Abstract
Aims
Impact of weight loss on cardiac structure has not been extensively investigated in large, multi-ethnic, community-based populations. We investigated the longitudinal impact of weight loss on cardiac structure by cardiac magnetic resonance (CMR).
Methods and results
2351 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) who underwent CMR at Exam 1 (2002) and Exam 5 (2011) were included. Primary outcomes were percentage change in LV mass (indexed to height) and LV mass-to-volume ratio (concentric LV remodelling). Multivariable linear regression was used to measure the association between outcomes and weight change. At median 9.4 years' follow-up, 639 individuals (27%) experienced >5% weight loss (median 6.9 kg) and 511 (22%) had >5% weight gain (median 6.4 kg). A >5% weight gain was associated with the greatest increase in LV mass (+5.4% median) and LV mass-to-volume ratio (+12.2% median). Adjusting for medications, hypertension/diabetes (and change in these risk factors), age, race and other risk factors, every 5% weight loss was associated with a 1.3% decrease in height-indexed LV mass and 1.3% decrease in LV mass-to-volume ratio (p <0.0001). There was no effect modification/confounding by age, race, gender or baseline BMI. Change in LV mass-to-volume ratio was roughly linear, specifically for modest degrees of weight loss (−10% to +10%). Change in LV mass was linear with weight loss, suggesting no threshold of weight loss is needed for LV mass regression.
Conclusions
In a large multi-ethnic population, weight loss is associated with beneficial effects on cardiac structure, independent of age, race, gender, BMI and obesity-related cardiometabolic risk. There is no threshold of weight loss required to produce these effects.
Keywords: Obesity, cardiac magnetic resonance, weight loss
Introduction
Obesity (body mass index BMI >30 kg/m2) is an independent risk factor for incident heart failure (HF).1 In cross-sectional studies of asymptomatic adults, obesity is associated with subclinical alterations in left and right ventricular structure and function that are harbingers of incident HF, independent of hypertension, coronary artery disease, obstructive sleep apnoea, and type 2 diabetes.2,3 However, investigations of the reversibility of this subclinical ‘obesity cardiomyopathy’ phenotype with weight loss (or worsening with weight gain) have been limited to small studies of nutritional interventions or bariatric surgery.4–8 In addition, these studies have generally focused on individuals with significant obesity (grades 2–3) with a large degree of surgically or dietary-induced weight loss. Therefore, whether these findings apply in a large, community-based population with a range of obesity phenotypes or to individuals with a more modest degree of weight loss remains relatively unexplored.
To investigate the effects of longitudinal weight change on cardiometabolic risk and left ventricular (LV) remodeling, we investigated 2351 patients in the Multi-Ethnic Study of Atherosclerosis (MESA) who underwent two serial cardiac magnetic resonance imaging (CMR) examinations at initial enrolment (2002) and at follow-up (2011). In light of prior evidence suggesting the cross-sectional impact of obesity on LV remodeling,9 we hypothesized that changes in LV mass and LV mass-to-volume ratio (a marker of adverse concentric LV remodelling) over long-term follow-up would be associated with weight changes, independent of body mass index, age, gender, race or other metabolic risk factors (e.g. hypertension or diabetes). We also hypothesized that there would be no threshold effect for weight change required to achieve a change in LV mass or concentric LV remodelling. Ultimately, our aim was to justify and extend current recommendations for weight loss by providing evidence for (1) the impact of weight changes on LV structure independent of potentially confounding clinical risk factors and (2) a lack of a threshold for weight change, implicating any degree of weight change as beneficial on cardiac structure.
Methods
Participant population
The overall design of the MESA study has been described previously.10 In brief, the MESA cohort consists of 6814 men and women of different ethnicities (white, African American, Chinese American and Hispanic) enrolled from six different national sites; all in the cohort were free of clinical cardiovascular disease (history of myocardial infarction, angina pectoris, prior revascularization, heart failure, atrial fibrillation, stroke or peripheral arterial disease) at the time of enrolment. Baseline demographics, medical history (including cardiac risk factors), medications (for hypertension, dyslipidaemia and diabetes) and physical examination were assessed at the index examination (Exam 1: July 2000–August 2002 cycle), the most recent follow-up examination (Exam 5: 2011), and at three additional examinations (Exams 2–4) at two-year intervals. At every MESA clinic visit, body mass index (in kg/m2), resting systolic blood pressure, fasting blood glucose and dysglycaemia status were assessed at every visit as has been described.11 Dysglycaemia status was defined at each visit as normal glucose (<100mg/dl), impaired fasting glucose (100–125 mg/dl) or diabetes. CMR exams at baseline and follow-up were performed during MESA clinic visits 1 and 5, respectively.
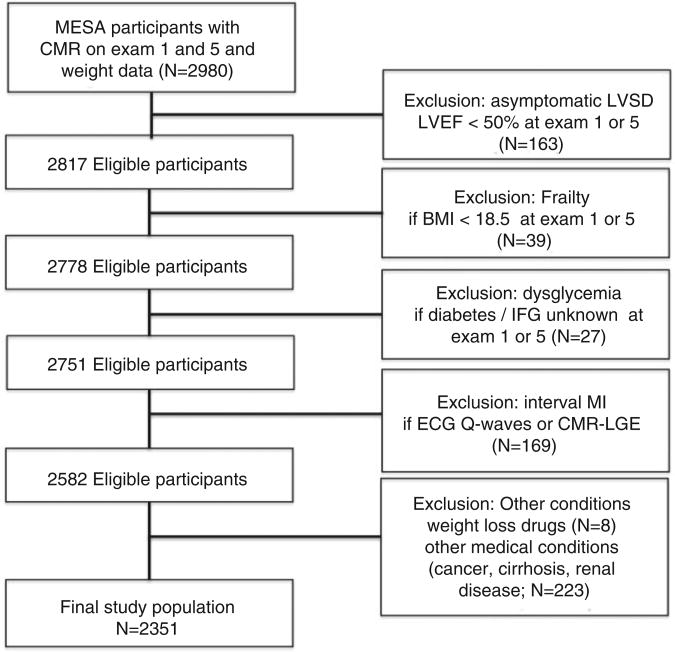
From the initial 6814 MESA participants enrolled in Exam 1, we excluded patients with (1) missing CMR data at Exam 1 or Exam 5 for LV ejection fraction (LVEF), LV end-diastolic or end-systolic volumes (LVEDV, LVESV) or LV mass; (2) missing weight data; (3) missing dysglycaemia status at Exam 1 or Exam 5. To limit confounding by development of sub-clinical/clinical cardiovascular disease, we excluded patients with LV dysfunction (defined by Exam 1 or Exam 5 CMR LVEF <50%) or evidence of prior myocardial infarction (by Q waves or late gadolinium enhancement by CMR at Exam 5, performed in a subgroup of patients undergoing CMR at Exam 5). In addition, to limit confounding by frailty and unintentional weight loss, we excluded patients with body mass index < 18.5 kg/m2 in Exam 1 or Exam 5, use of weight loss medications at Exam 1 or Exam 5, or self-reported history of cancer, cirrhosis or renal disease at Exam 1. Protocols were approved by the Institutional Review Board at each participating institution. All participants provided written informed consent.
Cardiac magnetic resonance assessment of left ventricular structure and function
In Exam 1, CMR was performed at 1.5 T as previously described12,13 to assess ventricular function using electrocardiographically gated fast gradient echo cine images (repetition time 6 ms, minimal echo time, flip angle 20°, 8 mm slice thickness with 2mm gap, matrix 256×160, field of view adjusted to body size, receiver bandwidth 32 kHz). LV volumes and mass were determined by short-axis volumetric coverage. Papillary muscles were included in the LV volumes and excluded from LV mass. Given advances in CMR technology between Exam 1 and Exam 5, steady-state free precession CMR was used to measure LV structure and function in Exam 5. Exam 1 CMR data were analysed with MASS software (Medis, The Netherlands), and Exam 5 CMR data were analysed with CIM 6.0 software (UniServices, Auckland, New Zealand), in both cases at the MESA CMR reading centre (Johns Hopkins University, Baltimore, MD) by readers blinded to clinical data. Changes in CMR pulse sequence technology and software that occurred between Exams 1 and 5 by the MESA CMR Core Laboratory were used to adjust Exam 1 to compare with Exam 5. The adjustment was based on the application of calibration curves, obtained by scanning a subset of participants in Exam 5 (N = 498) using both the gradient echo and steady state free precession technique and reading with different software.
Statistical analysis
Based on evidence outlining a cardiometabolic benefit with modest weight loss (e.g. between 5% and 10% of initial weight),14,15 we stratified MESA participants into three categories of weight change (defined as the percentage net change in weight between Exam 1 and Exam 5): ≥5% weight loss (‘loss’), ≥5% weight gain (‘gain’), and <5% weight change (i.e. 0–5% weight loss or gain – ‘stable’). Demographic, clinical and selected biochemical measures were compared by non-parametric tests (Kruskal Wallis for continuous covariates) or Chi-square testing (for categorical covariates). For the purposes of modelling, we indexed LV mass to the height raised to the power of 2.7, as has been described previously.16 For the purposes of the remainder of this work, ‘LV mass’ is height2.7 -indexed LV mass as specified above, unless otherwise specified.
Percentage change in LV mass and mass-to-volume ratio between Exam 1 and Exam 5 were our primary outcome measures of LV remodelling. Two generalized additive models (GAM using package ‘mgcv’ in R) for percentage LV mass change and percentage LV mass-to-volume change were used to assess their relationship with percentage body weight change between Exam 1 and Exam 5. GAM models are a flexible modelling framework that allow for linear and non-linear relationships between independent and dependent variables. This modelling framework was selected specifically for this study since it was not known a priori how weight change (the primary independent variable of interest) would be associated linearly with cardiac remodelling. In addition to a smoothing spline for percentage body weight change, the GAM models included gender, race and smoothing splines of age, body mass index and systolic blood pressure at baseline as additional covariates for simultaneous adjustment.
When a linear relationship between percentage change in LV mass and weight change appeared a reasonable approximation, a multivariable linear regression model was used to estimate the effect size of body weight change on percentage change in LV mass. To adjust for confounders involved in the pathogenesis of LV hypertrophy the following predictors were simultaneously included in the multi-variable linear models: (1) glycaemic status at Exam 1 (classified as normal, impaired fasting glucose and treated/ untreated diabetes); (2) change in glycaemic status between Exam 1 and 5 (where a ‘+’ value represents a worsening of glycaemic status); (3) hypertension stage at Exam 1 (as defined by JNC 7); (4) change in hypertension stage between Exam 1 and 5 (where a ‘+’ value represents a worsening of hypertension status); (5) change in the number of antihypertensive medication classes from Exam 1 to 5; (6) inflammation (CRP at Exam 1, log-transformed); (7) waist circumference at Exam 1; (7) initial BMI (log-transformed). These covariates were chosen based on prior published literature in large cohorts.17,18 In addition, the multivariable linear models were adjusted for age, gender, race, income, educational status, smoking, Exam 1 BMI and LV mass at Exam 1 (to account for regression to the mean effects). The coefficient for weight change, entered as continuous variable, was scaled so that it corresponded to the percentage change in LV mass for 5% of weight change. In addition, we performed general linear models for LV mass-to-volume change in a similar manner. Effect modification by age, gender, race and BMI was examined in each regression model. We did not find evidence for significant multicollinearity in either LV mass or LV mass-to-volume regressions by variance inflation factor statistics.
Examining overall weight changes between Exam 1 and Exam 5 does not account for the influence of patterns of weight change between Exam 1 and Exam 5 (e.g. sustained weight loss versus weight loss then regain) on LV structure and geometry. To address this potentially important aspect, we computed the change in weight at each interval Exam between 1 and 5 (e.g. between Exam 1 and 2, then 2 and 3, then 3 and 4, and finally 4 and 5) relative to Exam 1. Using the trapezoid rule, we integrated the total area under the weight versus time (in years) curve. When normalized to the total follow-up time and the baseline weight, this value represents a time-averaged weight change over the entire MESA study follow-up. We used this value as an alternative predictor to account for duration and extent of weight changes in multivariable regression.
Finally, in order to further explore the role of changes in adiposity distribution on LV remodelling, we used methods previously published in MESA to estimate fat mass.9,19 Fat mass was estimated as the difference of total weight and lean body mass, which was estimated using the methods of Kuch et al.,19 derived from bioelectrical impedance studies. In this study, lean body mass was estimated as 5.1 × [height (m)1.14] × [weight (kg)0.41] (males) and 5.34 × [height (m)1.47] × [weight (kg)0.33] (females).
All analyses were carried out in SAS 9.4 (SAS, Cary, NC) and R (version 2.15.1, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/). A two-tailed p value less than 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
The final cohort consisted of 2351 MESA participants with LV volumes, mass and ejection fraction at both Exam 1 and 5 (Figure 1). Baseline clinical, demographic, and biochemical characteristics at Exam 1 are shown in Table 1. The median time between Exam 1 and Exam 5 was 9.4 years (interquartile range 9.2–9.7 years). Of the 2351 MESA participants, 639 individuals (27%) experienced >5% weight loss (median weight change −6.9 kg), 1201 individuals (51%) had an Exam 5 weight within 5% of Exam 1 (classified as ‘weight stable’ in Table 1), and 511 individuals (22%) gained >5% weight between Exam 1 and 5 (median weight change +6.4 kg). Relative to stable or weight gain, individuals who experienced >5% weight loss were more likely to be older with a higher BMI and more prevalent metabolic syndrome.
Figure 1.
Derivation of the study cohort.
CMR, cardiac magnetic resonance; LVSD, left ventricular systolic dysfunction; LVEF, left ventricular ejection fraction; BMI, body mass index; IFG, impaired fasting glucose; ECG, electrocardiogram; LGE, late gadolinium enhancement.
Table 1.
Baseline clinical, biochemical and anthropometric indices from initial MESA examination, stratified by weight change. Values presented at median and interquartile range or frequency, as appropriate; p values are for a Kruskal Wallis (continuous) or Chi-square test (categorical) comparison for equality across all three weight change strata (weight stable, loss or gain).
| Variable | All patients (Exam 1) | |||
|---|---|---|---|---|
|
| ||||
| Weight loss (N=639) | Weight stable (N=1201) | Weight gain (N=511) | p-value | |
| Age (years) | 63 (54–69) | 57 (51–65) | 54 (49–61) | <0.0001 |
| Male gender, n (%) | 268 (42) | 581 (48) | 207 (41) | 0.002 |
| Race | ||||
| White, n (%) | 262 (41) | 469 (39) | 222 (44) | 0.005 |
| Chinese, n (%) | 67 (10) | 188 (16) | 62 (12) | |
| Black, n (%) | 185 (29) | 276 (23) | 124 (24) | |
| Hispanic, n (%) | 125 (20) | 268 (22) | 103 (20) | |
| Weight (lb) | 171 (149–199) | 166 (144–190) | 162 (142–194) | 0.0003 |
| Body-mass index (kg/m2) | 28.0 (24.9–31.5) | 26.9 (24.1–30.0) | 26.6 (23.8–29.9) | <0.0001 |
| Systolic BP (mmHg) | 125 (113–140) | 119 (108–134) | 116 (105–132) | <0.0001 |
| Glycemic control | ||||
| Normal, n (%) | 476 (75) | 990 (82) | 428 (84) | <0.0001 |
| Impaired, n (%) | 92 (14) | 131 (11) | 30 (6) | |
| Diabetes, n (%) | 71 (11) | 80 (7) | 53 (10) | |
| Hypertension, n (%) | 285 (45) | 414 (34) | 160 (31) | <0.0001 |
| Total cholesterol (mg/dl) | 194 (171–217) | 194 (172–216) | 191 (169–214) | 0.24 |
| LDL (mg/dl) | 117 (97–139) | 118 (97–138) | 113 (94–133) | 0.04 |
| HDL (mg/dl) | 49 (41–60) | 48 (40–59) | 51 (43–61) | 0.03 |
| Triglycerides (mg/dl) | 115 (82–162) | 113 (78–160) | 102 (75–149) | 0.04 |
| Medications | ||||
| Anti-Lipid, n (%) | 110 (17) | 176 (15) | 66 (13) | 0.11 |
| Anti-HTN, n (%) | 240 (38) | 354 (29) | 129 (25) | <0.0001 |
| Insulin, n (%) | 7 (1) | 17 (1) | 8 (2) | 0.77 |
| Waist circumference (cm) | 97.5 (89.5–106.7) | 95.0 (86.5–102.7) | 92.9 (84.9–101.5) | <0.0001 |
| Waist-to-hip ratio | 0.93 (0.88–0.97) | 0.92 (0.87–0.97) | 0.91 (0.85–0.96) | <0.0001 |
| Current/former smoking, n (%) | 300 (47) | 529 (44) | 252 (49) | 0.11 |
| Total daily intentional exercise (MET-min) | 840 (105–2089) | 1050 (315–2205) | 840 (210–2010) | 0.03 |
| C-reactive protein (mg/l) | 1.99 (0.85–4.38) | 1.61 (0.73–3.70) | 1.68 (0.69–1.57) | 0.004 |
| Interleukin-6 (pg/ml) | 1.15 (0.78–1.72) | 1.02 (0.69–1.61) | 1.01 (0.66–1.57) | 0.0001 |
| Metabolic syndrome, n (%) | 229 (36) | 321 (27) | 116 (23) | <0.0001 |
Weight loss is associated with decreases in LV mass and concentric LV remodeling over long-term follow-up
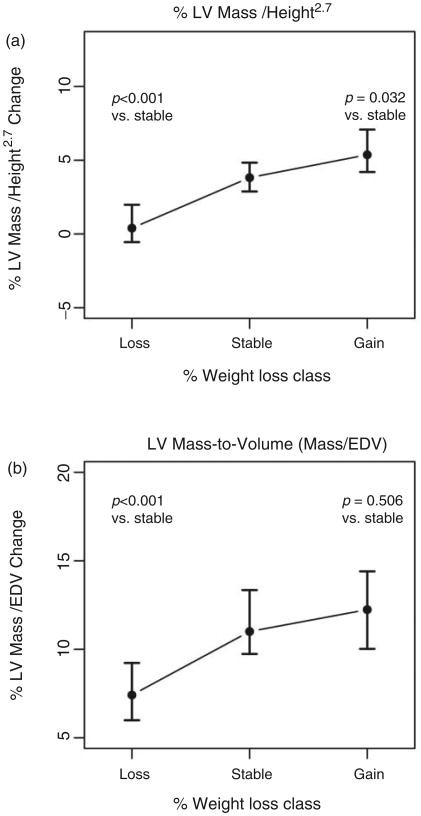
The aggregate baseline and percentage change between initial (Exam 1) and follow-up CMR (Exam 5) for our primary outcome of LV mass and LV mass-to-volume is shown in Figure 2 and Table 2. Overall, while LV ejection fraction was stable with weight changes, weight loss was associated with a decrease in both raw (un-indexed) and indexed LV mass and less concentric LV remodelling (p < 0.05 for both mass and mass-to-volume ratio compared to weight stable and weight gain, with Bonferroni adjustment), without a significant change in indexed LV volumes. Individuals who gained >5% weight between Exam 1 and 5 had the greatest increase in un-indexed LV mass (+4.1%) and greatest progression in concentric LV remodelling (+12.2%), with similar findings when LV mass was indexed to height2.7.
Figure 2.
Median percentage change in height-indexed LV mass (Figure 2a) and LV mass-to-volume ratio (Figure 2b), stratified by weight loss, stable or gain. The error bars represent 95% CI, obtained by bootstrap replications; p values refer to pairwise post hoc comparisons using the Wilcoxon testing with adjustment by the Holm method.
Table 2.
Cardiac magnetic resonance indices of left ventricular structure and function, stratified by obesity status and presence of weight loss from Exam 1 to Exam 5. Values presented at median and interquartile range.
| Variable | Weight loss (N=639) | Weight stable (N=1201) | Weight gain (N=511) | P |
|---|---|---|---|---|
| LV ejection fraction (Exam 1) (%) | 64.0 (60.1, 67.1) | 63.4 (59.6, 66.7) | 62.6 (58.8, 65.8) | 0.0003 |
| %ΔLV ejection fraction | −0.7 (−8.1, 6.5) | −0.9 (−7.4, 7.9) | 0.6 (−6.5, 8.5) | 0.04 |
| Raw (un-indexed) LV parameters | ||||
| LV mass (Exam 1) (g) | 116.4 (100.2, 134.6) | 113.5 (97.1, 134.7) | 113.1 (96.2, 134.2) | 0.07 |
| Δ LV mass (Exam 1 to 5) | −2.7 (−12.9, 7.8) | 1.9 (−7.8, 12.4) | 4.4 (−6.6, 15.3) | <0.0001 |
| %Δ LV mass (Exam 1 to 5) | −2.3 (−11, 6) | 1.8 (−7, 11) | 4.1 (−5.9, 13.8) | <0.0001 |
| LV end-diastolic volume (Exam 1) (ml) | 124.8 (108.8, 145.3) | 125.8 (108.9, 147.6) | 125.8 (107.8, 146.6) | 0.51 |
| %Δ LV end-diastolic volume (Exam 1 to 5) | −9.9 (−19, 0.3) | −8.6 (−18.7, 0.6) | −7.2 (−16, 3) | 0.05 |
| Height-indexed LV parameters | ||||
| Indexed LV mass (Exam 1) (g/m2.7) | 29.9 (26.4, 33.7) | 28.7 (25.6, 32.3) | 28.9 (25.6, 32.3) | <0.0001 |
| %Δ LV mass index (Exam 1 to 5) | 0.4 (−8.5, 10.5) | 3.8 (−5.0, 13.6) | 5.4 (−3.5, 16.0) | <0.0001 |
| LV end-diastolic volume (Exam 1) (ml/m2.7) | 32.2 (28.3, 36.1) | 32.1 (28.2, 35.6) | 32.3 (28.8, 35.6) | 0.76 |
| %Δ LV end-diastolic volume (Exam 1 to 5) | −7.3 (−16.2, 3.3) | −6.6 (−16.4, 2.8) | −5.3 (−14.5, 4.7) | 0.37 |
| LV mass to volume ratio (Exam 1) | 0.94 (0.84, 1.05) | 0.90 (0.81, 1.00) | 0.89 (0.81, 0.99) | <0.0001 |
| %Δ LV mass to volume ratio (Exam 1 to 5) | 7.4 (−4.1, 21.2) | 11.0 (−0.4, 26.6) | 12.2 (−0.4, 26.9) | 0.0001 |
The association of weight changes with LV mass and LV concentricity is linear across weight change
The relationship between the percentage change in LV mass and LV mass-to-volume ratio versus body weight change predicted by the GAM models is shown in Figure 3. In the case of LV mass change, the relationship with body weight change was linear. The terms for systolic blood pressure (p = 0.04) and BMI at baseline (p<0.001) and the parametric terms for gender (p<0.001) and African-American race (p = 0.04) were also significant in the GAM model for percentage change in LV mass. Overall, these results from the GAM model for percentage LV mass change indicated a constant incremental effect on LV mass with change in weight (e.g. no ‘threshold’ weight loss required to impact LV mass). Similarly, the GAM model for LV mass-to-volume demonstrated linearity with percentage body weight change, primarily between −10% and +10% weight change between Exam 1 and 5 (Figure 3). In addition, the terms for BMI at baseline (p < 0.001) and the parametric term for gender (p = 0.002) were significant in this GAM.
Figure 3.
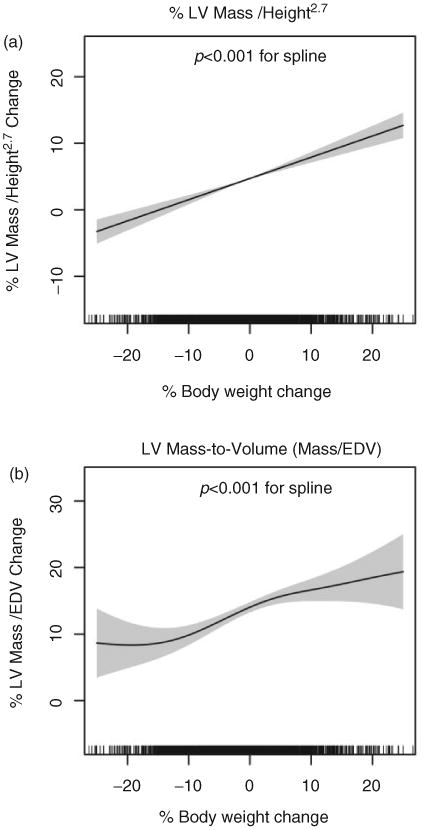
Smoothing splines obtained from fit of MESA data to general additive models (GAM) for percent change in height-indexed LV mass (Figure 3a) and LV mass-to-volume ratio (Figure 3b), with initial body mass index, age, gender, race and systolic blood pressure at baseline included as predictors for simultaneous adjustment. Weight loss and gain appear linearly associated with changes in LV mass (Figure 3a). LV mass-to-volume ratio changes linearly with body weight between −10% and +10% weight change, with significant non-linearity outside this regime. The density of hash marks on the x-axis represents the distribution of weight loss in the population studied. Grey bands represent 95% CI for the spline and p values refer to significance of the smooth term (spline) in the GAM that represents body weight change.
Weight change is associated with changes in LV mass and LV concentricity, regardless of race, gender, age or body mass index
We subsequently constructed multivariable linear models for percent change in LV mass and in LV mass-to-volume (Table 3). Female gender (p < 0.0001), baseline BMI (p < 0.0001), baseline hypertension stage and change in hypertension stage (P < 0.0001), and baseline LV mass (P < 0.0001) were independently associated with percentage change in LV mass. After further adjustment for obesity-related metabolic confounders that may impact LV remodelling and hypertrophy (hypertension, dysglycaemia, age, race, gender) and initial LV mass (regression to the mean), every 5% decrease in weight was still independently associated with a near 1.3% additional decline in height-indexed LV mass (p < 0.0001). Importantly, the effect size for every 5% weight loss on LV mass in multivariable models was similar to that in the univariable linear regression (β = 1.4, p < 0.0001; where percentage weight change was used as the only independent variable), suggesting little confounding by addition of the additional covariates in the multivariable model. When LV mass was indexed to height (as opposed to height2.7), we obtained a similar association between LV mass and changes in weight.
Table 3.
Multivariable linear regression for percentage change in height-indexed LV mass and percentage change in LV mass-to-volume ratio between Exam 1 and Exam 5 (where a negative effect estimate indicates lower LV mass or mass-to-volume ratio at Exam 5 versus Exam 1).
| Parameter | % Change in LV mass index | % Change in LV mass/volume ratio | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | |
| Age (years) | −0.07 | (−0.14−0) | 0.04 | 0.21 | (0.1–0.31) | 0.0001 |
| Female | −8.92 | (−10.15 to −7.69) | <0.0001 | −8.29 | (−10.16 to −6.42) | <0.0001 |
| Race | ||||||
| White | Ref | Ref | Ref | Ref | Ref | Ref |
| Chinese-American | 1.32 | (−0.5–3.13) | 0.16 | −0.13 | (−2.88–2.62) | 0.93 |
| African-American | 2.23 | (0.79–3.67) | 0.002 | 2.91 | (0.72–5.1) | 0.009 |
| Hispanic | 0.39 | (−1.27–2.05) | 0.65 | 0.17 | (−2.32–2.65) | 0.9 |
| Smoking | −0.89 | (−1.99–0.21) | 0.11 | 2.29 | (0.63–3.95) | 0.007 |
| Income | −0.17 | (−0.37–0.03) | 0.1 | −0.05 | (−0.35–0.25) | 0.76 |
| Education | −0.11 | (−0.39–0.17) | 0.45 | −0.63 | (−1.05 to –0.2) | 0.004 |
| Body mass index, log-transformed | 28.65 | (21.52–35.79) | <0.0001 | 7.55 | (−2.55–17.65) | 0.14 |
| Glycaemic control | ||||||
| Normal fasting glucose | Ref | Ref | Ref | Ref | Ref | Ref |
| Impaired fasting glucose | −0.77 | (−2.55–1) | 0.39 | 1.86 | (−0.83–4.56) | 0.18 |
| Untreated diabetes | −2.28 | (−6.64–2.07) | 0.3 | 6.44 | (−0.16–13.05) | 0.06 |
| Treated diabetes | 1.03 | (−1.22–3.28) | 0.37 | 3.52 | (0.1–6.94) | 0.04 |
| Change in glycaemic category | −0.19 | (−0.91–0.54) | 0.62 | 0.57 | (−0.53–1.67) | 0.31 |
| Hypertension stage | 2.78 | (2.22–3.34) | <0.0001 | 2.74 | (1.91–3.57) | <0.0001 |
| Change in hypertension stage | 2.96 | (2.5–3.42) | <0.0001 | 2.45 | (1.75–3.15) | <0.0001 |
| No. of antihypertensive Rx classes | 0.26 | (−0.38–0.91) | 0.42 | −1.22 | (−2.19 to –0.25) | 0.01 |
| Change in no. of antihypertensive Rx classes | −0.09 | (−0.7–0.52) | 0.78 | −0.65 | (−1.58–0.27) | 0.17 |
| C-reactive protein, log-transformed | −0.39 | (−0.92–0.14) | 0.15 | 0.25 | (−0.56–1.05) | 0.55 |
| Waist circumference (cm) | 0.01 | (−0.07–0.1) | 0.78 | 0.2 | (0.08–0.33) | 0.002 |
| Exercise (per 1000 MET/min/week) | 0.16 | (−0.06–0.38) | 0.15 | 0.12 | (−0.22–0.45) | 0.49 |
| LV mass/height2.7, Exam 1 | −1.3 | (−1.42 to – 1.17) | <0.0001 | NA | NA | NA |
| LV mass to volume ratio, Exam 1 | NA | NA | NA | −63.76 | (−69.21 to –58.31) | <0.0001 |
| Change in weight (per 5% decrease) | −1.33 | (−1.67 to –0.99) | <0.0001 | −1.27 | (−1.79 to –0.76) | <0.0001 |
Ref, referent category; NA, not included in regression.
Similarly, in linear regression models for change in LV mass-to-volume ratio, weight change was associated with LV geometry (1.1% decline in concentricity per 5% decrease in weight). In addition, given that LV mass-to-volume ratio observed most linearity between −10% and +10% body weight change (Figure 2), we repeated the multivariable linear regression analysis for the subset of MESA participants who experienced between −10% and +10% weight change between Exams 1 and 5. After full adjustment (with identical covariates as in our linear models), the association of weight loss with LV mass-to-volume ratio was more pronounced in those with moderate levels of weight loss (−1.72% per 5% weight loss; 95% CI −2.65 to −0.78g/ml; p = 0.0003). All associations were stable to exclusion of 2.5% of data from each tail of the distribution for LV mass in Exam 1 (to account for potential outliers). There was no effect modification in any model by age, gender, race, or initial BMI.
When we examined changes in fat mass (as defined in Methods), we found similar associations, with a 10% decrease in fat mass associated with a 1.25% decrease in indexed LV mass and 1.07% decrease in LV mass-to-volume ratio (both p < 0.0001; Supplementary Table 1).
Finally, to examine the influence of the pattern of weight changes (e.g. sustained weight loss or weight loss and regain) on LV remodelling, we constructed fully adjusted multivariable models for LV mass and LV mass-to-volume including time-averaged weight change. In these models, a 5% decrease in time-weighted average change in weight was independently associated with percentage change in LV mass (β = −1.40, 95% CI −1.97 to −0.82; p < 0.0001) and percentage change in LV mass-to-volume ratio (β=−1.30, 95% CI −2.16 to −0.44, p=0.003).
Discussion
In a large community-based, multi-ethnic, prospective cohort of patients, we found that reduction in weight is associated with LV mass and concentricity regression over time, regardless of age, gender, race, waist circumference, baseline BMI or comorbid status. Furthermore, the relationship between weight change and LV mass change was approximately linear, suggesting the absence of a ‘threshold effect’ of weight change needed to experience a significant change in LV mass. The relationship between a change in weight and changes in LV geometry revealed a significant linear relationship between weight change and LV geometry over a more limited range of weight change (−10% to +10%). Finally, we found that average weight decrease over the entire study follow-up was also associated with decreased LV mass and LV concentricity, suggesting that temporal patterns of weight change (e.g. sustained loss) may be as important as net weight change in terms of LV remodelling. These results describe for the first time the impact of weight change on important, prognostic indices of LV structure regardless of age, gender, race or BMI in a large, community-based cohort free of cardiovascular disease or heart failure using cardiac magnetic resonance for precise assessment of ventricular structure.
Although the implication of weight change on cardiometabolic risk factors and incident diabetes in obesity has been previously demonstrated,20 the impact of weight changes on cardiac structure independent of obesity-related comorbidities has not been extensively studied. Our results suggest that the benefits of weight change on LV mass is present independently of BMI and race and is seen even with small degrees of weight change. In contrast, we provide evidence that weight loss may differentially impact cardiac geometry (by LV mass-to-volume ratio) in normal vs overweight/obese individuals. These results justify current 2020 global strategic goals of the American Heart Association that intentional weight loss may directly impact cardiac structure across BMI, race and gender.21
Mechanistically, weight loss should have a favourable effect on LV remodelling: excess adiposity is associated with endothelial dysfunction,22 myocardial fibrosis,23 diastolic dysfunction24,25 and LV hypertrophy26 and may be reversible with significant weight loss.4,6,7 In a study of 1189 patients aged 23–35 in the Coronary Artery Risk Development in Young Adults (CARDIA) study, Gardin et al.27 measured LV mass using M-mode echocardiography and found a significant association between longitudinal changes in LV mass and BMI.18 Interestingly, they found no significant increase in LV mass over 10 years, except in a subgroup of young African-American women, where a 9.1 kg weight gain over 5 years was associated with a 9–12% increase in LV mass. In general, we did not observe any race or gender-related heterogeneity in the effects of weight loss on LV remodelling. The discordance in findings between these studies may owe to a younger population in CARDIA free of long-standing exposure to comorbidity (e.g. ageing, hypertension, diabetes) as well as the use of a gold-standard CMR measurement of LV mass in this study.
Our results are also in line with a report from Monsuez et al.28 in 280 participants from a primary prevention study of antioxidant therapy, who underwent echocardiography for LV mass and diastolic function over 6 years, as well as anthropometric measures of body composition. In this study, a 1 kg/m2 increase in BMI was associated with an increase in indexed LV mass by 2.3 g/m2.7 (95% CI 1.3–3.3; p < 0.0001). In addition, there were parallel increases in left atrial area but these were not accompanied by changes in E-wave deceleration time (a marker of diastolic dysfunction). Our study extends these results in a multi-ethnic cohort with a fully tomographic CMR assessment of LV mass and geometry (LV mass-to-volume) over a range of weight change.
In a comprehensive report from the Framingham Heart Study, Lieb et al.17 studied 4217 individuals with LV mass measured by M-mode echocardiography four times over 16 years to identify baseline correlates of longitudinal changes in LV mass. These investigators found that a progressive increase in LV mass was more prominent with male gender, older age, higher BMI at baseline, hypertension and smoking but not diabetes mellitus (after multivariable adjustment). More recent results from the Framingham study report similar secular trends in echocardiographic LV mass over a longer time frame (between 1987 and 2008, with a modest increase in LV mass similar to results in this report.29 Our results extend these observations from Framingham by (1) using CMR as a three-dimensional volumetric approach to LV mass quantification; (2) demonstrating that longitudinal changes in weight are associated with regression in LV mass and changes in LV geometry; (3) generalizing the therapeutic benefits of weight loss on LV mass across BMI, race, gender and age. These associations do not vary by race, gender or age, suggesting that the benefits of weight loss (and the maladaptive effects of weight gain) are ubiquitous. In the context of prior studies within MESA and in other cohorts demonstrating the prognostic import of LV mass and geometry on incident HF,30,31 our results provide mechanistic insight into the well-known association of weight on HF risk: weight changes impact LV remodelling, regardless of age, gender and ethnicity. Indeed, prior reports from MESA suggest that a 10% increase in body size-adjusted LV mass is associated with a 40% increased hazard of incident HF,30 suggesting the significant prognostic benefits of relatively modest changes in LV mass.
The results of this study need to be viewed in the context of its design. MESA is a prospective cohort study and weight changes within MESA may not necessarily be intentional. We did attempt to control for residual confounding by frailty and severe illness (as causes of unintentional weight loss or markers of advanced non-cardiac disease) by our exclusion of frail BMI (Exam 1 or 5 BMI <18.5 kg/m2), cancer, cirrhosis or renal disease. Moreover, only patients who survived until Exam 5 were included in our analysis. Therefore, despite some referral bias therefore inherent due to inclusion of a subset of the MESA cohort, associations between weight changes and LV remodelling were significant even after adjustment for covariates linked to LV remodelling. Although we did not have serial assessments of body composition, we found a significant association between changes in calculated fat mass (using formulae published in MESA previously)9 and LV remodelling (Supplementary Table 1). Although regression to the mean has been an issue in other studies,17 our analysis appears to be robust on this issue (see online Supplement for a detailed assessment of this aspect). Finally, given that MESA was a cohort study without specific intervention, potential aetiologies of weight change (e.g. diet–behavioural changes) that could not be addressed directly were assessed using survey measures of intentional exercise and not formal testing of fitness. More definitive analyses will require serial CMR studies at each time point (anticipated in the United Kingdom BioBank) and serial dietary and exercise assessments (potentially including formal physiological assessment of fitness). Further studies on cardiac structure/function and more intricate, cellular indices of cardiac remodelling (e.g. T1 mapping) in prospective cohort studies with weight loss/gain (e.g. United Kingdom BioBank) will be needed to address more subtle aspects of ventricular function and remodelling.
In summary, we demonstrate that weight loss of any degree is independently associated with LV mass and geometry, both prognostic indices of cardiac remodelling, regardless of age, gender, race, BMI or cardiometabolic risk factors. Fundamentally, there does not appear to be a threshold of weight loss necessary to alter global cardiovascular structure, as defined by LV mass or LV geometry. These results cement weight loss as a critical first-line preventive measure for myocardial remodelling that forecasts heart failure across the world.
Supplementary Material
Acknowledgments
We acknowledge the participants in the MESA study and the study coordinators for the tireless efforts in the prevention of cardiovascular disease in our nation.
Funding: MESA is supported by contracts NO1-HC-95159 to N01-HC-95169 from the National Heart, Lung, and Blood Institute. Dr Shah was supported by an American Heart Association Post-Doctoral Fellowship Award (11POST000002) and a training grant from the Heart Failure National Institutes of Health Clinical Research Network (U01-HL084877). Dr Jerosch-Herold receives support through R01-HL-65580.
Footnotes
All other authors have no financial disclosures relevant to the content of this manuscript.
Conflict of interest: There are no conflicts of interest relevant to the content of this manuscript.
References
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–813. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–443. doi: 10.1038/ncpcardio0943. [DOI] [PubMed] [Google Scholar]
- 3.Wong C, Marwick TH. Alterations in myocardial characteristics associated with obesity: detection, mechanisms, and implications. Trends Cardiovasc Med. 2007;17:1–5. doi: 10.1016/j.tcm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Algahim M, Lux T, Leichman J, et al. Progressive regression of left ventricular hypertrophy two years after bariatric surgery. Am J Med. 2010;123:549–555. doi: 10.1016/j.amjmed.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashrafian H, Roux C, Darzi A, et al. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118:2091–2102. doi: 10.1161/CIRCULATIONAHA.107.721027. [DOI] [PubMed] [Google Scholar]
- 6.Di Bello V, Santini F, Di Cori A, et al. Effects of bariatric surgery on early myocardial alterations in adult severely obese subjects. Cardiology. 2008;109:241–248. doi: 10.1159/000107787. [DOI] [PubMed] [Google Scholar]
- 7.Garza C, Pellikka P, Somers V, et al. Structural and functional changes in left and right ventricles after major weight loss following bariatric surgery for morbid obesity. Am J Cardiol. 2010;105:550–556. doi: 10.1016/j.amjcard.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 8.Rider O, Francis J, Ali M, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718–726. doi: 10.1016/j.jacc.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 9.Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Yeboah J, Bertoni AG, Herrington DM, et al. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:140–146. doi: 10.1016/j.jacc.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckbert SR, Post W, Pearson GD, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan RT, Bluemke D, Gomes A, et al. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2011;57:1735–1744. doi: 10.1016/j.jacc.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 15.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 16.Dewey FE, Rosenthal D, Murphy DJ, Jr, et al. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117:2279–2287. doi: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- 17.Lieb W, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the Framingham offspring study. Circulation. 2009;119:3085–3092. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–387. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 19.Kuch B, Gneiting B, Doring A, et al. Indexation of left ventricular mass in adults with a novel approximation for fat-free mass. Journal of hypertension. 2001;19:135–142. doi: 10.1097/00004872-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 22.Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 23.Quilliot D, Alla F, Bohme P, et al. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int J Obes (Lond) 2005;29:1321–1328. doi: 10.1038/sj.ijo.0803022. [DOI] [PubMed] [Google Scholar]
- 24.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Chakko S, Mayor M, Allison MD, et al. Abnormal left ventricular diastolic filling in eccentric left ventricular hypertrophy of obesity. Am J Cardiol. 1991;68:95–98. doi: 10.1016/0002-9149(91)90718-z. [DOI] [PubMed] [Google Scholar]
- 26.Lavie CJ, Milani RV, Ventura HO, et al. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100:1460–1464. doi: 10.1016/j.amjcard.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 27.Gardin JM, Brunner D, Schreiner PJ, et al. Demographics and correlates of five-year change in echocardiographic left ventricular mass in young black and white adult men and women: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 2002;40:529–535. doi: 10.1016/s0735-1097(02)01973-3. [DOI] [PubMed] [Google Scholar]
- 28.Monsuez JJ, Kesse-Guyot E, Fezeu L, et al. Impact of 6-year body weight change on cardiac geometry and function in ageing adults: the SUpplementation en Vitamines et Mineraux AntioXydants-2 (SU.VI.MAX-2) cardiovascular ultrasound substudy. J Hypertens. 2010;28:2309–2315. doi: 10.1097/HJH.0b013e32833d4576. [DOI] [PubMed] [Google Scholar]
- 29.Kaess BM, Gona P, Larson MG, et al. Secular trends in echocardiographic left ventricular mass in the community: the Framingham Heart Study. Heart. 2013;99:1693–1698. doi: 10.1136/heartjnl-2013-304600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.