Abstract
Arsenic (As) is a toxic heavy metal that can accumulate in crops and poses a threat to human health. The genetic mechanism of As accumulation is unclear. Herein, we used quantitative trait locus (QTL) mapping to unravel the genetic basis of As accumulation in a maize recombinant inbred line population derived from the Chinese crossbred variety Yuyu22. The kernels had the lowest As content among the different maize tissues, followed by the axes, stems, bracts and leaves. Fourteen QTLs were identified at each location. Some of these QTLs were identified in different environments and were also detected by joint analysis. Compared with the B73 RefGen v2 reference genome, the distributions and effects of some QTLs were closely linked to those of QTLs detected in a previous study; the QTLs were likely in strong linkage disequilibrium. Our findings could be used to help maintain maize production to satisfy the demand for edible corn and to decrease the As content in As-contaminated soil through the selection and breeding of As pollution-safe cultivars.
Arsenic (As), which is a highly toxic metalloid and is found ubiquitously in the environment, poses a serious risk to plants, animals and humans1. Arsenic content in soils has increased substantially in recent years because of irrigation with As-rich surface water or from anthropogenic activities, such as ore mining, smelting, burning of coal, use of As pesticides and the application of wastes2,3,4. Excess As in soil can inhibit seed germination and plant growth5,6,7, disturb plant metabolism8 and cause plant death9,10. Arsenic can be taken up by plants and vegetables from the soil and irrigated water, and subsequently enter the food chain11. Thus, humans may consume As. Arsenic exposure can cause human diseases such as skin lesions, neurological defects, atherosclerosis and even cancer12. In recent years, the most serious As pollution problems have occurred in Bangladesh and West Bengal, India13,14. Therefore, there is an urgent need to find suitable methods to reduce the transmission of As to humans. One option is to remove As from the soil. However, traditional methods and phytoremediation are limited by their own shortcomings15,16,17. Yu et al. have described the concept of the pollution-safe cultivar18. This concept refers to the use of cultivars that accumulate a very low level of a specific pollutant, which ensures the crop remains safe for human consumption, even when grown in contaminated soil. The application of pollution-safe cultivar selection and breeding is considered a practical and cost-effective approach to minimize the entry of heavy metals into the human food chain, and has received widespread attention19,20.
Human exposure to As occurs commonly by transfer from the crop–soil–water system1. Recently, the accumulation and distribution of As have been studied in different crops. Abedin et al. found that rice roots accumulated much more As than the straw and grain21. The trend of As concentration in different rice tissues was as follows: grain <husk <straw <root22. In maize, Baig et al. reported that the total As content in different tissues was in the order: grain <shoot <root23. Other studies have reported that the trend of As concentration in four different maize tissues was: kernels <bracts <stems <leaves24,25.
Marker-assisted selection is very useful to accelerate genetic improvement in crops. Many quantitative trait loci (QTLs) for important traits have been mapped in whole genomes26, forming the basis for rapid genetic improvement through marker-assisted selection. Four QTLs for As accumulation have been detected in rice27, and Tapash et al. identified an As tolerance gene on chromosome 6 of rice using a recombinant inbred line (RIL) population from a Bala × Azucena population28. In maize, many studies have focused on the physiological and biochemical responses to As accumulation. The majority of these studies demonstrated a trend of decreasing As content from the roots to the aerial parts, including the leaves, stems and seeds1. Maize takes up the arsenic naturally present in the soil or arsenic that is added through groundwater irrigation or by soil additives contaminated with arsenic. Several studies have described a significant relationship between the As concentration in the irrigation water or soil and the total As content accumulated by maize plants29. Gulz et al. observed that the correlation between the total accumulated As in maize plants and the water-soluble As fraction in the soil was higher than the total As content in the soil30. Several factors, including pH, redox potential, organic matter content, interaction/competition with other elements and chemical forms of the pollutant, can affect As solubility in soils31.
Maize is the most cultivated cereal in the world and is used as an important animal feed or a staple food crop for humans in many developing countries in Africa, Asia and Latin America1. Hence, maize grown on As-contaminated land could accumulate As and pose a risk to human health. Thus, methods to reduce As accumulation in maize are urgently needed. However, there have been few reports of mapped QTLs associated with As accumulation and distribution in maize. In the present study, a RIL population derived from parents with contrasting As tolerances was studied at two locations where the soil As levels substantially differed. The accumulation and distribution of As in different maize tissues were examined. Additionally, we generated data that may aid QTL mapping for important traits in breeding populations for the genetic improvement and production of maize pollution-safe cultivars.
Results
Performance of the measured traits at two locations
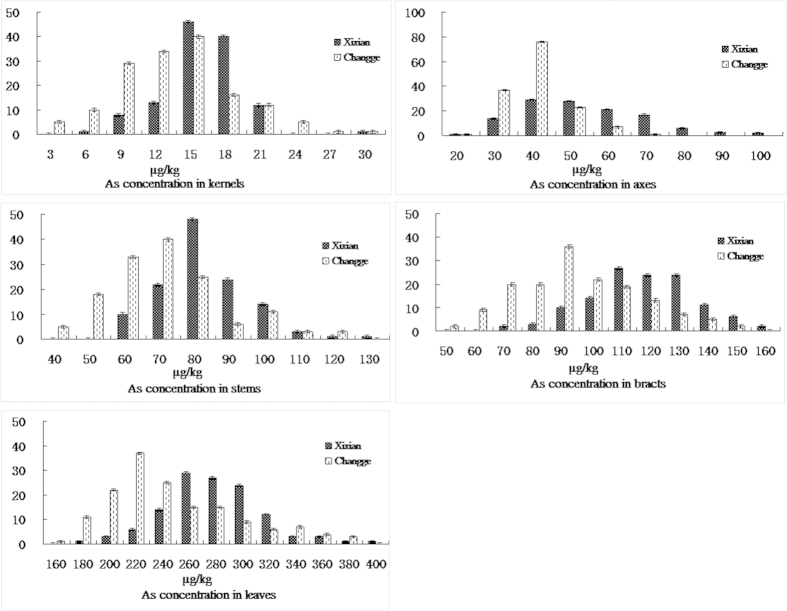
The soil As concentration at Xixian was 20.70 ± 0.37 mg/kg (pH = 6.5) because of irrigation with As-rich surface water. While the soil As concentration at Changge was 12.24 ± 0.21 mg/kg (pH = 6.5), which was used as a control. In terms of As concentration of the two parents and the hybrid, the five measured tissues showed higher levels at Xixian compared with Changge (Table 1, Fig. 1). The As content in the five maize tissues examined varied widely in the RIL population, which showed two-way transgressive segregation. In addition, the average As concentration in the RIL population was higher at Xixian than at Changge. The results indicated that soil As concentration is an important factor affecting the As content in maize tissues.
Table 1. Performance of five maize tissues in the RIL population.
| Location | Population | Trait | KAC (μg/kg) | AAC (μg/kg) | SAC (μg/kg) | BAC (μg/kg) | LAC (μg/kg) |
|---|---|---|---|---|---|---|---|
| Xixian | Zong3 | Mean | 11.54 ± 0.87 | 28.20 ± 1.18 | 96.63 ± 7.12 | 141.41 ± 10.50 | 239.59 ± 16.55 |
| 87-1 | Mean | 18.22 ± 1.45 | 53.17 ± 2.18 | 69.18 ± 1.12 | 99.62 ± 4.88 | 296.14 ± 17.58 | |
| F1 | Mean | 14.27 ± 0.55 | 78.95 ± 5.46 | 79.00 ± 5.50 | 113.81 ± 5.05 | 328.17 ± 20.53 | |
| RIL | Mean | 14.66 ± 3.48 | 47.59 ± 16.12 | 77.08 ± 12.05 | 112.08 ± 18.07 | 268.02 ± 36.18 | |
| Range | 5.66–29.00 | 19.81–97.70 | 52.35–123.99 | 60.20–155.40 | 170.21–384.49 | ||
| Skewness | 0.38 | 0.69 | 0.65 | −0.23 | 0.33 | ||
| Kurtosis | 1.94 | 0.19 | 1.36 | 0.06 | 0.82 | ||
| Changge | Zong3 | Mean | 8.63 ± 1.02 | 26.85 ± 3.96 | 67.28 ± 2.80 | 50.00 ± 2.26 | 260.44 ± 10.39 |
| 87-1 | Mean | 18.75 ± 2.74 | 39.87 ± 4.95 | 41.68 ± 7.20 | 78.48 ± 1.45 | 212.53 ± 10.32 | |
| F1 | Mean | 13.07 ± 3.39 | 32.48 ± 1.53 | 66.05 ± 8.90 | 98.03 ± 4.34 | 242.03 ± 12.33 | |
| RIL | Mean | 12.03 ± 4.88 | 35.04 ± 7.47 | 66.44 ± 16.80 | 89.62 ± 21.17 | 237.73 ± 50.19 | |
| Range | 0.88–29.38 | 21.57–64.69 | 35.91–114.68 | 47.67–142.74 | 152.04–377.28 | ||
| Skewness | 0.46 | 1.14 | 0.79 | 0.38 | 0.81 | ||
| Kurtosis | 0.53 | 1.58 | 0.47 | −0.30 | 0.08 |
KAC: kernel As concentration, AAC: axis As concentration, SAC: stem As concentration, BAC: bract As concentration, LAC: leaf As concentration, RIL; recombinant inbred line.
Figure 1. Histogram of As concentration in five maize tissues of the recombinant inbred line (RIL) population.
Variance analysis of As accumulation in different maize tissues
The trend of As concentration in different maize tissues at the two locations was as follows: kernels <axes <stems <bracts <leaves. Analysis of variance indicated the As concentration in the five measured tissues in the RIL population was significantly affected by environment, block, genotype and genotype-by-environment factors (Table 2). The As concentration in the kernels largely differed by genotype and genotype-by-environment factors, explaining 32.03% and 39.86% of the total variation, respectively. However, the environment and block factors only contributed 8.43% and 1.16% of the total variation, respectively. The same trend was observed for the As concentration in the axes, stems, bracts and leaves. These results indicated that the differences in As concentrations in different maize tissues mostly depended on the genotype and the interaction between the genotype and the environment. The Pearson correlation coefficient was used to calculate the correlation of As content among the different tissues. The results showed that there were no significant relationships among the As concentration in the five measured tissues at the two locations (Table 3).
Table 2. Variance analysis of the five measured tissues in the RIL population.
| Tissue | Variance | MS | F | SS/SSTa | p valued | h2b (%)c |
|---|---|---|---|---|---|---|
| Kernel | B | 93.66 | 13.66** | 1.16 | 1.72E–06 | 71.9 |
| L | 1363.24 | 210.35** | 8.43 | 1.5E–39 | ||
| G | 45.01 | 6.95** | 32.03 | 6.3E–52 | ||
| L × G | 56.02 | 8.64** | 39.86 | 1.49E–64 | ||
| Axis | B | 103.19 | 6.10** | 0.24 | 0.002 | 85.8 |
| L | 17375.41 | 1050.62** | 9.74 | 1.7E–121 | ||
| G | 639.64 | 38.68** | 41.58 | 3.9E–181 | ||
| L × G | 680.85 | 41.17** | 44.25 | 9.8E–187 | ||
| Stem | B | 183.47 | 6.70** | 0.14 | 0.001 | 88.7 |
| L | 13220.71 | 495.85** | 5.92 | 5.12E–74 | ||
| G | 1046.41 | 39.25** | 51.04 | 1.3E–171 | ||
| L × G | 771.82 | 28.95** | 37.65 | 1.7E–146 | ||
| Bract | B | 432.05 | 16.16** | 0.22 | 1.63E–07 | 69.3 |
| L | 106736.17 | 4267.72** | 27.42 | 5.9E–238 | ||
| G | 1089.78 | 43.57** | 32.76 | 1.7E–193 | ||
| L × G | 1216.58 | 48.64** | 36.57 | 1.3E–203 | ||
| Leave | B | 198.84 | 4.00* | 0.03 | 0.019 | 90.0 |
| L | 121193.22 | 2469.75** | 8.35 | 3.1E–189 | ||
| G | 5509.44 | 112.27** | 44.39 | 2.3E–283 | ||
| L × G | 5665.05 | 115.45** | 45.65 | 4.4E–286 |
**significant at α = 0.01, *significant at α = 0.05.
E: environment, B: block, G: genotype, G × E: genotype-by-environment interaction.
aSum of squares (SS) of each effect by total SS (SST).
dp value, statistical significance of five five measured tissues in the two locations.
ch2b, broad-sense heritability.
RIL; recombinant inbred line.
Table 3. Correlation coefficients among five maize tissues in the RIL population.
| Location | Trait | Kernel | Axis | Stem | Bract | Leave |
|---|---|---|---|---|---|---|
| Xixian | Kernels | 1.00 | ||||
| Axis | −0.12 | 1.00 | ||||
| Stem | −0.15 | −0.06 | 1.00 | |||
| Bract | 0.10 | −0.03 | 0.10 | 1.00 | ||
| Leaves | 0.04 | 0.07 | 0.14 | 0.10 | 1.00 | |
| Changge | Kernels | 1.00 | ||||
| Axis | −0.15 | 1.00 | ||||
| Stem | −0.14 | −0.02 | 1.00 | |||
| Bract | 0.08 | −0.03 | 0.07 | 1.00 | ||
| Leaves | 0.04 | 0.05 | 0.13 | 0.07 | 1.00 | |
| BLUP | Kernels | 1.00 | ||||
| Axis | 0.04 | 1.00 | ||||
| Stem | 0.07 | 0.11 | 1.00 | |||
| Bract | −0.01 | −0.05 | −0.06 | 1.00 | ||
| Leaves | 0.06 | −0.13 | 0.10 | −0.08 | 1.00 |
RIL; recombinant inbred line.
QTL analysis of As content in different maize tissues
QTLs identified in the two locations
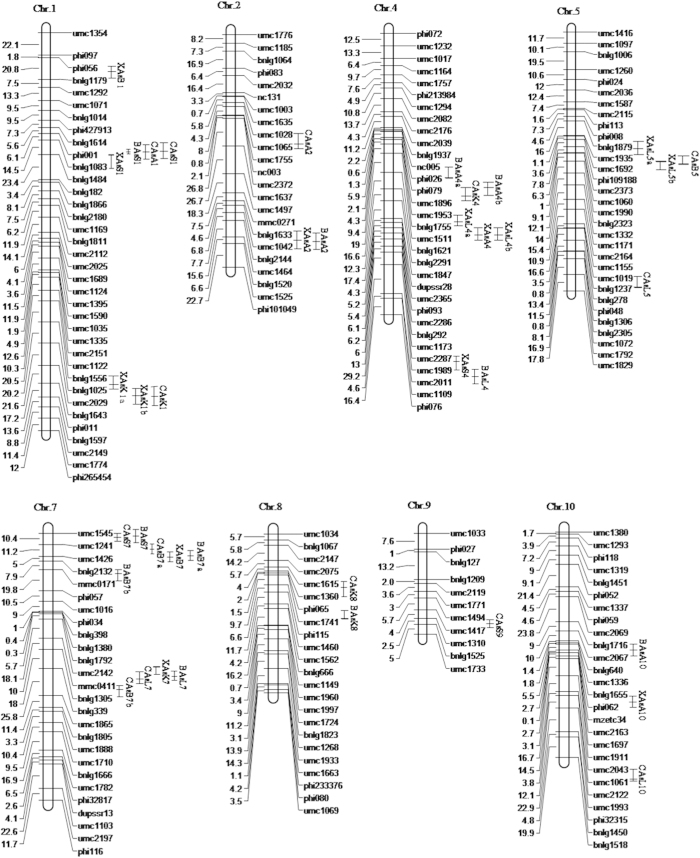
Twenty-eight QTLs related to the As concentration in the different maize tissues were detected in plants at the two locations (Table 4, Fig. 2). The QTLs were found on all chromosomes, except chromosomes 3 and 6. Six QTLs for the kernel As concentration (KAC) were identified at both locations. Two QTLs, XAsK1b and CAsK1, were detected in the common regions in the interval 220.20M–233.37M on chromosome 1. XAsK1a was identified at Xixian, which explained a relatively large proportion (26.50%) of the variance. For the six QTLs for KAC, the increasing effects of alleles came from both parents. Five QTLs were identified for the axes As concentration (AAC) at the two locations. However, there were no stable QTLs for AAC at the two locations. Of the five QTLs for AAC, three had positive additive effects, indicating that Zong3 contributed more to the AAC. Two QTLs for the stem As concentration (SAC), XAsS1 and CAsS1, were adjacent to each other on chromosome 1. All five identified QTLs for SAC had positive additive effects, which indicated that Zong3 contributed more to the SAC. For the bract As concentration (BAC), five QTLs were detected at the two locations. For XAsB7 and CAsB7a, one common region was found in the interval 2.27M–2.91M on chromosome 7. Four of the five QTLs for BAC had negative additive effects, which indicated that 87-1 contributed more to the BAC. Seven QTLs for the leaf As concentration (LAC) were identified at the two locations. Stable QTLs were not observed in the different environments. For these QTLs, the increasing effects of the alleles came from both parents.
Table 4. QTLs detected for As concentration in five maize tissues.
| Location | Trait | QTLa | Location | Confidence interval | Flanking-markers | Support interval | LODb | Ac | R2d |
|---|---|---|---|---|---|---|---|---|---|
| Xixian | Kernel | XAsK1a | 275.41 | 270.41–278.41 | bnlg1556–bnlg1025 | 209.87M–220.20M | 3.24 | −1.87 | 26.50 |
| XAsK1b | 282.91 | 281.91–285.91 | bnlg1025–umc2029 | 220.20M–233.37M | 2.85 | −1.77 | 23.93 | ||
| XAsK7 | 81.21 | 81.21–84.21 | umc2142–mmc0411 | 108.54M–126.46M | 2.77 | 0.98 | 7.86 | ||
| Axis | XAsA2 | 159.51 | 156.01–162.51 | bnlg1633–umc1042 | 199.17M–202.21M | 4.33 | −7.09 | 12.06 | |
| XAsA4 | 114.81 | 109.51–117.81 | bnlg1755–umc1511 | 81.19M–118.32M | 3.13 | −7.05 | 12.01 | ||
| XAsA10 | 108.41 | 106.61–110.41 | bnlg1655–phi062 | 85.27M–102.32M | 2.82 | 6.10 | 8.83 | ||
| Stem | XAsS1 | 103.51 | 103.41–112.51 | phi001–bnlg1083 | 27.92M–46.38M | 3.93 | 5.59 | 11.21 | |
| XAsS4 | 218.71 | 214.71–225.71 | umc2287–umc1989 | 230.38M–231.90M | 4.76 | 5.13 | 14.06 | ||
| Bract | XAsB1 | 36.91 | 32.91–42.91 | phi056–bnlg1179 | 2.19M–4.99M | 3.29 | 7.50 | 32.44 | |
| XAsB7 | 20.41 | 18.41–22.61 | umc1241–umc1426 | 2.27M–2.91M | 2.66 | −4.65 | 9.95 | ||
| Leave | XAsL4a | 108.51 | 106.41–109.51 | umc1953–bnlg1755 | 75.51M–118.32M | 2.95 | −11.54 | 9.95 | |
| XAsL4b | 115.81 | 111.81–118.81 | bnlg1755–umc1511 | 81.19M–118.32M | 2.82 | −12.20 | 11.19 | ||
| XAsL5a | 103.21 | 97.21–108.21 | bnlg1879–umc1935 | 14.27M–49.68M | 2.93 | 14.13 | 14.69 | ||
| XAsL5b | 114.31 | 114.21–116.31 | umc1935–umc1692 | 49.68M–53.91M | 3.00 | 10.97 | 8.82 | ||
| Changge | Kernel | CAsK1 | 280.91 | 272.41–288.91 | bnlg1025–umc2029 | 220.20M–233.37M | 3.12 | −3.46 | 12.30 |
| CAsK4 | 103.51 | 101.5–104.41 | phi079–umc1896 | 41.83M–65.70M | 2.79 | −2.70 | 7.59 | ||
| CAsK8 | 34.41 | 32.41–37.41 | umc1615–umc1360 | 71.28M–90.71M | 2.82 | 2.68 | 7.37 | ||
| Axis | CAsA1 | 96.81 | 92.81–100.41 | bnlg1614–phi001 | 13.51M–27.92M | 3.72 | 2.81 | 10.36 | |
| CAsA2 | 65.01 | 62.21–66.01 | umc1028–umc1065 | 150.12M–151.45M | 3.52 | 2.32 | 9.03 | ||
| Stem | CAsS1 | 93.81 | 89.51–96.81 | bnlg1614–phi001 | 13.51M–27.92M | 2.94 | 6.26 | 8.57 | |
| CAsS7 | 9.01 | 6.01–12.41 | umc1545–umc1241 | 1.56M-1.81M | 2.68 | 6.15 | 8.32 | ||
| CAsS9 | 55.21 | 54.51–56.21 | umc1494–umc1417 | 134.30M–134.38M | 2.57 | 6.00 | 6.81 | ||
| Bract | CAsB5 | 114.21 | 113.21–114.31 | umc1935–umc1692 | 49.68M–53.91M | 3.39 | −8.40 | 8.46 | |
| CAsB7a | 15.41 | 13.41–16.41 | umc1241–umc1426 | 2.27M–2.91M | 2.65 | −7.43 | 11.41 | ||
| CAsB7b | 109.31 | 107.31–113.31 | mmc0411–bnlg1305 | 126.46M–131.01M | 3.02 | 6.12 | 7.32 | ||
| Leave | CAsL5 | 211.11 | 208.51–211.1 | umc1019–bnlg1237 | 191.23M–192.73M | 2.68 | 13.09 | 6.70 | |
| CAsL7 | 87.21 | 78.51–92.21 | umc2142–mmc0411 | 108.54M–126.46M | 3.29 | −22.55 | 19.90 | ||
| CAsL10 | 141.21 | 133.51–143.21 | umc2043–umc1061 | 135.38M–139.10M | 2.72 | 17.81 | 11.53 | ||
| BLUP | Kernel | BAsK8 | 37.41 | 36.41–37.41 | phi065–umc1741 | 61.30M–70.97M | 2.63 | 0.93 | 6.41 |
| Axis | BAsA4a | 97.21 | 95.41–97.21 | phi026–phi079 | 36.88M–41.83M | 2.96 | −2.48 | 7.51 | |
| BAsA4b | 101.51 | 100.51–103.51 | phi079–umc1896 | 41.83M–65.70M | 2.57 | −2.71 | 8.96 | ||
| BAsA10 | 92.21 | 87.21–97.21 | bnlg1716–umc2067 | 58.13M–62.25M | 3.99 | 3.70 | 16.05 | ||
| Stem | BAsS1 | 100.41 | 95.81–103.51 | phi001–bnlg1083 | 27.92M–46.38M | 5.32 | 4.72 | 13.77 | |
| BAsA2 | 160.51 | 158.01–162.51 | bnlg1633–umc1042 | 199.17M–202.21M | 3.16 | 3.54 | 7.37 | ||
| BAsS7 | 7.01 | 1.01–14.41 | umc1545–umc1241 | 1.56M–1.81M | 3.84 | 4.31 | 11.44 | ||
| Bract | BAsB7a | 19.41 | 17.41–21.61 | umc1241–umc1426 | 2.27M–2.91M | 2.76 | −4.71 | 12.36 | |
| BAsB7b | 31.61 | 29.61–33.61 | bnlg2132–mmc0171 | 3.25M–4.69M | 2.70 | −4.31 | 10.57 | ||
| Leave | BAsL4 | 256.71 | 250.71–261.91 | umc1989–umc2011 | 230.38M–237.55M | 2.78 | −14.15 | 13.19 | |
| BAsL7 | 85.21 | 80.51–89.21 | umc2142–mmc0411 | 108.54M–126.46M | 2.70 | −13.10 | 11.45 |
aQuantitative trait loci (QTLs) detected for As concentration in five maize tissues.
bLogarithm of odds for each QTL.
cAdditive effect; positive values indicate that Zong3 alleles increase the rates.
dR2: contribution ratio.
Figure 2. Chromosomal locations of quantitative trait loci (QTLs) for As concentration in five maize tissues.
QTLs identified by best linear unbiased predictions
Eleven QTLs were detected in a joint analysis of the two locations (see the best linear unbiased predictions (BLUP) section of Table 4). Six QTLs including one for the axes, three for the stems, one for the bracts and one for the leaves were also detected in a single environment analysis in common marker intervals. Additionally, some QTLs detected in the joint analysis were not detected in the single environment analysis (e.g., BAsK8, BAsA4a, BAsA10, BAsB7b and BAsL4). These may have been only minor QTLs and were not stable across different environments.
Discussion
In the present study, we found that As levels in maize tissues followed the trend: leaves >bracts >stems >axis >kernels. Regarding environmental As content, Gulz et al. observed that the As content in maize roots was positively correlated with the total As content in the soil30. In a study conducted in Thailand, Prabpai et al. reported that there was a direct linear relationship between As accumulation in maize tissues and the total soil As content29. Additionally, in the present study, we observed that the maize tissue As concentrations were significantly affected by the genotype, environment and genotype-by-environment interactions. However, there were small environment-related differences in the As concentrations in the various maize tissues, and the genotype and genotype-by-environment interactions contributed more to the total variation. These results suggest that As concentration in different maize tissues could be reduced through genetic improvement.
To the best of our knowledge, only one article has reported QTLs related to As accumulation in different maize tissues. Ding et al. detected 11 QTLs for As accumulation in four different maize tissues24. In our study, 14 QTLs were identified at each location and 11 QTLs were identified in a joint analysis. Some of these QTLs were identified in different environments and were also detected by joint analysis. Compared with the B73 RefGen v2 reference genome, the distributions and effects of certain QTLs were closely linked to those of QTLs detected in previous studies. Most QTLs clustered at 27.92 M–46.38 M (chromosome 1), 209.87 M–233.37 M (chromosome 1), 75.51 M–118.32 M (chromosome 4), 1.56 M–2.91 M (chromosome 7) and 108.54 M–131.01 M (chromosome 7). XAsK1b and CAsK1 were detected in a common chromosomal region (209.87 M–233.37 M in chromosome 1) and were clustered with XAsK1a. Within the same genomic region, Qin et al. detected a QTL for Zn content in maize kernels using an F2:3 population32. Additionally, Liu et al. found two QTLs related to drought tolerance using a RIL population derived from the parents, Zong3 and 87-133. XAsL4a was found in common regions with a QTL for Hg accumulation, which had been identified using the same population in a previous study34. There may be strong linkage disequilibrium between QTLs and a genome-wide association study (GWAS) will be conducted to test the linkage disequilibrium in the future. Our results and those reported in previous studies identified certain chromosomal regions that should be analyzed further. These regions may represent targets for marker-assisted selection of maize cultivars with low As concentrations.
In maize, kernels are the main edible parts for humans and animals. In this study, the kernels contained the lowest As concentration, while the main biomass products including the leaves, bracts, stems and axes had relatively high As concentrations. Additionally, the As concentration in the kernels was considerably lower than the limit of 200 μg/kg specified in the National Standard of China (GB2762-2005). Maize is capable of adapting to its environment and is widely planted globally. Therefore, it is important to ensure maize production continue to satisfy the global demand for edible corn and to decrease the As content in As-contaminated soil by selecting and breeding As pollution-safe cultivars.
Materials and Methods
Experimental locations
The field experiments were conducted in 2012 at Xixian (E114° 72′, N32° 35′) and Changge (E113° 34′, N34° 09′) counties, which are located in northern China, with average temperatures of 15.2 °C and 14.3 °C, respectively, and rainfalls of 873.8 mm and 462.8 mm, respectively.
Plant materials
A mapping population of 194 F8 generation RILs was used. The RILs were derived from a cross between inbred line Zong3 (from a synthetic population of Chinese domestic germplasm) and 87-1 (from an exotic germplasm) using a single seed descent method. The RIL population, the two parents and the hybrid (Yuyu22) were grown in 2012 at Xixian and Changge using a randomized complete block design, with three replications at each location. Each plot included 15 plants with one 6 m × 0.67 m row, allowing a density of 45,000 plants per hectare.
Determination of the As concentration in maize
All plant materials were harvested when they reached physiological maturity and five consecutive plants from each plot were selected for further analysis. Mature plants were dissected into five parts: kernels, axes, stems, bracts and leaves. The collected plant materials were dried and ground into fine powder using a mortar and pestle. Powdered samples (0.5 g) were added to polypropylene tubes and digested with 5 mL HNO3/HClO4 (80/20 v/v) using a heating block (AIM500 Digestion System, A.I. Scientific, Australia). The concentrations of As in the different plant materials were then determined using atomic fluorescence spectrometry (AFS-3000, Beijing Haiguang Analytical Instrument Co., Beijing, China).
Statistical analysis
The expected genotypic variance (G), block variance (B), environmental variance (E) and G × E interaction were estimated by two-way ANOVA using the IBM SPSS Statistics package. The broad-sense heritabilities were calculated for genotype variance of each effect by the total sum of genotype variance and environmental variance. In the present study, 194 F8 generation RILs, which were derived from a cross between inbred line Zong3 and 87-1, were used as the materials. The two parents have contrasting levels of As accumulation. The soils of the test locations also have contrasting As contents. The materials and test locations were selected such that the G, E, and G × E terms were fitted as a fixed effect.
Linkage map construction and QTL analysis
The genetic linkage map, consisting of 263 simple sequence repeat markers, was constructed using Mapmaker 3.0 and covered 2,361 cM, with an average interval of 9 cM between markers35. The QTLs were identified with Model 6 of the Zmapqtl module of QTL Cartographer 2.5 using the composite interval mapping method36. The logarithm of odds thresholds for all measured traits were calculated by 1,000 random permutations at a significance level of α = 0.05, scanning intervals of 2 cM between markers and putative QTLs, with a 10 cM window37. The number of marker cofactors for background controls was determined by stepwise regression with five controlling markers. The phenotypic data for each measured material were based on the average values of three replicates. BLUP of arsenic concentration for each plant material at the two locations was calculated by a random effects model using the MIXED procedure in SAS. The QTLs were annotated as follows: for example, for XAsK1a, the “X” indicates the location at which the QTL was detected (Xixian, Changge and BLUP were abbreviated as X, C and B, respectively), “AsK” represents the arsenic concentration of the kernels (axes, stems, bracts and leaves were abbreviated as A, S, B and L, respectively), the number “1” is the serial number of the chromosome and “a” represents the serial number of the identified QTL.
Additional Information
How to cite this article: Fu, Z. et al. Genetic analysis of arsenic accumulation in maize using QTL mapping. Sci. Rep. 6, 21292; doi: 10.1038/srep21292 (2016).
Acknowledgments
This work was supported by the National High-tech Research and Development Program of China (2012AA10A305) and the State Key Basic Research and Development Plan of China (2014CB138203).
Footnotes
Author Contributions J.T., Z.L. and W.L. designed the experiments. Z.F., X.X., M.X., X.L., H.L. and Y.X. performed the experiments. Z.F. and W.L. analyzed the data. Z.F. and J.T. wrote the manuscript.
References
- Rosas-Castor J. M., Guzmán-Mar J. L., Hernández-Ramírez A., Garza-González M. T. & Hinojosa-Reyes L. Arsenic accumulation in maize crop (Zea mays L.): A review. Sci Total Environ. 488–489, 176–187 (2014). [DOI] [PubMed] [Google Scholar]
- Smith E., Naidu R. & Alston A. M. Arsenic in the Soil Environment: A Review. Adv Agr. 64, 149–195 (1998). [Google Scholar]
- Acharyya S. K. et al. Arsenic poisoning in the Ganges delta. Nature. 401, 545 (1999). [DOI] [PubMed] [Google Scholar]
- Lambkin D. C. & Alloway B. J. Arsenate-induced phosphate release from soils and its effect on plant phosphorus. Water Air Soil Poll. 144, 41–56 (2003). [Google Scholar]
- Joinal Abedin M. & Meharg A. A. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil. 243, 57–66 (2002). [Google Scholar]
- Azizur Rahman M. et al. Effect of arsenic on photosynthesis, growth and yield of five widely cultivated rice (Oryza sativa L.) varieties in Bangladesh. Chemosphere. 67, 1072–1079 (2007). [DOI] [PubMed] [Google Scholar]
- Shri M. et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotox Environ Safe. 72, 1102–1110 (2009). [DOI] [PubMed] [Google Scholar]
- Dixon H. B. F. The biochemical action of arsenic acids especially as phosphate analogues. Adv Inorg Chem. 44, 191–228 (1997). [Google Scholar]
- Baker S., Barrentine W. L., Bowmaan D. H., Haawthjorne W. L. & Pettiet J. V. Crop response and arsenic uptake following soil incorporation of MSMA. Weed sci. 24, 322–326 (1976). [Google Scholar]
- Marin A. R., Masscheleyn P. H. & Patrick W. H. J. The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant Soil. 139, 175–183 (1992). [Google Scholar]
- Meharg A. A. & Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 154, 29–43 (2002). [Google Scholar]
- Watts M. J., O’Reilly J., Marcilla A. L., Shaw R. A. & Ward N. I. Field based speciation of arsenic in UK and Argentinean water samples. Environ Geochem Hlth. 32, 479–490 (2010). [DOI] [PubMed] [Google Scholar]
- Chowdhury U. K., Rahman M. M., Mandal B. K., Paul K. & Lodh D. Groundwater arsenic contamination and human suffering in Wes Bengal, India and Bangladesh. Environ Sci. 8, 393–415 (2001). [Google Scholar]
- Bundschuh J. et al. Arsenic in the human food chain: the Latin American perspective. Sci Total Environ. 429, 92–106 (2012). [DOI] [PubMed] [Google Scholar]
- Karenlampi S. et al. Genetic engineering in the improvement of plants for phytoremediation of metal polluted soils. Environ Pollut. 107, 225–31 (2000). [DOI] [PubMed] [Google Scholar]
- Krämer U. Phytoremediation: novel approaches to cleaning up polluted soils. Curr Opin Biotech. 16, 133–141 (2005). [DOI] [PubMed] [Google Scholar]
- Pilon-Smits E. & Freeman J. Environmental cleanup using plants biotechnological advances and ecological considerations. Front Ecol Environ. 4, 203–210 (2006). [Google Scholar]
- Yu H., Wang J., Fang W., Yuan J. & Yang Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ. 370, 302–309 (2006). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Cadmium accumulation in different pakchoi cultivars and screening for pollution-safe cultivars. J Zhejiang Univ Sci B. 13, 494–502 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. A., Clarke J. M., Duguid S. & Chaney R. L. Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ. 390, 301–310 (2008). [DOI] [PubMed] [Google Scholar]
- Joinal Abedin M., Cotter-Howells J. & Meharg A. A. Arsenic uptake and accumulation in rice (Oryza sativa L.) irrigated with contaminated water. Plant Soil. 240, 311–319 (2002). [Google Scholar]
- Smith E., Juhasz A. L., Weber J. & Naidu R. Arsenic uptake and speciation in rice plants grown under greenhouse conditions with arsenic contaminated irrigation water. Sci Total Environ. 392, 277–283 (2008). [DOI] [PubMed] [Google Scholar]
- Baig J. A. et al. Evaluating the accumulation of arsenic in maize (Zea mays L.) plants from its growing media by cloud point extraction. Food Chem Toxicol. 48, 3051–3057 (2010). [DOI] [PubMed] [Google Scholar]
- Ding D. et al. Identification of QTLs for arsenic accumulation in maize (Zea mays L.) using a RIL population. Plos One. 6, e25646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. H. et al. Arsenic accumulation and distribution in the tissues of inbred lines in maize (Zea mays L.). Genet Resour Crop Ev. 59, 1705–1711 (2012). [Google Scholar]
- Guimaraes E. P., Ruane J., Scherf B. D., Sonnino A. & Dargie J. D. Marker assisted Selection: Current Status and Future Perspectives in Crops, Livestock, Forestry and Fish (FAO, Rome, 2007).
- Zhang J. et al. Mapping quantitative trait loci associated with arsenic accumulation in rice (Oryza sativa L.). New Phytol. 177, 350–355 (2008). [DOI] [PubMed] [Google Scholar]
- Dasgupta T., Hossain S. A., Meharg A. A. & Price A. H. An arsenate tolerance gene on chromosome 6 of rice. New Phytol. 163, 45–49 (2004). [DOI] [PubMed] [Google Scholar]
- Prabpai S., Charerntanyarak L., Siri B., Moore M. R. & Noller B. N. Effects of residues from municipal solid waste landfill on corn yield and heavy metal content. Waste Manage. 29, 2316–2320 (2009). [DOI] [PubMed] [Google Scholar]
- Gulz P. A., Gupta S. K. & Schulin R. Arsenic accumulation of common plants from contaminated soils. Plant Soil. 272, 337–347 (2005). [Google Scholar]
- Marwa E. M. M., Meharg A. A. & Rice C. M. Risk assessment of potentially toxic elements in agricultural soils and maize tissues from selected districts in Tanzania. Sci Total Environ. 416, 180–186 (2012). [DOI] [PubMed] [Google Scholar]
- Qin H. et al. Identification of QTL for zinc and iron concentration in maize kernel and cob. Euphytica. 187, 345–358 (2012). [Google Scholar]
- Liu Y. et al. Maize leaf temperature responses to drought: Thermal imaging and quantitative trait loci (QTL) mapping. Environ Exp Bot. 71, 158–165 (2011). [Google Scholar]
- Fu Z. J. et al. Quantitative Trait Loci for Mercury Accumulation in Maize (Zea mays L.) Identified Using a RIL Population. Plos One. 9, e107243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. H. et al. Genetic dissection of plant height by molecular markers using a population of recombinant inbred lines in maize. Euphytica. 155, 117–124 (2006). [Google Scholar]
- Zeng Z. B. Precision mapping of quantitative trait loci. Genetics. 136, 1457–1468 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill Gary A. & Rebecca W. Doerge. Empirical threshold values for quantitative trait mapping. Genetics. 138.3, 963–971(1994). [DOI] [PMC free article] [PubMed] [Google Scholar]