Geelen M. J., Parker R. A., Evenson K. J., Gibson D. M. Modulation of hydroxymethylglutaryl-CoA reductase activity, reductase kinase activity, and cholesterol synthesis in rat hepatocytes in response to insulin and glucagon. J Biol Chem. 1979 Oct 25;254(20):9986–9989. [PubMed] [Google Scholar]
- Liscum L., Finer-Moore J., Stroud R. M., Luskey K. L., Brown M. S., Goldstein J. L. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J Biol Chem. 1985 Jan 10;260(1):522–530. [PubMed] [Google Scholar]
- Luskey K. L., Stevens B. Human 3-hydroxy-3-methylglutaryl coenzyme A reductase. Conserved domains responsible for catalytic activity and sterol-regulated degradation. J Biol Chem. 1985 Aug 25;260(18):10271–10277. [PubMed] [Google Scholar]
- Marrero P. F., Haro D., Hegardt F. G. Phosphorylation of HMG-CoA reductase induced by mevalonate accelerates its rate of degradation in isolated rat hepatocytes. FEBS Lett. 1986 Mar 3;197(1-2):183–186. doi: 10.1016/0014-5793(86)80323-4. [DOI] [PubMed] [Google Scholar]
- Mosley S. T., Brown M. S., Anderson R. G., Goldstein J. L. Mutant clone of Chinese hamster ovary cells lacking 3-hydroxy-3 -methylglutaryl coenzyme A reductase. J Biol Chem. 1983 Nov 25;258(22):13875–13881. [PubMed] [Google Scholar]
- Nakanishi M., Goldstein J. L., Brown M. S. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J Biol Chem. 1988 Jun 25;263(18):8929–8937. [PubMed] [Google Scholar]
- Nordstrom J. L., Rodwell V. W., Mitschelen J. J. Interconversion of active and inactive forms of rat liver hydroxymethylglutaryl-CoA reductase. J Biol Chem. 1977 Dec 25;252(24):8924–8934. [PubMed] [Google Scholar]
- Parker R. A., Miller S. J., Gibson D. M. Phosphorylation of native 97-kDa 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Impact on activity and degradation of the enzyme. J Biol Chem. 1989 Mar 25;264(9):4877–4887. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Zammit V. A., Caldwell A. M. Direct demonstration that increased phosphorylation of 3-hydroxy-3-methylglutaryl-CoA reductase does not increase its rate of degradation in isolated rat hepatocytes. Biochem J. 1992 Jun 15;284(Pt 3):901–904. doi: 10.1042/bj2840901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]