Abstract
Background
Neuromuscular electrical stimulation (NMES) has been shown to reduce quadriceps activation failure (QAF), and eccentric exercise has been shown lessen muscle atrophy post-ACL reconstruction. Given that these are two critical components of quadriceps strength, intervention combining these therapies may be effective at reinstituting quadriceps function post-anterior cruciate ligament (ACL) reconstruction.
Objectives
To evaluate the effectiveness of a combined NMES and eccentric exercise intervention to improve the recovery of quadriceps activation and strength post-reconstruction.
Design
Parallel longitudinal design.
Setting
Laboratory.
Participants
Thirty-six individuals post-injury were placed into four treatment groups (N&E, NMES and eccentrics; E-only, eccentrics only; N-only, NMES-only; STND, standard of care) and ten healthy controls participated.
Intervention
N&E and N-only received the NMES protocol 2x per week for the first six weeks post-reconstruction. N&E and E-only received the eccentric exercise protocol 2x per week beginning six weeks post-reconstruction.
Main outcome measure
Quadriceps activation was assessed via the superimposed burst technique and quantified via the central activation ratio. Quadriceps strength was assessed via maximal voluntary isomeric contractions (Nm/kg). Data was gathered on three occasions: pre-operative, 12-weeks-post-surgery and at return-to-play.
Results
No differences in pre-operative measures existed (P>0.05). E-only recovered quadriceps activation better than N-only or STND (P<0.05). N&E and E-only recovered strength better than N-only or the STND (P<0.05) and had strength values that were similar to healthy individuals at return-to-play (P>0.05).
Conclusion
Eccentric exercise was capable of restoring levels of quadriceps activation and strength that were similar to those of healthy adults and better than NMES alone.
Keywords: ACL, eccentric, rehabilitation, electrostimulation, strength training
1. Introduction
Quadriceps weakness is common following anterior cruciate ligament (ACL) reconstruction. Despite aggressive rehabilitation programs directed at improving quadriceps function, a universally effective treatment approach to reverse this muscle weakness has yet to be identified [1]. Quadriceps weakness that accompanies ACL injury is related to reduced functional performance [2, 3], the potential for re-injury [2, 4], and the development of post-traumatic osteoarthritis [5]. Given the short-and long-term consequences of quadriceps weakness, it is critical that rehabilitation approaches capable of reinstituting complete quadriceps strength are developed.
Although the precise mechanism(s) of quadriceps weakness are unknown, there is evidence to suggest that quadriceps activation failure (QAF) and muscle atrophy contribute to persistent strength deficits [1, 6]. Activation failure is the inability to completely volitionally contract the muscle due to alterations in neural signaling [7], and is common following ACL reconstruction [8, 9]. Specifically, these changes in neural signaling are thought to be caused by a reduction in alpha motorneuron recruitment and/or firing rate [7]. If left untreated, QAF can significantly impede strength gains[10] by only allowing portions of the muscles to be volitionally utilized during active exercise [7]. Quadriceps muscle atrophy that occurs following ACL reconstruction is also thought to contribute to persistent muscle weakness [11, 6] due to alterations in muscle architecture [12, 13], selective fiber atrophy [14-16], or even neural deficits such as QAF [1]. Together, QAF and muscle atrophy have been reported to account for approximately 60% of the variance in quadriceps strength post-ACL injury [6]. As such, QAF and muscle atrophy are crucial factors to target in order to improve the recovery of quadriceps strength following ACL reconstruction.
Neuromuscular electrical stimulation (NMES) is a clinical modality that has the potential to treat QAF by initiating action potentials in intramuscular nerve branches, resulting in an involuntary contraction of the muscle [17].Because NMES exogenously stimulates the muscle, large diameter, Type II muscle fibers are thought to be selectively recruited, resulting in a greater potential for muscle force production [18]. Importantly, in ACL reconstructed individuals, NMES has been found to be more effective than exercise alone at improving quadriceps activation [19].
Eccentric exercise, whereby the muscle is lengthened and an external force exceeds that produced by the muscle, has been shown to be more effective than traditional concentric strengthening at minimizing muscle atrophy and improving muscle force production [20]. Though the application of early post-operative eccentric resistance exercise to the ACL reconstructed limb has traditionally been contraindicated, as there is potential for injury to the graft, articular cartilage, or surrounding soft tissue structure [21], recent evidence has shown that the application of early eccentrics to the ACL limb can be used to safely increase quadriceps muscle volume [22, 23] and strength [24, 25].
As NMES and eccentric exercise have been shown to successfully improve two critical components of quadriceps weakness, it seems plausible that a rehabilitation protocol utilizing both of these therapies will be effective at reinstituting quadriceps strength. Understanding the benefit of this combined therapy approach will help to provide preliminary evidence of therapies that can positively influence post-operative quadriceps function and will provide data that will help to justify the necessity of this intervention to be examined using a large randomized clinical trial. Therefore, the goal of the current study was to determine if the combination of NMES and eccentric exercise would be effective at improving quadriceps muscle strength in patients following ACL reconstruction. We hypothesized that the greatest improvements in quadriceps muscle strength would be achieved in patients receiving the combined intervention, when compared to patients receiving either therapy in isolation or the standard of care following ACL reconstruction. Furthermore, we anticipated that improvements in quadriceps activation would be significantly related to gains in quadriceps strength.
2. Materials and Methods
2.1 Participants
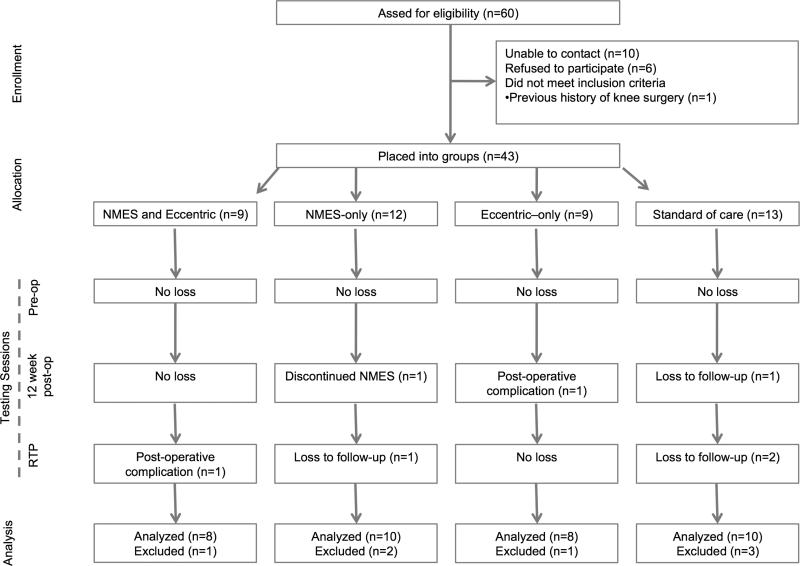
Prior to the recruitment of participants, this trial was prospectively registered in a public registry (NCT01555567). Individuals scheduled for ACL reconstruction were invited to participate in this study (Figure 1, Table 1). Potential participants were excluded if they: had a previous history of surgery to either knee, suffered a previous ACL injury, or had a known heart condition. Pregnant females were also excluded. Surgical reports were obtained to report any concomitant meniscal damage that required surgery (Table 1). Sample size for this investigation was estimated using an a priori power analysis based on previous work examining the effects of NMES on volitional muscle activation post-ACL reconstruction. Based on this previous work, a projected effect size of 3.66 was calculated for quadriceps strength. Thus, in order to detect associations with a α-level of 0.05 and the 1-β of 0.80, it was determined that at least six patients per group would be needed for this investigation [26]. Additionally, ten healthy controls were also enrolled in this investigation so that we could estimate the effectiveness of these interventions to restore healthy levels of quadriceps function. Informed consent and was obtained from all subjects approved by the University's Institutional Review Board prior to testing.
Figure 1.
Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) diagram. NMES= neuromuscular electrical stimulation, RTP=return-to-play.
Table 1.
Participant demographics (mean (SD))
| Pre-operative Quadriceps Function | Surgery Details | Time-to-Test (days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | Gender | Age (yrs) | Height (m) | Mass (kg) | Strength (Nm/kg) | QAF (CAR) | Graft | Meniscus | ACL injury to pre-operative | 12-weeks post-surgery | Return to play |
| N&E | 8 | 3f/5m | 23.2(6.3) | 1.45(0.6) | 77.8(16.5) | 2.7(0.8) | 95.7(3.5) | PT=7 STG=1 |
ACL-only=7 Meniscal Repair=1 |
73.2(54.4) | 85.8(3.4) | 208.5(26.3) |
| N-only | 10 | 2f/8m | 21.8(4.4) | 1.76(0.1) | 81.65(22.6) | 2.6(0.6) | 97.4(3.0) | PT=10 STG=0 |
ACL-only=5 Meniscal Repair=4 Meniscectomy=1 |
51.5(12.5) | 85.1(2.8) | 215.9(30.2) |
| E-only | 8 | 3f/5m | 23.2(5.4) | 1.75(0.1) | 77.7(10.4) | 2.8(1.1) | 95.9(4.5) | PT=6 STG=2 |
ACL-only=6 Meniscal Repair=2 |
94.1(84.7) | 83.5(4.3) | 228.8(39.4) |
| STND | 10 | 5f/5m | 18.3(3.7) | 1.73(0.1) | 75.5(24.1) | 2.6(0.6) | 94.9(5.3) | PT=8 STG=2 |
ACL-only=5 Meniscal Repair=4 Meniscectomy=1 |
95.7(57.9) | 96.2(26.4) | 220.1(27.5) |
| Healthy | 10 | 3f/7m | 23.5(3.4) | 1.73(0.1) | 71.7(9.9) | - | - | - | - | - | - | - |
| Total | 46 | 17f/29m | 21.9(4.9) | 1.69(0.2) | 76.8(17.6) | 2.74(0.8) | 96.0(4.1) | PT=31 STG=5 |
ACL-only=23 Meniscal Repair=11 Meniscectomy=2 |
78.0(59.1) | 87.3(13.1) | 218.7(31.3) |
Abbreviations: CAR, central activation ratio; E-only, eccentric-only; f, female; m, male; N-only; NMES-only; N&E, NMES and eccentrics; PT, bone-patellar-tendon-bone graft; STG, semitendinosus/gracilis graft; STND, standard of care
2.2 Design overview
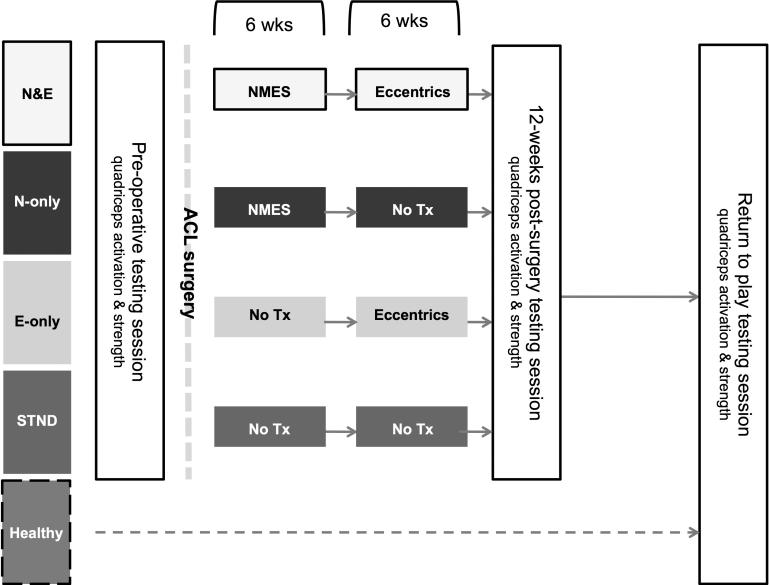
This study utilized a parallel longitudinal design, wherein ACL patients were placed into one of four treatment groups (Figure 2). All ACL patients were required to complete quadriceps activation and strength measures at three testing sessions (pre-operative, 12-week post-surgery and at return-to-play [RTP]). At the first post-operative rehabilitation appointment, patients in the combined NMES and eccentric exercise (N&E) and NMES (N-only) groups began the 6-week NMES therapy protocol. At six weeks post-operative, the N&E and eccentric (E-only) groups began the eccentric strengthening program. Patients in the standard of care (STND) group did not receive either the NMES or eccentric strengthening study protocol and underwent the standard ACL rehabilitation protocol that is utilized at our institution. Healthy participants participated in only one data collection where quadriceps activation and strength were assessed.
Figure 2.
Study testing timeline. Upon confirmation of ACL rupture, ACL patients were place into one of four groups. Prior to ACL reconstruction, all ACL patients underwent a pre-operative testing session consisting of quadriceps muscle strength and activation measurements. ACL patients in the combined NMES and eccentric exercise (N&E) and NMES (N-only) groups began the 6-week NMES therapy protocol immediately post-surgery. At six weeks post-operative, the N&E and eccentric (E-only) groups began the eccentric strengthening program. Patients in the standard of care (STND) group did not receive either intervention. Quadriceps strength and activation measurements were collected again at 12-weeks post-surgery as well as return-to-play. Healthy participants participated in only one testing session (return-to-play) where quadriceps activation and strength were measured.
Patients in all groups received the same basic rehabilitation protocol (Appendix A). The NMES and eccentric treatments were considered to be adjunct treatment(s) to the overall post-operative treatment plan. As such, we did not strictly control the basic rehabilitation protocol utilized at our institution. The NMES intervention was initiated immediately post-surgery at the first physical therapy appointment (average 6.5±2.9 days post-surgery to start of NMES) and the eccentric exercise intervention was delayed until each patient was at least six weeks post-reconstruction (average 42.5±3.4 days post-surgery to start of eccentrics). This was done to ensure each patient had adequate time to manage his or her pain, effusion, range of motion and quadriceps function.
2.3 Interventions
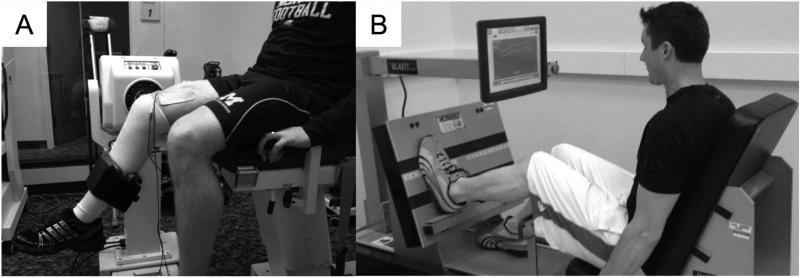
Patients placed into groups N&E and N-only received the NMES intervention two times per week for six weeks following ACL reconstruction (Figure 3, A). Patients in the STND and E-only groups did not receive this treatment. To deliver the therapy, patients were positioned in a Biodex Dynamometer (Biodex System 3, Biodex Medical Systems, Shirley, NY, USA) with their hips flexed to 90°, ACL knee flexed to 60° and their back supported. Self-adhesive, stimulating electrodes (Dura-Stick II [7 × 13cm] Chattanooga Group, Hixson, TN, USA) were placed over the vastus lateralis proximally and the vastus medialis distally. The Intelect Legend XT (Chattanooga Medical Supply, Chattanooga, TN) was set to deliver a 2500 Hz alternating current, modulated at 75 bursts per second, with a ramp-up time of 2 seconds, followed by a 50-second rest period [27]. Patients were encouraged to tolerate the stimulus at maximal tolerance level and to relax while the NMES was delivered in order to avoid voluntary quadriceps contraction and hamstring co-contraction (Appendix B: NMES intensity). Ten isometric actions lasting 10 seconds each were elicited during each session. This post-operative protocol has been demonstrated to improve quadriceps strength and activation in patients that have undergone total knee arthroplasty [28] and ACL reconstruction [29, 27].
Figure 3.
Patient receiving the NMES treatment (A) and performing the eccentric leg press (B).
Patients placed into Group N&E and E-only received eccentric exercise two times per week for six weeks, beginning at six weeks post-ACL reconstruction (Figure 3, B). N-only and STND patients did not receive this intervention. To perform the exercise, patients were positioned in a BLAST!™ Leg Press (BLAST! Leg Press version 1.2, Bio Logic Engineering Inc., Dexter, MI, USA) with their ACL-reconstructed knee range of motion limited to approximately 20 to 60° of knee flexion, following guidelines established by Gerber et al. [24]. Once correctly positioned in the Blast!™ Leg Press, patients performed a warm-up trial consisting of a series of ten isokinetic concentric and eccentric quadriceps actions with the ACL reconstructed limb. Following the warm-up trial, each participant performed ten isokinetic eccentric quadriceps actions with the ACL limb. In total, patients completed four sets of ten eccentric actions with two minutes rest in between each set. During the eccentric actions, patients were encouraged to train at an intensity equal to 60% of their eccentric one-repetition maximum (recorded at the beginning of each week) (Appendix C: Eccentric intensity) [30].
2.4 Outcomes
To assess quadriceps strength, patients were positioned with their hips flexed to 90°, their back supported, and their testing leg and torso strapped into the dynamometer. At each testing session, patients performed three knee extension maximal voluntary isometric (MVIC) trials with the knee flexed to 90°. The maximal knee extension torque produced across the three trials was normalized to body weight and used for analysis.
To quantify quadriceps activation the superimposed burst technique was utilized, wherein the peak torque recorded from the MVIC trials was inputted into a custom written program (LabVIEW version 8.5, National Instruments, Austin, TX, USA) set to deliver a supramaximal electrical stimulus (100 pulses/sec, 600μsec pulse duration, 10 pulse titanic train, 130 V) to the quadriceps muscle once the maximal knee extension torque had been reached and then subsequently dropped by one Nm [31]. The electrical stimulus was delivered through two self-adhesive stimulating electrodes (Dura-Stick II [7 × 13cm] Chattanooga Group, Hixson, TN, USA) applied over the vastus lateralis muscle proximally and the vastus medialis distally using a Grass S88 Dual Output Square Pulse Stimulator (S88, Grass Technologies, West Warick, RI, USA) with an SIU8T Transformer Stimulus Isolation Unit (SIU8T, Grass Technologies, West Warick, RI, USA) attached. Volitional activation of the quadriceps was determined using the central activation ratio (CAR) formula (Equation 1) wherein, the subject's peak torque generated immediately prior to the delivery of the stimulus was divided by the peak torque generated as a result of the electrical stimulus and multiplied by 100. A CAR of 100 was used to represent complete quadriceps activation [32]. The maximal CAR value (i.e. trial that displayed the least amount of QAF) that was collected was used for statistical analysis.
Equation 1 Central Activation Ratio
2.5 Statistical analysis
To ensure there were no differences in baseline parameters one-way ANOVAs were used examine the following variables between groups: time between sessions (post-injury to pre-operative test, post-surgery to 12-week test and post-surgery to return-to-play test), pre-operative quadriceps activation, strength, and subject demographics).
The primary outcome measures for this study were quadriceps strength (MVIC) and quadriceps activation (CAR). To detect the immediate and prolonged effectiveness of interventions, percent change scores (Equation 2) were calculated for quadriceps strength and activation between the 1) pre-operative and 12-week post-surgery time points (Pre-to-12wks), and between the 2) pre-operative and RTP (Preto-RTP) time points. The percent change score were then subsequently analyzed using one-way ANOVAs with post hoc independent t-tests to determine between group differences where appropriate. To determine if gains in quadriceps activation were a mechanism leading towards gains in strength, several separate linear regressions were utilized to assess the relationship between change in quadriceps activation and strength from 1) Pre-to-12wks and 2) Pre-to-RTP. To determine the effectiveness of interventions to restore ‘healthy and/or normal’ levels of quadriceps function, each treatment group's quadriceps strength and activation at RTP was compared to Healthy controls utilizing independent t-tests. Lastly, standardized effect sizes and 95% confidence intervals (CI) were calculated to determine if gains in quadriceps function that were detected between groups were clinically meaningful. Effect sizes were interpreted using the guidelines described by Cohen [33]. The α-level was set a priori at P≤0.05 for all tests. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software version 21.0 (IBM Corp., Armonk, NY, USA).
Equation 2 Percent Change
3. Results
3.1 Pre-operative quadriceps function and demographics
No significant differences in participant demographics were found between groups (P>0.05). Furthermore, no difference in pre-operative quadriceps function (Strength: P=0.94; QAF: P=0.61), time between injury and surgery (P=0.32) or between testing sessions (12-week post-surgery: P=0.17; RTP: P=0.59) were detected between treatment groups (Table 1).
3.2 Percent change scores- quadriceps activation
A significant change in quadriceps activation was detected between groups at the pre-operative to RTP time points (P=0.04), but not between groups at the pre-operative and 12-weeks post-surgery (P=0.21, Table 2) time points. Post hoc tests and effect sizes calculations revealed that E-only demonstrated significantly greater, and strong-clinically meaningful gains in quadriceps activation at RTP as compared to STND (P=0.01, d=1.25, CI=0.23, 2.26) and N-only (P=0.05, d=0.99, CI=0.01, 1.97). N&E demonstrated significantly greater, and strong-clinically meaningful gains at RTP as compared to STND (P=0.04, d=1.05, CI=0.06, 2.05). Compared to N-only, N&E demonstrated a trend towards improved quadriceps activation (P=0.09, d=0.84, CI=−0.13, 1.81) that was found to be clinically meaningful, but not statistically significant. No difference in quadriceps activation was detected between N&E and E-only (P=0.63, d=0.24, CI=−0.74, 1.23) or between N-only and STND (P=0.93, d=0.03, CI=−0.84, 0.91).
Table 2.
Quadriceps Central activation ratio (CAR [mean (SD)])
| Quadriceps CAR | Percent Change | ||||
|---|---|---|---|---|---|
| Group | Pre-operative | 12-weeks | RTP | Pre-to-12wks | Pre-to-RTP |
| N&E (n=8) | 95.7(3.5) | 94.8(4.2) | 97.6(2.8) | −1.1(6.4) | 1.6(3.9)† |
| N-only (n=10) | 97.4(3.0) | 91.4(8.4) | 91.8(4.6) | −7.2(8.3) | −3.4(7.3) |
| E-only (n=8) | 95.9(4.5) | 95.0(3.8) | 98.1(1.2) | −1.2(7.8) | 2.6(4.1)* |
| STND (n=10) | 94.9±(5.3) | 91.7(6.4) | 91.8(9.0) | −3.6(4.2) | −3.2(5.0) |
| Healthy (n=10) | - | - | 96.6(2.8)‡ | - | - |
P<0.05, compared to N-only and STND for Pre-to-RTP
P=0.04, compared to STND for Pre-to-RTP
P<0.05, compared to N-only and STND at RTP
3.3 Percent change scores- quadriceps strength
Significant changes in quadriceps strength were detected between groups at the pre-operative to 12-week post-surgery time points (P=0.02) and between groups at the pre-operative to RTP time points (P=0.02, Table 3). Post hoc tests and effect sizes calculations revealed that N&E demonstrated significantly greater, and strong-clinically meaningful gains in quadriceps strength at all post-operative time points as compared to N-only (Pre-op to 12wks: P=0.04, d=1.05, CI=0.06, 2.04; Pre-op to RTP: P=0.03, d=1.07, CI=0.08, 2.07) and STND (Pre-op to 12wks: P=0.01, d=1.41, CI=0.37, 2.45; Pre-op to RTP: P=0.02, d=1.14, CI=0.14, 2.14). No difference in quadriceps strength was detected between N&E and E-only (Pre-op to 12wks: P=0.29, d=0.51, CI=−0.45, 1.54; Pre-op to RTP: P=0.32, d=0.51, CI=−0.49, 1.50). E-only was found to be statistically and clinically stronger than STND (Pre-op to 12wks: P=0.08, d=0.87, CI=−0.10, 1.84; Pre-op to RTP: P=0.03, d=1.13, CI=0.13, 2.13) and N-only (Pre-op to 12wks: P=0.34, d=0.46, CI=−0.48, 1.40; Pre-op to RTP: P=0.05, d=0.97, CI=−0.01, 1.96) at RTP. Lastly, no differences in strength were detected between N-only and STND (Pre-op to 12wks: P=0.26, d=0.52, CI=−0.37, 1.41; Preop to RTP: P=0.92, d=0.04, CI=−0.83, 0.92).
Table 3.
Quadriceps strength (mean (SD))
| Quadriceps Strength (Nm/kg) | Percent Change | ||||
|---|---|---|---|---|---|
| Group | Pre-operative | 12-weeks | RTP | Pre-to-12wks | Pre-to-RTP |
| N&E (n=8) | 2.7(0.8) | 2.3(0.6) | 2.9(0.6) | −8.7(27.4)* | 15.9(39.4)* |
| N-only (n=10) | 2.6(0.6) | 1.7(0.3) | 2.1(0.6) | −31.3(15.2) | −17.4(22.5) |
| E-only (n=8) | 2.8(1.1) | 2.1(0.6) | 2.8(0.9) | −22.6(23.3) | 1.0(13.0)† |
| STND (n=10) | 2.6(0.6) | 1.5(0.5) | 2.1(0.6) | −39.4(16.1) | −18.3(19.8) |
| Healthy (n=10) | - | - | 3.1(0.9)‡ | - | - |
P<0.05, compared to N-only and STND for Pre-to-12wks and Pre-to-RTP
P=0.03, compared to STND for Pre-to-RTP
P<0.05, compared to N-only and STND at RTP
3.4 Relationship between change in quadriceps activation and strength
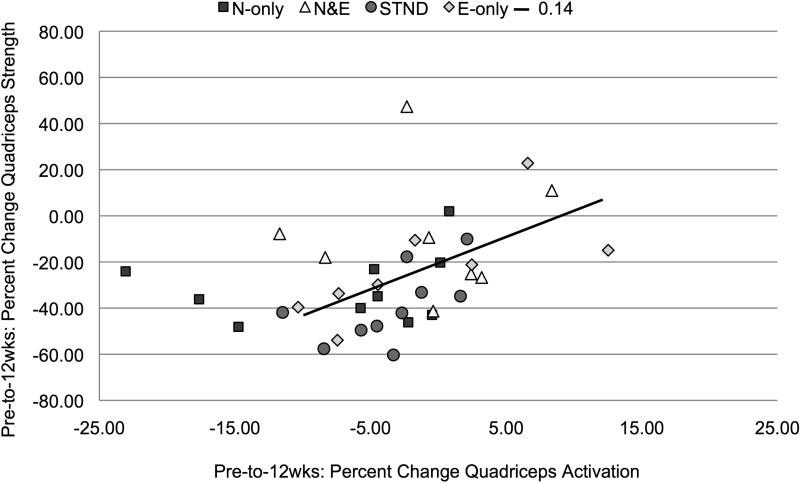
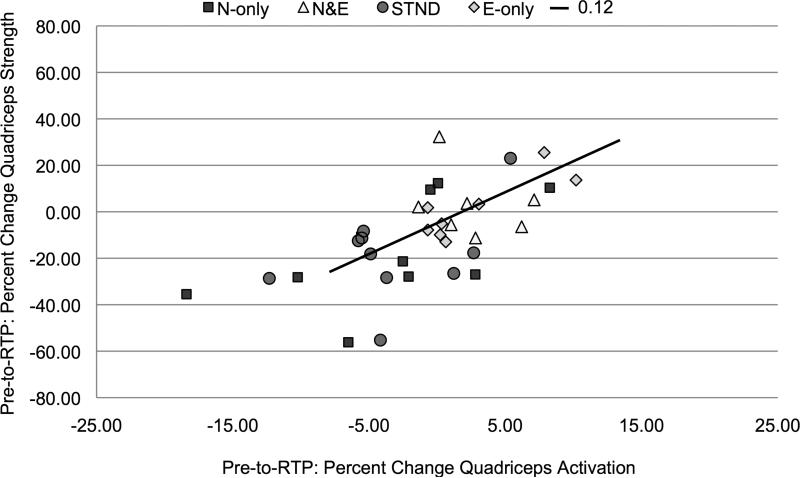
The change in quadriceps activation from Pre-to-12wks (R2=0.14, b=0.38, P=0.02, Figure 4) and from Pre-to-RTP (R2=0.12, b=0.35, P=0.03, Figure 5) was significantly related to improvements in quadriceps strength.
Figure 4.
Pre-operative-to-12-weeks post-surgery percent change in quadriceps activation plotted against percent change in quadriceps strength (P=0.02)
Figure 5.
Pre-operative-to-Return-to-Play percent change in quadriceps activation plotted against percent change in quadriceps strength (P=0.03).
3.5 Quadriceps activation and strength- compared to Healthy
As compared to Healthy, no differences in quadriceps strength or activation were detected for groups N&E (Strength: P=0.55, d=0.29, 95%CI=−0.65, 1.22, Table 2; QAF: P=0.46, d=0.36, CI=−0.58, 1.29, Table 3) and E-only (Strength: P=0.48, d=0.34, CI=−0.59, 1.28; QAF: P=0.18, d=0.66, CI=−0.29, 1.61) at RTP. At RTP, group N-only demonstrated significant, strong-clinically meaningful strength deficits and QAF as compared to Healthy (Strength: P=0.01, d=1.32, 95%CI=0.35, 2.29; QAF: P=0.01, d=1.24, CI=0.28, 2.20). Healthy was statistically and clinically stronger than STND at RTP (Strength: P=0.01, d=1.32, CI=0.35, 2.29), but no difference in activation was detected (QAF: P=0.12, d=0.71, CI=−0.19, 1.61).
4. Discussion
This prospective study used a combined NMES and eccentric exercise intervention to improve the recovery of quadriceps strength. It was found that individuals receiving the combined NMES and eccentric exercise intervention or eccentric exercise alone recovered quadriceps strength better than individuals that received just the NMES therapy or the standard of care following ACL reconstruction. Importantly, the combined intervention and eccentrics-only were capable of restoring levels of quadriceps activation and strength similar to those of healthy adults.
4.1 Quadriceps activation
The eccentric exercise therapy, not NMES, was the driving factor behind improvements in quadriceps activation post-ACL reconstruction (Table 2). This is a novel finding, and to our knowledge, this is the first study to directly compare these treatments. Our data agree with previous research performed by Brasileiro and colleagues [23] wherein improvements in quadriceps activation, measured via EMG, was found after six weeks of eccentric exercise post-ACL reconstruction and correlated with gains in quadriceps strength. As such, eccentric exercise seems to be an effective therapy that is capable of combating QAF, which aids in the restoration of quadriceps strength, better than NMES alone post-ACL reconstruction.
Our measure of quadriceps activation, assessed via the superimposed burst technique, indicates that there is an improvement in an individual's ability to volitionally utilize their quadriceps muscle, possibly occurring through improved alpha motorneuron recruitment and/or firing [32]. However, the CAR does not directly measure alpha motorneuron pool excitability[34], as such, the mechanisms leading to greater volitional activation post-eccentrics are unknown. Eccentric exercise is capable of improving quadriceps activation through selective recruitment of Type II muscle fibers [35]. Given that Type II fibers are thought to be selectively inhibited post-ACL reconstruction [36, 15], it seems plausible that eccentrics may improve the ability volitionally recruit the inhibited portions of the quadriceps, resulting in improved activation and greater force production.
Our results illustrate that NMES-only therapy produced a change in quadriceps activation similar to the STND group (Table 2). Given that previous research has shown NMES to be more effective than exercise alone at improving QAF [29, 26, 27], we had expected NMES patients to perform better than those receiving the standard of care. Furthermore, given that our NMES intervention was based on protocols that have improved quadriceps function in ACL reconstructed individuals previously [29, 27] and that all our patients were able to receive this therapy at maximal tolerance and in many instances at the equipment's maximal stimulus capacity (100 milliamps, Appendix B), this result was unexpected. Though many studies have found that NMES has a positive effect on quadriceps activation [19], there are some similar to ours demonstrating that NMES does not provide any additional benefits to quadriceps function above traditional exercise [37, 38]. We contend the disagreement in the literature in part exists due to the equipment utilized to deliver the NMES treatment. Specific to our study, the Intelect Legend XT was not powerful enough to override and recruit the inhibited alpha motoneurons despite being delivered at maximal output. In clinical trials, where NMES has been found to be effective, the VersaStim 380 and Empi 300 PV electrical stimulators (VersaStim 380, Electro-Med Healthy Industries, Miami Florida; Empi 300 PV, Empi, St Paul, MN) have been utilized [39, 40, 29, 27]. These devices are capable of producing significantly more stimulus output then the device we used. For instance, at maximal output (100 milliamps), our equipment was only capable of generating a training intensity of ~44% of the MVIC on the ACL reconstructed limb (Appendix B). In contrast, others have found that the VersaStim is capable of generating actions up to ~70% of the non-injured limb [40]. Thus, clinicians utilizing NMES to improve quadriceps activation may need to employ devices capable of generating high stimulus intensity (200 milliamps), which tend to be greater than the typical clinical electrical stimulation unit is able to deliver. Notably, the production of both the VersaStim 380 and Empi 300 PV have been discontinued and we are unaware of another device with similar stimulation capabilities to these units, which have proven beneficial post-ACL reconstruction.
4.2 Quadriceps strength
It is clear that eccentric exercise was the driving factor behind improvements in quadriceps strength, given that patients exposed to this therapy recovered quadriceps strength better than NMES-only or the standard of care post-ACL reconstruction (Table 3). Furthermore, patients that received eccentric treatments were able to restore quadriceps strength to pre-intervention levels (or better) and did not exhibit any strength differences when compared to healthy adults (Table 3). Taken together, these results suggest that eccentrics are an effective therapeutic approach to combating post-operative quadriceps strength deficits. Importantly, this finding is in agreement with previous research conducted in ACL reconstructed populations wherein eccentrics was found to be a superior strength training method as compared to concentrics [24, 25], and can last up to one year post-surgery [25]. From a clinical perspective, this is an significant finding, as quadriceps weakness is a factor that contributes to joint degeneration [5], leads to reduce knee function [41] and quality of life [42] post-ACL reconstruction.
4.3 Relationship of quadriceps activation and strength
Changes in quadriceps activation were found to positively influence the recovery of quadriceps strength, however, the relationship between these two variables was relatively low (Figures 4 and 5), suggesting that other mechanisms to improve strength were likely at work. Based on previous work in ACL reconstructed individuals [25, 23], and the proposed mechanisms of eccentrics exercise, it seems plausible that changes in quadriceps morphology may have contributed to changes in quadriceps muscle strength. Although alterations in muscle architecture and volume were not measured in our study, previous authors have found significant improvements in quadriceps muscle volume post-ACL reconstruction [25, 22]. Other morphological changes that may have aided in the restoration of quadriceps strength included differences in muscle architecture, such as a change in the angle of pennation, fiber length and possible sarcomeres adaptations [43]. In rats, eccentric exercise has been shown to increase the number of sarcomeres in a series [44], as well as decrease myostatin levels [45]. Myostatin is a protein that inhibits muscle growth, and has recently been found to be elevated in human quadriceps muscle following ACL reconstruction [46].
4.4 Limitations
This study is not without limitations. First, ACL patients enrolled in this study had a variety of graft types and concomitant meniscal injuries that in some cases required surgical intervention (Table 1). Based on our sample size, we are not powered to consider these variables as factors that may have affected the recovery of quadriceps function. Data emerging from our lab indicates that concomitant meniscal surgeries (meniscectomy or meniscal repair) does not affect the post-operative recovery of quadriceps strength or activation [47]. However, it is unclear whether or not graft type influences postoperative quadriceps strength, as the current literature reports inconsistent results. Thus, future investigations should continue to take graft type into consideration. It is also important to note that the leg press device that we utilized to deliver the eccentric treatment in this investigation is unique, and therefore is not a readily available clinical tool. Lastly, going forward, it is ideal for these treatments to be evaluated using a randomized control trial. Although no pre-operative differences in quadriceps function were detected, suggesting our study did not suffer from selection bias, to determine the true clinical effect, larger sample sizes and patient randomization is needed.
4.5 Clinical recommendations
Based on these results, our recommendation is that clinicians consider utilizing eccentric exercise as a means to improve quadriceps activation and strength post-ACL reconstruction. From a patient perspective, the utilization of eccentrics post-reconstruction to improve quadriceps function may be an attractive alternative to NMES, given that this type of intervention can be easily delivered (two treatment per week for six weeks), is safe, and is well tolerated by patients. In comparison, in order for NMES to be beneficial it must be delivered at high intensities (that may not be achievable by all clinical units) that are often uncomfortable and may reduce patient compliance. With that, it is important for therapists to consider the devices and time in which they decide to initiate the eccentric-strengthening program post-reconstruction. Our work utilized a closed kinetic chain device, which afforded us the ability to initiate an early post-operative intervention given that the potential for strain on the ACL graft was reduced as compared to an open-chain device [48]. In addition to these factors, the intensity of the treatment and dose is another important clinical consideration. Our work indicates that 6-weeks of eccentric strengthening (12 treatments in total) is adequate to produce prolonged strength gains. Further, we encouraged our patients to train at, or above 60% of their one repetition maximum.
5. Conclusion
Eccentric exercise post-ACL reconstruction was found to positively improve quadriceps activation and strength. Changes in quadriceps activation were positively related to changes in quadriceps strength, suggesting that by removing QAF, quadriceps strength should improve. NMES was not found to improve QAF or strength post-reconstruction. The inability of NMES to improve quadriceps muscle function may be the result of an inability to generate powerful muscle actions due to device limitations and post-operative pain. Importantly, when compared to healthy individuals, patients that were exposed to eccentric exercise were capable of restoring healthy levels of quadriceps activation and strength, whereas deficits in these measures still persisted for individuals not exposed to eccentric exercise.
Supplementary Material
Highlights.
Eccentric exercise restored levels of quadriceps function that were better than NMES.
Eccentrics are attractive alternative to NMES, as it is easily delivered, safe and well tolerated.
We report preliminary results of therapies that positiviely influence quadriceps function.
To determine the true clinical effect, larger sample sizes and patient randomization is needed.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number K08 AR05315201A2 to Dr. Palmieri-Smith. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3):405–24. doi: 10.1016/j.csm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt LC, Paterno MV, Hewett TE. The Impact of Quadriceps Femoris Strength Asymmetry on Functional Performance at Return to Sport Following Anterior Cruciate Ligament Reconstruction. J Orthop Sports Phys Ther. 2012;42(9):750–9. doi: 10.2519/jospt.2012.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keays SL, Bullock-Saxton J, Newcombe P, Keays AC. The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res. 2003;21:231–7. doi: 10.1016/S0736-0266(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 4.Hurley MV, Jones DW, Wilson D, Newham DJ. Rehabilitation of quadriceps inhibition due to isolated rupture of the anterior cruciate ligament. Journal of Orthopaedic Rheumatology. 1992;5:145–54. [Google Scholar]
- 5.Tourville TW, Jarrell KM, Naud S, Slauterbeck JR, Johnson RJ, Beynnon BD. Relationship Between Isokinetic Strength and Tibiofemoral Joint Space Width Changes After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2014;42(2):302–11. doi: 10.1177/0363546513510672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33(3):402–7. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: A limiting factor in joint rehabiliation. J Sport Rehab. 2000;9:135–59. [Google Scholar]
- 8.Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clinics in Sports Medicine. 2008;27(3):383–404. doi: 10.1016/j.csm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurley MV, Jones DW, Newham DJ. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Colch) 1994;86(3):305–10. doi: 10.1042/cs0860305. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29(5):633–40. doi: 10.1002/jor.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narici M, Cerretelli P. Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. Journal of gravitational physiology: a journal of the International Society for Gravitational Physiology. 1998;5(1):P73. [PubMed] [Google Scholar]
- 13.Kawakami Y, Muraoka Y, Kubo K, Suzuki Y, Fukunaga T. Changes in muscle size and architecture following 20 days of bed rest. Journal of gravitational physiology: a journal of the International Society for Gravitational Physiology. 2000;7(3):53. [PubMed] [Google Scholar]
- 14.Edstrom L. Selective atrophy of red muscle fibres in the quadriceps in long-standing knee-joint dysfunction:: Injuries to the anterior cruciate ligament. Journal of the neurological sciences. 1970;11(6):551–8. doi: 10.1016/0022-510x(70)90105-x. [DOI] [PubMed] [Google Scholar]
- 15.Lopresti C, Kirkendall DT, Street GM, Dudley AW. Quadriceps Insufficiency following Repair of the Anterior Cruciate Ligament. JOSPT. 1988;9(7):245–9. doi: 10.2519/jospt.1988.9.7.245. [DOI] [PubMed] [Google Scholar]
- 16.Lorentzon R, Elmqvist LG, Sjostrom M, Fagerlund M, Fuglmeyer AR. Thigh musculature in relation to chronic anterior cruciate ligament tear: muscle size, morphology, and mechanical output before reconstruction. Am J Sports Med. 1989;17(3):423–9. doi: 10.1177/036354658901700318. [DOI] [PubMed] [Google Scholar]
- 17.Trimble MH, Enoka RM. Mechanism underlying the training effects associated with neuromuscular electrical stimulation. Phys Ther. 1991;71:273–80. doi: 10.1093/ptj/71.4.273. [DOI] [PubMed] [Google Scholar]
- 18.Lake DA. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports medicine (Auckland, NZ) 1992;13(5):320. doi: 10.2165/00007256-199213050-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kim KM, Croy T, Hertel J, Saliba S. Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. The Journal of orthopaedic and sports physical therapy. 2010;40(7):383. doi: 10.2519/jospt.2010.3184. [DOI] [PubMed] [Google Scholar]
- 20.LaStayo PC, Woolf JM, Lewek MD, Snyder-Mackler L, Reich T, Lindstedt SL. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. The Journal of orthopaedic and sports physical therapy. 2003;33(10):557–71. doi: 10.2519/jospt.2003.33.10.557. [DOI] [PubMed] [Google Scholar]
- 21.Wilk KE, Reinold MM, Hooks TR. Recent advances in the rehabilitation of isolated and combined anterior cruciate ligament injuries. Orthop Clin North Am. 2003;34(1):107–37. doi: 10.1016/s0030-5898(02)00064-0. [DOI] [PubMed] [Google Scholar]
- 22.Gerber JP, Marcus RL, Dibble LE, Greis PE, Burks RT, LaStayo PC. Effects of early progressive eccentric exercise on muscle structure after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(3):559–70. doi: 10.2106/JBJS.F.00385. [DOI] [PubMed] [Google Scholar]
- 23.Brasileiro JS, Pinto OMSF, Avila MA, Salvini TF. Functional and morphological changes in the quadriceps muscle induced by eccentric training after ACL reconstruction. Revista Brasileira de Fisioterapia. 2011;15(4):284–90. doi: 10.1590/s1413-35552011005000012. [DOI] [PubMed] [Google Scholar]
- 24.Gerber JP, Marcus RL, Dibble LE, Greis PE, Burks RT, Lastayo PC. Safety, feasibility, and efficacy of negative work exercise via eccentric muscle activity following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2007;37(1):10–8. doi: 10.2519/jospt.2007.2362. [DOI] [PubMed] [Google Scholar]
- 25.Gerber JP, Marcus RL, Dibble LE, Greis PE, Burks RT, LaStayo PC. Effects of early progressive eccentric exercise on muscle size and function after anterior cruciate ligament reconstruction: a 1-year follow-up study of a randomized clinical trial. Physical Therapy. 2009;89(1):51–9. doi: 10.2522/ptj.20070189. [DOI] [PubMed] [Google Scholar]
- 26.Wigerstad-Lossing I, Grimby G, Jonsson T, Morelli B, Perterson L, Renstrom P. Effects of electrical muscle stimulation combined with voluntary contractions after knee ligament surgery. Med Sci Sports Exerc. 1988;20:93–8. doi: 10.1249/00005768-198802000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald GK, Piva SR, Irrgang JJ. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2003;33(9):492–501. doi: 10.2519/jospt.2003.33.9.492. [DOI] [PubMed] [Google Scholar]
- 28.Stevens JE, Mizner RL, Snyder-Mackler L. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004;34(1):21–9. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 29.Snyder-Mackler L, Ladin L, Schepsis AA, Young JC. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1991;73:1025–36. [PubMed] [Google Scholar]
- 30.Dwyer GB, Davis SE. ACSM's health-related physical fitness assessment manual. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 31.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. A clinical trial of neuromuscular electrical stimulation in improving quadriceps muscle strength and activation among women with mild and moderate osteoarthritis. Phys Ther. 2010;90(10):1441–52. doi: 10.2522/ptj.20090330. [DOI] [PubMed] [Google Scholar]
- 32.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19(7):861–9. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Analysis for the Behavioral Sciences. Academic Press; New York: 1977. [Google Scholar]
- 34.Stackhouse SK, Dean JC, Lee SC, Binder-MacLeod SA. Measurement of central activation failure of the quadriceps femoris in healthy adults. Muscle Nerve. 2000;23(11):1706–12. doi: 10.1002/1097-4598(200011)23:11<1706::aid-mus6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Hortobagyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG. Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol. 1996;80(3):765–72. doi: 10.1152/jappl.1996.80.3.765. [DOI] [PubMed] [Google Scholar]
- 36.Snyder-Mackler L, Binder-Macleod SA, Williams PR. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 1993;25:783–9. doi: 10.1249/00005768-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Draper V, Ballard L. Electrical stimulation versus electromyographic biofeedback in the recovery of quadriceps femoris muscle function following anterior cruciate ligament surgery. Phys Ther. 1991;71(6):455–61. doi: 10.1093/ptj/71.6.455. discussion 61-4. [DOI] [PubMed] [Google Scholar]
- 38.Paternostro-Sluga M, Fialka C, Alacamliogliu Y, Saradeth T, Fialka-Moser V. Neuromuscular electrical stimulation after anterior cruciate ligament surgery. Clin Orthop Relat Res. 1999;368:166–75. [PubMed] [Google Scholar]
- 39.Delitto AS, Rose J, Mckowen JM, Lehman RC, Thomas JA, Shively RA. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Phys Ther. 1988;71:455–64. doi: 10.1093/ptj/68.5.660. [DOI] [PubMed] [Google Scholar]
- 40.Snyder-Mackler L, Delitto A, Stralka S, Balley SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74(10):901–7. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 41.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 42.Logerstedt D, Lynch A, Axe MJ, Snyder-Mackler L. Pre-operative quadriceps strength predicts IKDC2000 scores 6 months after anterior cruciate ligament reconstruction. The Knee. 2013;(20):208–12. doi: 10.1016/j.knee.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle & Nerve. 2000;23:1647–66. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield TA, Leonard TR, Herzog W. Differential serial sarcomere number adaptations in knee extensor muscles of rats is contraction type dependent. Journal of Applied Physiology. 2005;99(4):1352–8. doi: 10.1152/japplphysiol.00481.2005. [DOI] [PubMed] [Google Scholar]
- 45.Ochi E, Nakazato K, Ishii N. Muscular hypertrophy and changes in cytokine production after eccentric training in the rat skeletal muscle. The Journal of Strength & Conditioning Research. 25(8):2283–92. doi: 10.1519/JSC.0b013e3181f1592e. [DOI] [PubMed] [Google Scholar]
- 46.Mendias CL, Lynch EB, Davis ME, Enselman ERS, Harning JA, DeWolf PD, et al. Changes in Circulating Biomarkers of Muscle Atrophy, Inflammation, and Cartilage Turnover in Patients Undergoing Anterior Cruciate Ligament Reconstruction and Rehabilitation. The American journal of sports medicine. 2013 doi: 10.1177/0363546513490651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lepley LK, Wojtys EM, Palmieri-Smith RM. Does Concomitant Meniscectomy or Meniscal Repair Affect the Recovery of Quadriceps Function Post-ACL Reconstruction? Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-014-3093-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henning CE, Lynch MA, Glick KR. An in vivo strain gage study of elongation of the anterior cruciate ligament. The American journal of sports medicine. 1985;13(1):22–6. doi: 10.1177/036354658501300104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.