Abstract
Understanding the genetics of neuropsychiatric disorders requires an understanding of the genetics of brain structure and function. The Vietnam Era Twin Study of Aging (VETSA) is a longitudinal behavioral genetic study focused on cognitive and brain aging. Here, we describe basic science work carried out within the VETSA MRI study that provides meaningful contributions toward the study of neuropsychiatric disorders. VETSA produced the first comprehensive assessment of the heritability of cortical and subcortical brain structure sizes, all within the same individuals. We showed that neocortical thickness and surface area are largely genetically distinct. With continuous neocortical thickness maps, we demonstrated regional specificity of genetic influences, and that genetic factors did not conform to traditional regions of interest (ROIs). However, there was some evidence for different genetic factors accounting for different types of cortex, and for genetic relationships across cortical regions corresponding to anatomical and functional connectivity and brain maturation patterns. With continuous neocortical surface area maps, we confirmed the anterior–posterior gradient of genetic influences on cortical area patterning demonstrated in animal models. Finally, we used twin methods to create the first map of cortical ROIs based entirely on genetically informative data. We conclude that these genetically based cortical phenotypes may be more appropriate for genetic studies than traditional ROIs based on structure or function. Our results also suggest that cortical volume—the product of thickness and surface area is a problematic phenotype for genetic studies because two independent sets of genes may be obscured. Examples supporting the validity of these conclusions are provided.
Keywords: heritability, cortical surface area, cortical thickness, cortical maps, APOE
IMPORTANCE OF TWIN STUDIES IN PSYCHIATRIC GENETICS
Understanding the genetics of psychiatric disorders requires an understanding of the genetics of brain structure and function. Twin studies have had a long history of contribution to psychiatric research [Kendler and Eaves, 2005; Kremen et al., 2012a]. In the late twentieth century, a strong focus on psychiatric disorders as brain diseases took hold as well [Henn and Nasrallah, 1982; McHugh and McKusick, 1991; Kendler and Eaves, 2005], with the National Institute of Mental Health designating the 1990s as the decade of the brain. Given the small sample sizes of most neuroimaging studies, the approach to twin studies was often a co-twin control design in which monozygotic (MZ) twins discordant for a disorder are compared. This approach is primarily informative about individual-specific environmental factors while controlling for genetic and common environmental factors. The larger contribution of twin studies—determining the proportion of variance due to genetic and environmental factors, and the genetic relatedness of different traits—was limited with respect to twin studies of the brain because of the large sample sizes required. Moreover, to reduce bias in heritability estimates, samples should be relatively representative of the population.
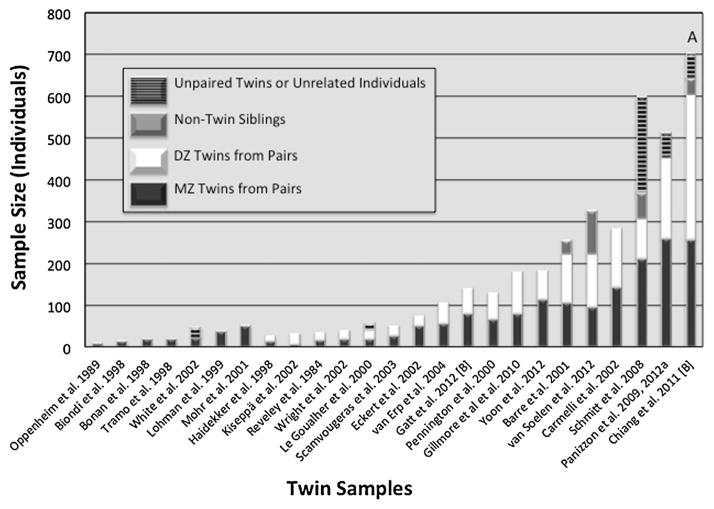
Blokland et al. [2012] conducted an extensive meta-analysis of the heritability of brain structure measures, yet studies with both adequate sample sizes and measurement of more than just a handful of brain structures have been conducted only relatively recently [see also Fig. 1; Peper et al., 2007; Schmitt et al., 2007]. These larger studies comprise community-based samples, which yield relatively unbiased heritability estimates. Thus, although it is widely agreed that genetics and brain are important for most psychiatric disorders, the two have been linked in relatively limited ways with respect to twin studies. With small samples, genome-wide association (GWA) studies of brain and psychiatric illness have not been feasible, so studies have been mostly limited to candidate gene studies.
Fig. 1.
Size of MRI twin samples (number of individuals). A: DZ twin category includes 15 individuals from five sets of trizygotic triplets. B: Sample recruitment is still ongoing. References cited in this figure are marked by an asterisk in the reference list.
Another development in this area was the application of the study of endophenotypes or intermediate phenotypes to psychiatric disorders [Gottesman and Gould, 2003]. However, psychiatric endophenotypes do not appear to be any less genetically complex than psychiatric disorders based on effect sizes of genetic loci [Flint and Munafò, 2007]. One view is that the value of brain endophenotypes may be not in aiding gene discovery, but in examining their association with genetic variants for psychiatric symptoms and diagnoses after those variants have been identified [de Geus, 2010]. Alternatively, the ability of twin studies to elucidate the complexity of phenotypes before knowing the specific gene variants may help in guiding molecular genetic approaches [Kremen et al., 2013b; Panizzon et al., 2011; Papassotiropoulos and de Quervain, 2011]. For example, verbal memory is one of many schizophrenia endophenotypes listed by Flint and Munafò [2007]. It is also an important phenotype for Alzheimer’s disease and other conditions. Although the heritability of verbal memory and most other neuropsychiatric endophenotypes is well established, univariate heritability estimates do not address the fact that memory is a complex, multi-component phenotype. Using multivariate twin analyses, we showed that the same genes influence short- and long-delay free recall, whereas there are some genes that are specific to acquisition and independent of free recall [Panizzon et al., 2011]. These findings provide crucial information about whether creating composite or separately examined measures is appropriate for GWA studies.
A variant of this approach is also possible for structural brain measures. Rather than examine interrelationships among already existing phenotypes, it is possible to use the continuous neocortical surface as a starting point and use twin methods to determine if novel regions of interest (ROIs) emerge. Being determined on the basis of genetically informative data, these latter ROIs may be more suitable for genetic association studies. We describe some of these multivariate twin approaches to the analysis of brain phenotypes in this article.
It is also the case that standard approaches to GWA studies are likely to be of limited success because behavioral, brain, and psychiatric phenotypes appear to be highly polygenic with individual genes having very small effects. New approaches to GWA studies such as genome-wide complex trait analysis [GCTA; Yang et al., 2010] essentially apply twin approaches to examine the contribution of all single nucleotide polymorphisms (SNPs) to phenotypic variation in aggregate. GCTA has captured a much larger portion of heritability than the conventional GWA study approach. Genic category annotation and pleiotropy-informed enrichment methodology recently developed by members of our group [Andreassen et al., 2013; Liu et al., 2013; Schork et al., 2013] is effective for finding regions of the genome enriched for effects in one or more phenotypes simultaneously, thereby elucidating common genetic mechanisms. This approach has much greater statistical power when conducted in twins compared to its typical application to unrelated individuals [Thompson and Kremen, unpublished data]. Using standard twin methodology to elucidate the genetic architecture of phenotypes not only provides information as to the most appropriate phenotypes, but it can also provide the basis for a priori hypotheses about SNP overlap or separation across phenotypes.
OVERVIEW OF THE VIETNAM ERA TWIN STUDY OF AGING (VETSA)
The VETSA—a longitudinal behavioral genetic study focused primarily on cognitive and brain aging—began in 2003. Detailed description of the VETSA has been provided elsewhere [Kremen et al., 2006, 2013a]. At the baseline assessment, 1,237 individual twins(349 MZ pairs, 265 dizygotic [DZ] pairs, and 9 unpaired twins) were assessed. The average age was 55.4 years (SD = 2.5; range 51–60) and the average educational attainment was 13.8 years (SD = 2.1). At the time of this writing (December 2012) data collection in the project’s first 5-year follow-up assessment is nearly complete.
Some key aspects of the VETSA design are as follows: first, all subjects were in midlife at baseline, before many substantial age-related changes may have taken place. Second, the study covers important transition points (e.g., from middle age to early old age) when genetic regulation of cognitive abilities or brain structure may become more variable due to factors such as impinging health burdens. Third, the large cohort and narrow age range means that power is maximized for longitudinal analysis of change within individuals over time. These first three points may also be particularly relevant for psychiatric illness. For example, there is strong evidence for different aging trajectories in schizophrenia versus psychiatrically normal individuals [Jeste et al., 2011], and in Alzheimer’s disease and mild cognitive impairment versus normal aging [Sperling et al., 2011]. Fourth, data are available from previous studies in which these and other twins in the Vietnam Era Twin (VET) Registry have participated. In particular, general cognitive ability scores were available dating back to age 20 on average, and the same test was administered an average of 35 and 40 years later in the wave 1 and 2 VETSA assessments [Lyons et al., 2009]. Fifth, extensive and detailed phenotypic data, including cognitive, psychosocial, biomedical, psychiatric diagnostic, treatment history, and current emotional functioning measures have been collected on all participants. Because there were very few women in the military during the Vietnam era, the VETSA is an all-male sample. Hence, we do not know how well the results may generalize to women.
Because VETSA participants are all veterans, one might assume that it is a rather atypical sample; however, that is not the case. Approximately 25% of men nationwide in this age range served in the military. The study participants are Vietnam era, not necessarily Vietnam War, veterans; 78% report having had no combat exposure. Indeed, they are a reasonably representative, community-dwelling sample of middle-aged men living throughout the United States. The fact that they live throughout the entire country enhances the representativeness of the sample as compared to a local sample. Representativeness is also increased because participants were not screened for any psychiatric, medical, or other characteristics. Furthermore, a look at U.S. census and Center for Disease Control data indicates that they are similar to the overall population of American men in their age range with respect to many sociodemographic (e.g., education, self-income, marital and employment status, % Caucasian) and health characteristics (e.g., % with diabetes, % overweight or obese) [National Health and Nutrition Examination Survey (NHANES III), 1999–2004; Kremen et al., 2006].
In 2003, we initiated the VETSA magnetic resonance imaging (MRI) study in which twins coming for the primary study were invited to stay an extra day and undergo MR scanning. Scanning in the VETSA MRI 5-year follow-up is underway in parallel with the follow-up of the primary project. At the baseline assessment, 94% of those invited agreed to participate in the MRI study. After exclusions for MRI safety concerns such as metal in the body or claustrophobia, inability to travel on the scanning day, or exclusion of a co-twin, 59% of those invited did participate. The final sample of participants with analyzable MRI data was 514 twins (130 MZ pairs, 97 DZ pairs, 60 unpaired twins). The MRI sample was similar to the larger VETSA sample with respect to age, ethnicity, employment, and health status [Panizzon et al., 2009]. MRI acquisition and image processing has been described in the VETSA studies reviewed in this article. The publicly available FreeSurfer software package was used to carry out volumetric segmentation of 30 subcortical, cerebellar, and global ROIs, and parcellation of 66 neocortical regions.
NEOCORTICAL VOLUME COMPONENTS: THICKNESS AND SURFACE AREA
Here, we call particular attention to our view that cortical volumes are phenotypes of highly questionable utility for genetic studies. It is important to clarify that we refer here specifically to neocortex (the cortical ribbon); for the hippocampus, for example, we use volume measures only. It has long been recognized that neocortical neurons are organized into columns running perpendicular to the brain’s surface [Mountcastle, 1997]. According to the radial unit hypothesis—perhaps the leading model of neocortical development—cells within a column share a common origin; whereas cortical thickness is influenced by the number of cells in a column, cortical surface area is driven by the number of columns [Rakic, 1988]. A review summarizing 20 years of research demonstrates considerable support for this model of two relatively independent mechanisms in the development of cortical thickness and surface area [Rakic, 2009]. Despite the support for this model, by far the most frequently studied neocortex phenotype has been volume. There is a rapidly growing number of studies of cortical thickness, but still little examination of cortical surface area in the MRI literature, and, virtually no human behavioral genetic studies of surface area. For example, the meta-analysis of Blokland et al. [2012] included heritabilities of volume and thickness, but not surface area measures. This omission may have been due largely to the lack of available imaging software packages to measure surface area.
Nevertheless, cortical surface area is of obvious importance. Individual differences in human cortical volume are largely attributable to variability in surface area rather than thickness [Pakkenberg and Gundersen, 1997; Im et al., 2008]. Surface area increases also appear to be the primary driver of the increased size of the human cortex relative to that of other animals [Rakic, 2009]. Cross-species differences in relative cortical surface area expansion also highlight the fact that functional specialization is linked to cortical surface area [Van Essen et al., 1998]. A simple example is the tremendous difference in relative area expansion of frontal versus somatosensory cortex in humans versus rodents [Chen et al., 2011]. Late fetal growth of cortical surface area in infants born preterm has been shown to be strongly correlated with overall cognitive ability at ages 2 and 6 years [Kapellou et al., 2006; Rathbone et al., 2011]. However, there is evidence in children of a changing relationship between cortical thickness and intelligence [Shaw et al., 2006], and in young adults of cortical thinning in some regions but thickening in others [Brans et al., 2010]. Thus, the longitudinal relationship of cortical thickness and surface area to different abilities or behaviors may be more complex.
Following Rakic’s [1988, 2009] radial unit developmental hypothesis, we tested whether there would be distinct genetic influences on cortical thickness and surface area. We found that the genetic correlation—which indicates shared genetic variance—between cortical thickness and surface measures was not significantly different from 0 [Panizzon et al., 2009]. Thus, cortical thickness and surface area are influenced by primarily distinct genetic factors. These results have been replicated in a large, multi-generation family study [Winkler et al., 2010]. Effects consistent with this VETSA finding have also been noted in nonhuman primates [Balzeau and Gilissen, 2010].
This finding has important implications for genetic association studies of the cerebral cortex. The key point is that volume is the product of surface area and thickness. As such, volume conflates two different sets of genetic influences. Neocortical volume measures have clearly been of great value in psychiatric research, but our findings strongly suggest that their value is at the phenotypic level. Given what we now know, we think it is problematic to focus genetic studies on neocortical volume measures. In our view, thickness and surface area should be examined first as separate phenotypes in genetic studies. After that, it could be the case that no associations are found. One might then reasonably examine cortical volume based on the idea that there could be additive or interactive effects of thickness and surface area associated with a particular phenotype, even if the effect of either alone is small.
COMPREHENSIVE ASSESSMENT OF HERITABILITY OF INDIVIDUAL BRAIN STRUCTURES
As we were examining the genetics of cortical thickness and surface area, we also characterized the heritability of brain structures in a relatively comprehensive fashion, all within the same individuals. Two things were particularly notable in reviews of the MRI twin literature by our group and others [Peper et al., 2007; Schmitt et al., 2007]. First, beyond global measures such as intracranial volume or total and left and right hemisphere gray and white matter volume, there were few specific ROIs measured. Consequently, there were few replications of the same specific ROIs, and a lack of studies in which multiple specific ROIs were measured in the same individuals.
Second, most studies had sample sizes that were rather small for twin analysis. Hence, estimates from those samples would be of uncertain reliability. We searched for published reports that, to the best of our knowledge, were based on independent twin samples. When there was more than one published report from the same sample, we selected the one with the largest sample size; for this purpose, sample size was based only on the number of normal paired twins. Figure 1 shows the number of normal (non-patient) participants in MRI twin samples of what are, to the best of our knowledge, different samples with normal twins. The first seven are samples with no DZ twins, precluding the ability to determine precise heritability estimates. These are followed by samples with both MZ and DZ twin pairs. Within each of these categories, the samples are ordered according to the total number of MZ and DZ twin pairs (although non-twin siblings and unpaired twins are also shown).
We found reports from 19 different samples prior to our study. Of the seven with no DZ twins, the median sample size was 20 (range: 10–52). Of the remaining 12 with both MZ and DZ twin pairs, the median sample size was 64 (range: 28–284). Including our sample [Panizzon et al., 2009, 2012a] and five other more recent samples [Gilmore et al., 2010; Chiang et al., 2011; Gatt et al., 2012; Yoon et al., 2012; van Soelen et al., 2012], there are 18 samples with both MZ and DZ twins; these 18 have a median sample size of 137 twins (range: 28–603). Out of the total of 25 samples, only three have more than 300 twins, and six now have more than 200. There is rather unequal representation of the age range in these samples. All but two comprise primarily children or relatively young adults. Only the VETSA and the sample of Carmelli et al. [2002] focus on adults that are over 50 years of age.
To address these issues, we utilized the VETSA MRI sample to conduct the first large-scale study in which there was a comprehensive analysis of genetic and environmental influences on the size of cortical, subcortical, and ventricular ROIs all in the same individuals [Kremen et al., 2010]. Genetic influences accounted for an average of 70% of the variance in global, subcortical, and ventricular ROI volumes. The heritability of thickness for cortical ROIs was moderate by comparison, with an average of approximately 49%. Cortical thickness ROIs also had greater variability in their heritability estimates compared with subcortical and ventricular ROIs (0.00–0.75 vs. 0.48–0.85). There was no evidence for larger heritabilities in homologous regions in one hemisphere or the other, or for regions associated with higher cognitive functions.
We then examined the heritability of cortical surface area ROIs [Eyler et al., 2011b]. In comparing cortical thickness and surface area ROIs, it is important to consider how to account for global versus regional effects. It is most common in studies of cortical thickness and surface area for researchers to adjust for total brain volume or intracranial volume. As we have noted, there are independent genetic influences on cortical thickness and surface area, and the association between intracranial or cortical volumes is very different for surface area and thickness; it is primarily driven by surface area and not cortical thickness. Adjusting thickness or surface area measures for total volume is likely to be problematic, and it blurs the distinction between the two. Consequently, when we refer to controlling for global effects of cortical thickness or cortical surface area, these are adjusted for average cortical thickness and total cortical surface area, respectively.
We showed that there were both global effects and regional differences in genetic influences on the surface area of cortical structures [Eyler et al., 2011b]. Total surface area was highly heritable. In addition, we found significantly higher heritability for left frontal surface area compared with most other lobes. We speculated that aging-relevant genes may have strong influences on the frontal lobes, as this broad region shows the most age-related shrinkage [Raz, 2000; Fjell et al., 2009]. The heritabilities of the regional surface area ROIs averaged 0.44, but they were substantially reduced (to 0.22) after controlling for total surface area. This 50% reduction, on average, in the heritability of regional surface area after controlling for total surface area was greater than the 27% average reduction (0.49 to 0.36) in the heritability of regional thickness ROIs after controlling for average thickness [Eyler et al., 2011b; Kremen et al., 2010]. Also, taking into account the phenotypic and genetic correlations between unadjusted surface area ROIs and total surface area, it appears that many of the genes influencing regional surface areas are the same genes that influence global surface area [Eyler et al., 2011b]. Unadjusted regional thickness had less genetic overlap with average thickness, and regional thickness had very little relationship to total brain volume or intracranial volume [Kremen et al., 2010].
One perplexing issue that arose from these findings was the tremendous inconsistency of heritability estimates for lateral ventricle volumes across published studies. Our estimate was 0.78, but three other studies yielded estimates at or very near 0 [Baaré et al., 2001; Wright et al., 2002; Chou et al., 2008]. Such discrepancies were particularly puzzling given that the ventricles are one of the easier ROIs to measure reliably. Intuitively, it seemed unlikely that the size of all of the surrounding ROIs had moderate to high heritability, yet ventricle size would be entirely environmentally determined. To address this issue, we re-examined eight twin studies of the lateral ventricles with samples ranging in average age from 9 to 72 [Kremen et al., 2012b]. After removing some problematic cases in two studies and revising the estimates, the weighted average heritability was 0.54. We, therefore, conclude that ventricular volume is indeed heritable.
The data also indicated that heritability increased with the age of the sample, but the increase was not due to age-associated ventricular enlargement. This suggested that individual differences within samples might be accounted for more by genetic influences as people get older. On the other hand, it might be that heritability increased because environmental variance was lower in the older samples. To determine what was driving the heritability increases, it was necessary to examine the absolute phenotypic variances rather the standardized variances used to create heritability estimates. Ventricular enlargement was associated with greater total phenotypic variance in older individuals. The average unique environmental variance was over two times as large in adults compared to children and adolescents and adults, but the average genetic variance was nearly eight times as large. Although it might be expected that with accumulated environmental insults over time, environmental factors would have more of an influence on ventricular volume in older individuals, the results indicate, perhaps counter-intuitively, that genetic factors play a larger role in determining individual differences in ventricular volume in older adults than they do in younger individuals.
APOE
As a study of aging, we were interested in the effects of the Apolipoprotein E (APOE) genotype on brain structure because of the well-replicated finding that the presence of an ε4 allele is associated with elevated risk for Alzheimer’s disease [Farrer et al., 1997]. After finding no direct association between APOE genotype and hippocampal volume in the middle-aged VETSA sample, we began to explore possible interaction effects. Protective effects of androgens against the deleterious effects of the APOE ε4 allele have been documented in both male and female mice [Raber et al., 2002]. Paralleling the animal model findings, we found a significant interaction of APOE genotype and testosterone with respect to hippocampal volume [Panizzon et al., 2010]. Having at least one ε4 allele was associated with smaller hippocampal volume, but only in the subgroup with low testosterone (defined as >1 SD below the mean). We also found that testosterone moderates the heritability of hippocampal volume [Panizzon et al., 2012b]; genetic influences played a greater role in determining hippocampal volume for men with higher levels of testosterone. The high concentration of androgen receptors in the hippocampus raises the possibility that these gene-by-hormone interactions may well reflect gene–gene interactions (e.g., with the androgen receptor [AR] gene). Because testosterone declines steadily with age, its interaction with APOE genotype may be an important factor in age-related hippocampal-dependent memory function.
APOE-ε4 effects in non-demented adults have not been limited to memory [Small et al., 2004]. In another analysis, we found that the ε4 allele was associated with thinner cortex in several prefrontal subregions within our sample of men in their 50s [Fennema-Notestine et al., 2011]. These later-developing cortical regions may be more susceptible to natural aging processes than mesial temporal regions. The impact of the ε4 allele on mesial temporal regions may not become manifest until later in life (except perhaps in the context of low testosterone).
GENETIC COVARIATION AMONG ROIS
After examining individual regional heritability estimates and the influence of APOE, we then sought to understand patterns of covariation and specificity of genetic effects [Eyler et al., 2011a]. These investigations began with a look at genetic associations among 19 subcortical volumetric ROIs, which included the hippocampus. Factors based on a promax (oblique) rotation were derived from these multivariate twin analyses to group together ROIs with shared genetic underpinnings that determine individual differences in volume. We found four genetic factors: basal ganglia/thalamus (putamen, pallidum thalamus, caudate); ventricles (lateral, temporal horn, third); limbic (hippocampus, amygdala); and nucleus accumbens. Left and right hemisphere measures of the same structure always loaded on the same factor. Overall, these results indicated different genetic factors influencing volumes of different subcortical ROIs; thus, there was not a single global set of genes influencing the size of all subcortical structures. Genetic correlations between homologous regions in different hemispheres were very high, indicating an absence of lateralization effects in the genetics of subcortical volumes. As already noted, the ventricles were highly heritable, but one might assume a priori that they would load negatively on the brain tissue factors. In other words, it could be posited that the same genes that cause decreases in gray matter structures would cause ventricular volumes to increase, and vice versa. Interestingly, the ventricular factor had very small and nonsignificant genetic correlations with the other factors indicating that the genetic influences on gray matter and ventricular volume are different and relatively independent of one another.
CONTINUOUS MAPS OF THE CORTICAL SURFACE
Estimating Thickness and Surface Area Heritabilities
To a certain extent, all cortical ROIs are based on arbitrary, imposed boundaries. The ROI definitions, such as Brodmann areas or the Desikan et al. [2006] sulcal-based parcellation system, were not created on the basis of distinct genetic factors. Hence, there is no necessary reason that genetic influences would conform to those boundaries. We created continuous, vertex-wise maps of both cortical thickness and surface area that were not constrained by any pre-defined boundaries in order to circumvent this shortcoming and provide some new insights. These maps were based on the heritability of thickness or surface area at each vertex with the magnitude of the heritability depicted on a color scale. The surface area maps reflect vertex-wise surface area expansion/contraction. Essentially, each vertex is a tiny triangle that is registered to a template, and the expansion/contraction is based on how much each vertex must be expanded or contracted to fit the template. The heritability of area expansion across the cortex was high, and slightly lower for cortical thickness [Eyler et al., 2012]. However, heritabilities were more modest for region-specific surface area measures because genetic correlations between global and regional surface area measures were high.
We also examined something that is not generally considered in the imaging genetics literature. When estimating ROI-based heritabilities, one generally estimates the heritability for a measure for which the unit of analysis is the ROI (e.g., mean thickness of the left superior frontal region). But one could also estimate the heritability for every individual vertex within the ROI, and then calculate the average of those individual heritabilities. This is not a trivial distinction given that we found that these two approaches result in different heritability estimates. The former approach tended to overestimate inter-regional variability in heritabilities and underestimate the heritability of small ROIs. Discrepancies between the two approaches were also greater for surface area than for thickness measures, and they were greatest in small ROIs [Eyler et al., 2012]. We speculated that surface area measures would show a greater discrepancy between these two approaches due to the effects of “inaccurate” boundary delineation. By inaccurate, we refer here to boundaries that may conform well to structural or functional, but not genetic, divisions. Because thickness measures are based on the average thickness, boundary placement will determine which vertices are included in the estimate but the thickness measure will not be greater simply because the ROI size is greater. In contrast, boundary placement directly affects the ROI size for surface area.
If one’s research goal is to examine the genetics of specific brain regions, using the latter method (averaging each individual vertex-wise heritability) may be preferable, particularly for small regions. On the other hand, using this approach would present problems for calculating confidence intervals. These results also have implications for gene finding. Particularly for surface area phenotypes, global surface area may account for most of the genetic variance for some specific regions. As such, those regions may not be good candidates for finding regionally specific genes.
Patterning of Genetic Influences on Thickness Across the Cortex
We created a continuous map of cortical thickness with the heritability of thickness at each vertex [Rimol et al., 2010]. Any heritability differences in that map are agnostic with respect to the question of whether the same or different genes are involved. Genetic correlations are needed to address that question. So we also created genetic correlation maps (rather than heritability maps) based on three seed point analyses in which we mapped the genetic correlations between thickness at each seed point and at every other vertex on the cortical surface.
With these cortical thickness maps, we addressed five research questions [Rimol et al., 2010]. First, we examined whether a single set of underlying genetic factors influenced cortical thickness or whether different sets of genes influenced the thickness of different regions. Our map showed that heritability varied from 0.16 to 0.73, strongly suggesting regional variability in the magnitude of genetic influences. With a seed point in primary visual cortex (area V1), we observed high genetic correlations with medial occipital and other primary sensory regions, but near-zero genetic correlations with anterior temporal and anterior frontal regions. With a middle temporal gyrus seed point, we observed very low genetic correlations with primary sensory regions, but very high genetic correlations in anterior temporal and anterior frontal cortices. This double dissociation pattern meant that individual differences in the thickness of different regions must be due to the effects of different sets of genes.
Second, we asked whether the different sets of genetic factors corresponded to traditional boundaries such as Brodmann areas or lobar divisions. The answer appeared to be “no.” An example was the high genetic correlations between anterior temporal and anterior frontal regions, which did not conform to Brodmann areas or sulcal-gyral boundaries. Third, we asked whether different genetic factors accounted for different cortex types (e.g., primary vs. association, or different sensory modalities). The V1 seed point map provided some support for this idea, with high genetic correlations between visual and somatosensory cortex but low genetic correlations between visual and association cortex. Fourth, we sought to determine whether genetic correlation patterns among thickness values corresponded to anatomical or functional connectivity. This idea was partially supported. For example, the middle frontal gyrus seed point map resulted in high genetic correlations that corresponded to roughly to the opposite ends of the dorsal component of the superior longitudinal fasciculus [Makris et al., 2005], and in the anterior temporal seed point map there were high genetic correlations corresponding to regions connected by the uncinate fasciculus [Ebeling and von Cramon, 1992].
Fifth, we considered the possibility that the genetic correlation patterns might correspond to white and gray matter maturation patterns [Yakovlev and Lecours, 1967; Barkovich et al., 1988]. The patterns did largely parallel this developmental trajectory, which proceeds from primary sensory cortices (corresponding to the V1 seed point map) to parietal and midfrontal regions (corresponding to the midfrontal seed point map) to the frontal and temporal poles (corresponding to the anterior temporal seed point map). None of these proposed explanations could alone account for the observed patterns. Therefore, it may that genetic factors influence some combination of these, and possibly other, processes in determining regional cortical thickness and connectivity.
Patterning of Genetic Influences on Surface Area Expansion/Contraction Across the Cortex
Given that different genetic factors are at work in determining thickness and area, we also sought to understand patterns of genetic influence for surface arealization. For this measure, our investigations were guided by animal (primarily mouse) data based on a small number of specific genes that have shown two key processes in cortical surface area expansion/contraction. This work has provided direct evidence of genetic control of cortical area patterning [Fukuchi-Shimogori and Grove, 2001; O’Leary et al., 2007]. Expression of Fgf8, for example, results in area expansion of anterior brain regions while simultaneously resulting in contraction of posterior regions. This well-replicated phenomenon in which anterior regions increase at the expense of posterior regions, and vice versa, is known as the anterior–posterior (A–P) gradient of gene expression. At a later point in development, gene expression gradients are translated into discontinuous patterns such that discontinuous, sharply bordered gene expression regions are formed; these define anatomically and functionally distinct areas [O’Leary et al., 2007]. It is obviously not possible to alter gene expression in the normal living human brain, but we used the twin design to test the idea that there would be evidence of similar genetic patterning in humans [Chen et al., 2011]. O’Leary and Sahara depicted four regions based on gene expression patterns in the mouse brain: primary visual cortex (V1); primary auditory cortex (A1); primary somatosensory cortex (S1); and frontal/motor cortex. We created genetic correlation maps of surface area expansion/contraction based on four seed points corresponding to those in the mouse study. Corresponding to the frontal/motor and A1 regions, we used seed points in frontal and temporal poles. We specifically selected very anterior regions in order to see if the patterns would extend to the full extent of the frontal and temporal lobes, which are far more expansive in the human brain compared to the mouse brain. As in the animal models, surface arealization measures were adjusted for total surface area. The frontal pole seed point map provided strong support for the A–P gradient. Genetic correlations anterior to the central sulcus were positive, and they were negative posterior to the central sulcus. This pattern indicates that the same genetic factors that increase pre-central cortical area also reduce post-central cortical area. All four seed point maps supported the animal data, indicating sharply bordered regions based on positive versus negative correlations. It is noteworthy that these regions were not the same as traditional lobar divisions; boundaries of the genetic correlations corresponded to known anatomical landmarks in some cases but not others.
To address the possibility that the results could be due to selected seed points, we created maps based on 34 different seed points across the cortex. The four regions held up regardless of seed point placement. For example, a seed point in the most anterior–inferior portion of the frontal lobe resulted in essentially the same divisions as a seed point in the most posterior-superior portion of the frontal lobe. We then conducted a fuzzy-cluster analysis so as to have an analytic procedure that would be unbiased; that is, it is a purely data-driven approach with no seed points. In this approach, clusters are formed so as to maximize shared genetic influences within clusters and minimize them between clusters. We constrained the clustering algorithm to have four clusters in order to be comparable to the cortical regionalization in the mouse model of O’Leary et al. The four clusters corresponded extremely closely to that of the mouse model except for expected proportional species-associated differences. For example, the frontal region was proportionally much larger and the somatosensory region was proportionally much smaller in the human compared to the mouse brain. These results suggest a general conservation of genetic influences on cortical area patterning across mammalian species, including human cortex. On the other hand, observed differences such as the relative size differences in frontal and somatosensory regions are consistent with species-specific functional specialization.
One possibility is that the development or pattern of the A–P gradient or of these sharply bordered gene expression regions is altered in some psychiatric disorders. This may be most likely for neurodevelopmental disorders such as autism or schizophrenia.
In a subsequent set of analyses, we extended our use of fuzzy clustering of vertex-wise genetic correlations to determine how many surface area clusters there are in the normal adult human brain [Chen et al., 2012]. We began with two clusters and continued to increase the number of clusters. We plotted silhouette coefficients to determine the optimal or natural number of clusters. There was a clear peak at 12 clusters. There were several noteworthy features about these clusters. The registration specified no landmarks or constraints against contiguity, yet the clusters that emerged did correspond to regions that were meaningful structurally and functionally. Vertices in both hemispheres were included together in the analysis with no constraints for bilateral symmetry. Nevertheless, despite some possible asymmetry in perisylvian regions, the clusters were bilateral and predominantly symmetric. In conjunction with this symmetry, the spatially contiguous within-hemisphere clusters were consistent with a modular pattern. The clusters also appeared to have a hierarchical organization because each successive set of clusters tended to include subdivisions of previous clusters [Tan et al., 2006].
These surface area clusters have important implications for genetic association studies. Previous cortical parcellation systems have been based on structure or function, but this work was the first human cortical parcellation system based solely on genetically informative data. As such, the clusters represent novel phenotypes with boundaries that differ from those proposed in previous systems. It is our hypothesis that because they are genetically based, these cortical subdivisions may be more useful for identifying specific genes associated with brain structure or function than traditional, non-genetically informative regions.
REDEFINED PHENOTYPES, ENDOPHENOTYPES, AND GENETIC ASSOCIATION STUDIES
It has so far proved difficult to replicate genetic associations with clinical or neuropsychiatric endophenotypes. One limiting factor may be their genetic complexity (polygenicity) [Flint and Munafò, 2007]. Another may be the fact that the phenotypes used have not been optimized for genetic analysis because they are based primarily on structure or function rather than genetically informative data [Panizzon et al., 2011; Papassotiropoulos and de Quervain, 2011; Chen et al., 2012]. Our findings that there are distinct genetic factors influencing cortical thickness and surface area, and that genetic effects on brain morphology do not always respect traditional anatomical boundaries are two key examples of the problem of suboptimal morphometric phenotypes [Panizzon et al., 2009; Chen et al., 2012].
Cognitive performance tends to decline in normal aging, and that in part coincides with the fact that total surface area and lobar surface area are negatively associated with age in humans [Pakkenberg and Gundersen, 1997; Østby et al., 2009]. Surface area features may also differ in normal and pathological aging. Age-related medial temporal differences in healthy younger versus older adults appear to be most prominent in volume and surface area, whereas the most prominent differences between healthy older adults and those with Alzheimer’s disease tend to be in volume and thickness [Dickerson et al., 2009]. In adults with autism, older age has been associated with thicker cortical regions but not greater surface area [Raznahan et al., 2010]. These are not findings from genetically informative studies, but they are consistent with the idea that separate examination of cortical thickness and surface area is important for autism and Alzheimer’s disease.
There are as yet few genetically informative studies addressing these issues, but they do appear to be building support for the validity of these concepts for helping to elucidate the genetics of neuropsychiatric disorders, which are often highly polymorphous. There is evidence from studies in schizophrenia that potential risk genes may be preferentially related to either cortical thickness or area [Schultz et al., 2011; Kähler et al., 2012]. In addition, using surface maps of thickness and area expansion proved more powerful than ROI-based analyses in identifying shared and distinct abnormalities in schizophrenia and bipolar disorder [Rimol et al., 2012]. The results of the latter study also emphasized that there are distinct patterns of regional specificity for thickness, surface area, and volume deficits in schizophrenia. Measures of cortical surface area and thickness also seem to have dissociable relationships with level of risk for schizophrenia [Prasad et al., 2010; Bhojraj et al., 2011; Jung et al., 2011] and show different trajectories of change with age [Cobia et al., 2012].
Acknowledgments
This work was supported by grants from the National Institute on Aging (R01 AG022381, R01AG018386, R01 AG018384, R01 AG022982), National Institute of Mental Health (T32 MH20030), National Institute for Drug Abuse (R01 DA18673), National Institute for Biomedical Imaging and Bioengineering (R01 EB006758), National Institute for Neurological Disorder and Stroke (R01 NS052585), and the National Center for Research Resources (P41 RR14705, BIRN002, U24 RR021382). It was also, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health, the Autism and Dyslexia Project of the Ellison Medical Foundation, and the VA Desert Pacific Mental Illness Research and Clinical Center. The Cooperative Studies Program of the Office of Research and Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the VET Registry. All statements, opinions, or views are solely of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government, the NIA, or the NIH. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible. We also appreciate the time and energy of many staff and students on the VETSA projects. Dr. Dale is a founder and holds equity in CorTechs Laboratories, Inc., and also serves on the Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
Footnotes
Conflict of interest: All other authors have no conflicts of interest. Grant sponsor: National Institute on Aging; Grant numbers: R01 AG022381; R01 AG018386; R01 AG018384; R01 AG022982; Grant sponsor: National Institute of Mental Health; Grant number: T32 MH20030; Grant sponsor: National Institute for Drug Abuse; Grant number: R01 DA18673; Grant sponsor: National Institute for Biomedical Imaging and Bioengineering; Grant number: R01 EB006758; Grant sponsor: National Institute for Neurological Disorder and Stroke; Grant number: R01 NS052585; Grant sponsor: National Center for Research Resources; Grant numbers: P41 RR14705; BIRN002; U24 RR021382; Grant sponsor: VA San Diego Center of Excellence for Stress and Mental Health; Grant sponsor: Autism and Dyslexia Project of the Ellison Medical Foundation; Grant sponsor: VA Desert Pacific Mental Illness Research and Clinical Center; Grant sponsor: The Cooperative Studies Program of the Office of Research and Development of the United States Department of Veterans Affairs.
References
- Andreassen OA, Djurovic S, Thompson WK, Schork AJ, Kendler KS, O’Donovan MC, Rujescu D, Werge T, van de Bunt M, Morris AP, et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular disease risk factors. Am J Hum Genet. 2013;92:197–209. doi: 10.1016/j.ajhg.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaré WFC, Hulshoff Pol HF, Boomsma DI, Posthuma D, de Geus EJC, Schnack HG, van Haren NEM, van Oel CJ, Kahn RS. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Balzeau A, Gilissen E. Endocranial shape asymmetries in Pan paniscus, Pan troglodytes and Gorilla gorilla assessed via skull based landmark analysis. J Hum Evol. 2010;59:54–69. doi: 10.1016/j.jhevol.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, Eack S, Rajarethinam R, Francis AN, Montrose DM, Keshavan MS. Progressive alterations of the auditory association areas in young non-psychotic offspring of schizophrenia patients. J Psychiatr Res. 2011;45:205–212. doi: 10.1016/j.jpsychires.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi A, Nogueira H, Dormont D, Duyme M, Hasboun D, Zouaoui A, Chantome M, Marsault C. Are the brains of monozygotic twins similar? A three-dimensional MR study. AJNR Am J Neuroradiol. 1998;19:1361–1367. [PMC free article] [PubMed] [Google Scholar]
- Blokland GA, de Zubicaray GI, McMahon KL, Wright MJ. Genetic and environmental influences on neuroimaging phenotypes: A meta-analytical perspective on twin imaging studies. Twin Res Hum Genet. 2012;15:351–371. doi: 10.1017/thg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonan I, Argenti AM, Duyme M, Hasboun D, Dorion A, Marsault C, Zouaoui A. Magnetic resonance imaging of cerebral central sulci: A study of monozygotic twins. Acta Genet Med Gemellol (Roma) 1998;47:89–100. doi: 10.1017/s000156600000026x. [DOI] [PubMed] [Google Scholar]
- Brans RG, Kahn RS, Schnack HG, van Baal GC, Posthuma D, van Haren NE, Lepage C, Lerch JP, Collins DL, Evans AC, et al. Brain plasticity and intellectual ability are influenced by shared genes. J Neurosci. 2010;30:5519–5524. doi: 10.1523/JNEUROSCI.5841-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins. Biol Psychol. 2002;61:139–155. doi: 10.1016/s0301-0511(02)00056-x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Panizzon MS, Eyler LT, Jernigan TL, Thompson W, Fennema-Notestine C, Jak AJ, Neale MC, Franz CE, Hamza S, et al. Genetic influences on cortical regionalization in the human brain. Neuron. 2011;72:537–544. doi: 10.1016/j.neuron.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, Fennema-Notestine C, Jak AJ, Neale MC, Franz CE, et al. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, Wright MJ, Thompson PM. Genetics of white matter development: A DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage. 2011;54:2308–2317. doi: 10.1016/j.neuroimage.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lepore N, Chiang MC, Avedissian C, Barysheva M, McMahon KL, de Zubicaray GI, Meredith M, Wright MJ, Toga AW, et al. Mapping genetic influences on ventricular structure in twins. Neuroimage. 2008;44:1312–1323. doi: 10.1016/j.neuroimage.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobia DJ, Smith MJ, Wang L, Csernansky JG. Longitudinal progression of frontal and temporal lobe changes in schizophrenia. Schizophr Res. 2012;139(1–3):1–6. doi: 10.1016/j.schres.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJ. From genotype to EEG endophenotype: A route for post-genomic understanding of complex psychiatric disease? Genome Med. 2010;2:63. doi: 10.1186/gm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Molloy EA, Blumenthal J, Zijdenbos A, Giedd JN. The epigenesis of planum temporale asymmetry in twins. Cereb Cortex. 2002;12(7):749–755. doi: 10.1093/cercor/12.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Prom-Wormley E, Fennema-Notestine C, Panizzon MS, Neale MC, Jernigan TL, Fischl B, Franz CE, Lyons MJ, Stevens A, et al. Genetic patterns of correlation among subcortical volumes in humans: Results from a magnetic resonance imaging twin study. Hum Brain Mapp. 2011a;32:641–653. doi: 10.1002/hbm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Prom-Wormley E, Panizzon MS, Kaup AR, Fennema-Notestine C, Neale MC, Jernigan TL, Fischl B, Franz CE, Lyons MJ, et al. Genetic and environmental contributions to regional cortical surface area in humans: A magnetic resonance imaging twin study. Cereb Cortex. 2011b;21:2313–2321. doi: 10.1093/cercor/bhr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Chen CH, Panizzon MS, Fennema-Notestine C, Neale MC, Jak A, Jernigan TL, Fischl B, Franz CE, Lyons MJ, et al. A comparison of heritability maps of cortical surface area and thickness and the influence of adjustment for whole brain measures: A magnetic resonance imaging twin study. Twin Res Hum Genet. 2012;15:304–314. doi: 10.1017/thg.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Jak AJ, Jernigan TL, et al. Presence of APOE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimer Dis. 2011;26(Suppl 3):49–60. doi: 10.3233/JAD-2011-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve D, Fischl B, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294(5544):1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Korgaonkar MS, Schofield PR, Harris A, Clark CR, Oakley KL, Ram K, Michaelson H, Yap S, Stanners M, et al. The TWIN-E project in emotional wellbeing: Study protocol and preliminary heritability results across four MRI and DTI measures. Twin Res Hum Genet. 2012;15:419–441. doi: 10.1017/thg.2012.12. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Schmitt JE, Knickmeyer RC, Smith JK, Lin W, Styner M, Gerig G, Neale MC. Genetic and environmental contributions to neonatal brain structure: A twin study. Hum Brain Mapp. 2010;31:1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Haidekker MA, Evertsz CJ, Fitzek C, Boor S, Andresen R, Falkai P, Stoeter P, Peitgen HO. Projecting the sulcal pattern of human brains onto a 2D plane: A new approach using potential theory and MRI. Psychiatry Res. 1998;83:75–84. doi: 10.1016/s0925-4927(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Henn FA, Nasrallah HA. Schizophrenia as a brain disease. New York: Oxford University Press; 1982. [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18(9):2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37:451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Kim JS, Jang JH, Choi JS, Jung MH, Park JY, Han JY, Choi CH, Kang DH, Chung CK, et al. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr Bull. 2011;37:839–849. doi: 10.1093/schbul/sbp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähler AK, Rimol LM, Brown AA, Djurovic S, Hartberg CB, Melle I, Dale AM, Andreassen OA, Agartz I. Effect of DISC1 SNPs on brain structure in healthy controls and patients with a history of psychosis. Am J Med Genet B. 2012;159B:722–730. doi: 10.1002/ajmg.b.32076. [DOI] [PubMed] [Google Scholar]
- Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, Maalouf E, Duggan P, Ajayi-Obe M, Hajnal J, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ. Psychiatric genetics. Washington, D.C: American Psychiatric Publishing; 2005. [Google Scholar]
- Kieseppä T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, Kapri J, Lonnqvist J. The volumetric findings in MRI brain study of bipolar twins and their healthy co-twins. Bipolar Disord. 2002;4(Suppl 1):29–30. doi: 10.1034/j.1399-5618.4.s1.6.x. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Res Hum Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, Franz CE, Lyons MJ, Pacheco J, Perry ME, et al. Genetic and environmental influences on the size of specific brain regions in midlife: The VETSA MRI study. Neuroimage. 2010;49:1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: Differentiating vulnerability factors from sequelae. Neuropharmacology. 2012a;62:647–653. doi: 10.1016/j.neuropharm.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Panizzon MS, Neale MC, Fennema-Notestine C, Prom-Wormley E, Eyler LT, Stevens A, Franz CE, Lyons MJ, Grant MD, et al. Heritability of brain ventricle volume: Converging evidence from inconsistent results. Neurobiol Aging. 2012b;33:1–8. doi: 10.1016/j.neurobiolaging.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Franz CE, Lyons MJ. VETSA: The Vietnam Era Twin Study of Aging. Twin Res Hum Genet. 2013a;16:399–402. doi: 10.1017/thg.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Moore CS, Franz CE, Panizzon MS, Lyons MJ. Cognition in middle adulthood. In: Finkel D, Reynolds CA, editors. Behavior genetics of cognition across the lifespan. New York: Springer; 2013b. [Google Scholar]
- Le Goualher G, Argenti AM, Duyme M, Baare WF, Hulshoff Pol HE, Boomsma DI, Zouaoui A, Barillot C, Evans AC. Statistical sulcal shape comparisons: Application to the detection of genetic encoding of the central sulcus shape. Neuroimage. 2000;11:564–574. doi: 10.1006/nimg.2000.0559. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, Andreassen OA, Weersma RK, Weismüller TJ, Eksteen B, et al. Dense genotyping of known autoimmune disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013 Apr 21; doi: 10.1038/ng.2616. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cereb Cortex. 1999;9:754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, Schaie KW, Panizzon MS, Boake C, Xian H, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol Sci. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- McHugh PR, McKusick VA. Genes, brain, and behavior. New York: Raven Press; 1991. [Google Scholar]
- Mohr A, Knauth M, Weisbrod M, Stippich C, Sartor K. Similarity of the brains of twins. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2001;173(6):515–521. doi: 10.1055/s-2001-14994. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- National Health and, Nutrition Examination Survey (NHANES III) Trends in health and aging. National Center for Health Statistics; 1999–2004. [Google Scholar]
- O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Oppenheim JS, Skerry JE, Tramo MJ, Gazzaniga MS. Magnetic resonance imaging morphology of the corpus callosum in monozygotic twins. Ann Neurol. 1989;26:100–104. doi: 10.1002/ana.410260117. [DOI] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen H. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale MC, Jacobson KC, Lyons MJ, Grant MD, Franz CE, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Dale AM, Eaves LJ, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, Jak AJ, et al. Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology. 2010;75:874–880. doi: 10.1212/WNL.0b013e3181f11deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Lyons MJ, Jacobson KC, Franz CE, Grant MD, Eisen SA, Xian H, Kremen WS. Genetic architecture of learning and delayed recall: A twin study of episodic memory. Neuropsychology. 2011;25:488–498. doi: 10.1037/a0022569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Kubarych TS, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Hamza S, Jak A, et al. Genetic and environmental influences of white and gray matter signal contrast: A new phenotype for imaging genetics? Neuroimage. 2012a;60:1686–1695. doi: 10.1016/j.neuroimage.2012.01.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger RL, Eaves LJ, Chen CH, Dale AM, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, et al. Genetic influences on hippocampal volume differ as a function of testosterone level in middle-aged men. Neuroimage. 2012b;59:1123–1131. doi: 10.1016/j.neuroimage.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papassotiropoulos A, de Quervain DJ. Genetics of human episodic memory: Dealing with complexity. Trends Cogn Sci. 2011;15:381–387. doi: 10.1016/j.tics.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Filley CM, Galabura A, DeFries JC. A twin MRI study of size variations in the human brain. J Cogn Neurosci. 2000;12(1):223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: A review of brain imaging studies in twins. Hum Brain Mapp. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Goradia D, Eack S, Rajagopalan M, Nutche J, Magge T, Rajarethinam R, Keshavan MS. Cortical surface characteristics among offspring of schizophrenia subjects. Schizophr Res. 2010;116:143–151. doi: 10.1016/j.schres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone R, Counsell SJ, Kapellou O, Dyet L, Kennea N, Hajnal J, Allsop JM, Cowan F, Edwards AD. Perinatal cortical growth and childhood neurocognitive abilities. Neurology. 2011;77:1510–1517. doi: 10.1212/WNL.0b013e318233b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salt-house TA, editors. The handbook of aging and cognition. 2. Hillsdale, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Bolton PF, Paus T, Murphy DG. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cereb Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- Reveley AM, Reveley MA, Chitkara B, Clifford C. The genetic basis of cerebral ventricular volume. Psychiatry Res. 1984;13:261–266. doi: 10.1016/0165-1781(84)90041-6. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Panizzon MS, Fennema-Notestine C, Eyler LT, Fischl B, Franz CE, Hagler DJ, Lyons MJ, Neale MC, Pacheco J, et al. Cortical thickness is influenced by regionally-specific genetic factors. Biol Psychiatry. 2010;67:493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, Hagler DJ, Jr, Bergmann O, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–660. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Scamvougeras A, Kigar DL, Jones D, Weinberger DR, Witelson SF. Size of the human corpus callosum is genetically determined: An MRI study in mono and dizygotic twins. Neurosci Lett. 2003;338:91–94. doi: 10.1016/s0304-3940(02)01333-2. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res Hum Genet. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, Greenstein D, Lerch JP, Kendler KS, Neale MC, et al. Identification of genetically mediated cortical networks: A multivariate study of pediatric twins and siblings. Cereb Cortex. 2008;18:1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schork AJ, Thompson WK, Pham P, Torkamani A, Roddey JC, Sullivan PF, Kelsoe JR, O’Donovan MC, Furberg H, Schork NJ, et al. All SNPs are not created equal: Genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet. 2013;9:e1003449. doi: 10.1371/journal.pgen.1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CC, Nenadic I, Koch K, Wagner G, Roebel M, Schachtzabel C, Mühleisen TW, Nöthen MM, Cichon S, Deufel T, et al. Reduced cortical thickness is associated with the glutamatergic regulatory gene risk variant DAOA Arg30Lys in schizophrenia. Neuropsychopharmacology. 2011;36:1747–1753. doi: 10.1038/npp.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychol Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P-N, Steinbach M, Kumar V. Introduction to data mining. Upper Saddle River, NJ: Pearson Education; 2006. [Google Scholar]
- Tramo MJ, Loftus WC, Stukel TA, Green RI, Weaver JB, Gazzaniga MS. Brain size, head size, and intelligence quotient in monozygotic twins. Neurology. 1998;50:1246–1252. doi: 10.1212/wnl.50.5.1246. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Cannon TD. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Joshi S, Miller MI. Functional and structural mapping of human cerebral cortex: Solutions are in the surfaces. Proc Natl Acad Sci USA. 1998;95:788–795. doi: 10.1073/pnas.95.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen IL, Brouwer RM, Peper JS, van Leeuwen M, Koenis MM, van Beijsterveldt TC, Swagerman SC, Kahn RS, Hulshoff Pol HE, Boomsma DI. Brain SCALE: Brain structure and cognition: An adolescent longitudinal twin study into the genetic etiology of individual differences. Twin Res Hum Genet. 2012;15:453–467. doi: 10.1017/thg.2012.4. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cereb Cortex. 2002;12:486–493. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET. Genetic contributions to regional variability in human brain structure: Methods and preliminary results. Neuroimage. 2002;17:256–271. doi: 10.1006/nimg.2002.1163. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Mankowski A, editor. Regional development of the brain in early life. Philadelphia: Davis; 1967. pp. 3–69. [Google Scholar]
- Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon U, Perusse D, Evans AC. Mapping genetic and environmental influences on cortical surface area of pediatric twins. Neuroscience. 2012;220:169–178. doi: 10.1016/j.neuroscience.2012.06.030. [DOI] [PubMed] [Google Scholar]