Abstract
We tested the hypothesis that Pseudomonas aeruginosa type 3 secretion system effectors exoenzymes Y and U (ExoY and ExoU) induce release of a high-molecular-weight endothelial tau, causing transmissible cell injury characteristic of an infectious proteinopathy. Both the bacterial delivery of ExoY and ExoU and the conditional expression of an activity-attenuated ExoU induced time-dependent pulmonary microvascular endothelial cell gap formation that was paralleled by the loss of intracellular tau and the concomitant appearance of high-molecular-weight extracellular tau. Transfer of the high-molecular-weight tau in filtered supernatant to naïve endothelial cells resulted in intracellular accumulation of tau clusters, which was accompanied by cell injury, interendothelial gap formation, decreased endothelial network stability in Matrigel, and increased lung permeability. Tau oligomer monoclonal antibodies captured monomeric tau from filtered supernatant but did not retrieve higher-molecular-weight endothelial tau and did not rescue the injurious effects of tau. Enrichment and transfer of high-molecular-weight tau to naïve cells was sufficient to cause injury. Thus we provide the first evidence for a pathophysiological stimulus that induces release and transmissibility of high-molecular-weight endothelial tau characteristic of an endothelial proteinopathy.
Keywords: proteinopathy, aggregation, microtubules, infection, pneumonia
pulmonary microvascular endothelium forms a semipermeable barrier that separates blood from the underlying tissue and optimizes gas exchange across capillaries (43, 44, 47, 62). Endothelial barrier integrity is maintained by adherens junctions and focal adhesions, which oppose the inward tension that is generated by actomyosin interactions (47). Microtubules provide an outward (centrifugal) force that also opposes endothelial contraction. Loss of junctional apposition, increased centripetal tension, and microtubule breakdown disrupt the endothelial barrier, which allows macromolecules, solutes, and water to access the interstitial compartment and, ultimately, the alveoli, resulting in alveolar flooding, which represents an important cause of arterial hypoxemia. Bacteria and viruses possess virulence factors that disrupt the endothelial cell barrier and contribute to development of acute lung injury. In many of these cases, however, the nature of the interaction between pathogen-encoded factors and the host cell is poorly understood; consequently, the mechanisms responsible for barrier disruption remain unclear.
Pseudomonas aeruginosa infection is a principal cause of acute pneumonia that can progress to sepsis and acute lung injury (32), especially in immunocompromised patients (12, 22, 37). P. aeruginosa is also responsible for chronic colonization of the airways of cystic fibrosis patients, where it resides in a mucoid biofilm (61). In the acute form of the infection, virulence is highly dependent on expression of a type 3 secretion system (T3SS) (14, 34). The T3SS is a needle apparatus that extends across the bacterial membrane to insert pore proteins into the host cell membrane (see Ref. 24 for review and references). This needle-pore protein complex is used to introduce or inject linear exoenzyme proteins directly into host cells. Once inside the host cell, exoenzymes form their tertiary structure, associate with mammalian cofactors, and acquire activity that modifies cellular physiology. These changes are postulated to favor bacterial replication, survival, or dissemination by inhibiting innate immunity, although the molecular events responsible for such interkingdom communication remain largely unknown.
Four P. aeruginosa T3SS effectors, exoenzyme S (ExoS), exoenzyme T (ExoT), exoenzyme U (ExoU), and exoenzyme Y (ExoY), have been described (16). Among these effector proteins, ExoU and ExoY have recently garnered considerable attention, because ExoU is a phospholipase that is highly cytotoxic (71) and because ExoY is a soluble purine and pyrimidine cyclase (41, 59, 72) that is found in ∼90% of P. aeruginosa isolates (17). P. aeruginosa gains access to pulmonary endothelium through the general circulation or following disruption of the alveolar epithelium. Under these conditions, infection causes extensive endothelial barrier disruption, with fluid accumulation in the interstitial compartments and alveoli.
ExoY's enzymatic activity is sufficient to disrupt the endothelial cell barrier; it causes endothelial cell rounding, loss of cellular adhesions, generation of interendothelial cell gaps, and tissue edema (41, 55, 72). These cellular effects depend on the ability of ExoY to generate intracellular cyclic nucleotides, including cAMP, cGMP, and cUMP (41, 59, 72). While we know that the ExoY-dependent production of cAMP best correlates with cell rounding (41, 46, 55) and that activation of other soluble adenylyl cyclases mimics these cellular effects (46, 54), the physiological function(s) of cGMP and cUMP remain(s) poorly understood. Nonetheless, the ExoY cyclic nucleotide signature activates intracellular protein kinases A and G (41), which cause endothelial tau phosphorylation and insolubility. Hyperphosphorylation of tau dissociates it from microtubules, leading to microtubule breakdown; this is the only known bacterial virulence mechanism targeting microtubules. Microtubule breakdown is not caused by an increase in the rate of microtubule disassembly or a decrease in the rate of centrosome nucleation; rather, it is due to impairment of microtubule assembly (5). Hence, the ExoY-microtubule interaction represents an important node for host-pathogen communication.
This host-pathogen interaction elicits long-lasting deleterious effects. ExoY exposure reduces endothelial cell migration and proliferation, and it decreases endothelial cell barrier function, even 1 wk after infection (63). The reason for such long-lasting deleterious effects is unclear, although studies in dementia models may provide some insight. Hyperphosphorylated, insoluble tau oligomerizes within neurons (8, 48) and can be released into the extracellular space (52). Nearby cells endocytose oligomerized tau, and the abnormal oligomer nucleates monomeric tau as a mechanism of disease propagation (19, 28). These data suggest that ExoY-induced tau hyperphosphorylation could generate high-molecular-weight forms of tau that are released as a mechanism of disease propagation.
While hyperphosphorylation causes tau insolubility and oligomerization, phosphorylation is not the only stimulus for tau oligomer formation. In biochemical assays, addition of free arachidonic acid to purified tau also induces oligomerization (30, 70). Although free arachidonic acid is commonly used to generate tau oligomers in vitro, a physiologically relevant arachidonic acid stimulus responsible for tau oligomerization has not been identified in intact cells or in tissues.
In the studies described here, two separate stimuli were used to initiate extracellular high-molecular-weight endothelial tau: hyperphosphorylation and arachidonic acid exposure; ExoY induces tau hyperphosphorylation (41), while ExoU generates arachidonic acid (51). Therefore, in the present study we tested the hypothesis that the P. aeruginosa T3SS effectors ExoY and ExoU are sufficient to induce interendothelial cell gaps with concomitant release of high-molecular-weight endothelial tau into the extracellular space. We also tested whether supernatant containing high-molecular-weight endothelial tau is injurious to uninfected control cells in cell culture and the intact lung, characteristic of a transmissible proteinopathy. Our findings support this idea and suggest that P. aeruginosa T3SS effectors cause a transmissible endothelial proteinopathy that may contribute to end-organ dysfunction.
MATERIALS AND METHODS
Cell culture.
Rat pulmonary microvascular endothelial cells (PMVECs) were obtained from the cell culture core at the University of South Alabama Center for Lung Biology. Isolation and characterization of these cells are described elsewhere (29, 42). Cells were cultured as described previously (41). Briefly, they were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (catalog no. 10082, Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (catalog no. 15140, Invitrogen) at 37°C in 21% O2 and 5% CO2.
Bacterial strains.
P. aeruginosa strains are described in detail elsewhere (55, 72). Five strains of P. aeruginosa were used: one that expresses ExoU and ExoT toxins (PA103), one with an active ExoY toxin (PA103 exoUexoT::Tc pUCPexoY; ExoY+), one with an inactive ExoY exotoxin (PA103 ΔexoUexoT::Tc pUCPexoYK81M; ExoYK81M), one that lacks PcrV required for a functional T3SS (ΔPcrV), and one that has a functional T3SS but contains none of the four effector toxins (PA103 exoUexoT::Tc; ΔUΔT). Bacteria were taken from frozen stocks, grown overnight on solid agar-carbenicillin (400 μg/ml), and resuspended in PBS to an optical density at 540 nm of 0.25, which was previously determined to equal 2 × 108 bacteria/ml (55). Bacteria were subsequently diluted in Hanks' balanced salt solution (HBSS) to achieve the desired multiplicity of infection (MOI).
For bacterial infection, endothelial cells were trypsinized and counted using a Countess automated cell counter (model C10227, Invitrogen) according to the manufacturer's instructions. Endothelial cells were grown to 12–24 h postconfluence and then infected with P. aeruginosa ExoY+, PA103, ΔPcrV, or ΔUΔT at a MOI of 20:1 in HBSS and incubated for ≤8 h at 37°C in 21% O2 and 5% CO2.
Generation of the L618 ExoU mutant.
ExoUL618 was identified as an insertional mutation within exoU, as described previously (56). Briefly, 15 nucleotides were inserted randomly by transposon mutagenesis into an expression plasmid encoding a parental copy of exoU. Transposon sequences were resolved, and the resulting plasmids were transformed into P. aeruginosa strain PA103 ΔexoUexoT::Tc. Molecules with in-frame five-amino acid insertions were phenotypically characterized. Linker insertion L618 encodes a derivative of ExoU that contains an in-frame insertion encoding the five amino acids CLNTL, followed by the remaining COOH-terminal region of the parental molecule. This insertion diminishes, but does not eliminate, phospholipase activity, allowing examination of the initial stages of ExoU cellular intoxication.
Preparation of microtubule pellets.
Microtubules were isolated from PMVECs by procedures described elsewhere (4, 67). Briefly, cells were collected by trypsinization of four 150-cm2 confluent plates of PMVECs. Cells were rinsed once in 4°C PBS and once in 4°C PEM microtubule buffer (80 mM PIPES, pH 6.8, 1 mM EGTA, and 1 mM MgCl2) and then homogenized at 4°C in an equal volume of PEM buffer supplemented with 0.1 mg/ml GTP and 1 μg/ml each of chymostatin, leupeptin, antipain, and pepstatin. After centrifugation at 100,000 g for 90 min at 4°C, the supernatant was collected and then made 0.5 mg/ml in GTP and 10 μM with taxol. The supernatant was warmed to 37°C for 30 min to induce assembly of microtubules and then was overlaid on a cushion of 10% sucrose in PEM buffer that was supplemented with GTP and taxol. The preparation was centrifuged at 100,000 g for 40 min at 30°C, and the pellet was rinsed once with PEM buffer supplemented with taxol and GTP and then collected.
Mass spectrometry analysis.
The proteins in the microtubule pellet were separated by one-dimensional SDS-PAGE and stained with Coomassie blue. Another SDS-polyacrylamide gel was used for Western blotting with tau-specific antibodies. Bands of interest were excised, and the proteins were digested in situ with trypsin (Promega). The digests were analyzed by capillary high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry on a Thermo Fisher LTQ Orbitrap Velos mass spectrometer, as described elsewhere (58). Identified proteins were cross-referenced with the Mascot results, and protein and peptide identity probabilities were determined using Scaffold (Proteome Software). Two peptides were assigned to rat brain tau in the database analysis of the mass spectrometry results.
Generation of the ExoU-myc-FKBP-inducible cells.
To conditionally control gene and protein expression, we developed a two-tier control protein expression system to conditionally express ExoU mutants by combining doxycyline-inducible gene expression (2) with protein stability regulation with small molecules (7, 21, 36). This system is described in detail elsewhere (41). The lentiviral vector pMA3236 was constructed as follows. First, translation termination codons were removed from an L618 mutant of the P. aeruginosa exoU gene. Next, a terminatorless ExoU L618 was inserted between MluI and SpeI of pMA3174, a lentiviral vector that allows for the COOH-terminal fusions to myc-tag and destabilizes the E31G R71G K105E mutant FKBP (41). This construct also contains a doxycycline-inducible promoter for conditional expression of ExoU L618. Lentivirus-containing supernatant (ltMA3236) was produced as described previously (2) and used to infect MV2641 cells (2), thereby creating a PMVEC line transduced with two viruses, rv2641 and lt3236. Internal institutional labeling of the resulting cell line is MV2641/ltMA3236 for ExoUL618. Cells expressing both constructs were selected with puromycin at 10 μg/ml until a homogeneous population was established.
ExoU induction.
PMVECs were engineered to conditionally (Tet-On) express an activity-attenuated mutant ExoU encoding both myc and protein degradation domain epitopes, as described above. Confluent cells were washed once for 5 min at 37°C in HBSS. Thereafter, ExoU transcription was induced over a range of doxycycline concentrations (0.01–0.5 μg/ml), and protein degradation was prevented using Shield1 (2 μM; catalog no. 632189, Clontech Laboratories, Mountain View, CA) diluted in HBSS.
Supernatant transfer experiments.
Supernatant was generated under four separate conditions. All inductions/infections were performed in HBSS buffer. PMVECs were inoculated with the ExoY+, ExoYK81M, ΔPcrV, ΔUΔT, or PA103 strain at a MOI of 20:1. ExoU expression was induced using 0.1 μg/ml doxycyline and 2 μM Shield1. When the PMVEC monolayer exhibited significant gap formation, yet was healthy enough to remain attached to the dish, supernatants were obtained 7–8 h after ExoY+ or ExoYK81M infection, 4 h after PA103 or ΔUΔT infection, and 6 h after ExoU induction. Control supernatant was generated by incubation of PMVECs in HBSS for a length of time appropriate for the condition. Supernatant was collected and centrifuged at 4,500 g for 10 min at room temperature to pellet cell debris. Supernatant was collected, and phosphatase and protease inhibitors were added (see Antibodies and immunoblot). Then supernatant was passed through a 0.22-μm low-protein-binding filter (catalog no. SLGPM33RS, EMD Millipore, Billerica, MA) and either added directly to PMVECs or stored at −20°C. Naïve PMVECs (cells that were not subjected to an infection or induction) were grown to 12–24 h postconfluence and rinsed in HBSS at 37°C for 15 min before addition of supernatant. Images were captured at 6–24 h after supernatant addition to monitor injurious effects. For network formation assays, growth factor-reduced Matrigel (catalog no. 356237, Corning Life Sciences, Tewksbury, MA) at 120 μl/well was used to coat a 48-well plate. After 1 h at 37°C to solidify Matrigel, 40,000 PMVECs per well were added in 200 μl of full DMEM culture medium. Networks were allowed to form for 6–24 h at 37°C, culture medium was removed, 100 μl of supernatant were mixed with 100 μl of fresh culture medium, and the mixture was added to PMVEC networks. Each supernatant was tested in duplicate, and images (4 per well) were captured at 24 h after supernatant addition to monitor network stability.
Antibody capture experiments.
Supernatant was taken from ExoU induction experiments and treated as described above (see Supernatant transfer experiments). Aliquots of filtered supernatant were added to separate tubes for each condition. Increasing concentrations (10–50 μg/ml) of tau oligomer complex (TOC1), pan tau (Tau-5), and caspase-cleaved specific tau (TauC3) antibodies were added separately to supernatant aliquots and incubated for 1 h at 37°C on a rocker. Confluent PMVECs were rinsed for 15 min at 37°C with HBSS and treated with supernatant, and images were captured to evaluate injury. A subsequent experiment was performed with TOC1 where the 1-h incubation step was followed by addition of 100 μl of protein A agarose beads (catalog no. 20333, Thermo Scientific, Rockford, IL) before another 1-h incubation at 37°C on a rocker. Then the beads were pelleted by centrifugation, and supernatant was collected. Confluent PMVECs were rinsed for 15 min at 37°C with HBSS before treatment with supernatant, and images were captured to evaluate injury.
Electroelution experiments.
Supernatant (ExoU and control; see Supernatant transfer experiments) was generated in HBSS and collected and centrifuged at 4,500 g for 10 min at room temperature to pellet cell debris, and phosphatase and protease inhibitors were added. After addition of inhibitor cocktail, supernatant was passed through a 0.22-μm low-protein-binding filter and stored at −20°C. Supernatant was concentrated 10-fold using Amicon spin filters (catalog no. UFC801024, Millipore) by centrifugation at 3,100 g at 4°C, 6× Laemmli sample buffer (catalog no. BP-111R, Boston BioProducts, Worcester, MA) was added, and supernatant was resolved in nine wells of three 4–12% Bis-Tris polyacrylamide gels. After electrophoresis, a scalpel was used to excise a 74- and/or 37-kDa protein band (∼0.3–0.4 cm from top to bottom) from all gels, and gel pieces were minced. Gel pieces were added to a dialysis bag in the presence of ∼1 ml of PBS. Proteins were eluted at 40 mA for 45 min out of the gel into the PBS buffer in the dialysis bag. Buffer was collected and centrifuged at 4,500 g for 10 min at room temperature to pellet remaining gel pieces. Buffer was placed in a fresh dialysis bag, dialyzed at 4°C for 24–36 h in HBSS, diluted to ∼3 ml with HBSS, and filter-sterilized. This buffer was further diluted 1:1 with sterile HBSS before transfer to naïve PMVECs. Naïve PMVECs were grown to 12–24 h postconfluence and rinsed in HBSS at 37°C for 15 min before addition of supernatant.
Antibodies and immunoblot.
Cell lysates were generated by rinsing with cold (4°C) 1× PBS followed by lysis with RIPA buffer (catalog no. BP-115, Boston BioProducts) with protease inhibitor cocktail (1:10 dilution; catalog no. 11697498001, Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitors (1:100 dilution; phosphatase inhibitor cocktails I and II, catalog nos. BP-479 and BP-480, Boston BioProducts). Cell lysates were normalized for protein concentration using the Lowry protein assay kit (procedure no. P5656, Sigma-Aldrich, St. Louis, MO), resolved in 4–12% Bis-Tris polyacrylamide gels (catalog no. NP0321, Invitrogen), and then transferred to 0.2-μm polyvinylidene difluoride membranes (catalog no. 162-0177, Bio-Rad, Hercules, CA). Membranes were incubated with appropriate antibodies [Tau-5 at 1:1,000 dilution (catalog no. AT-5004, MBL International, Woburn, MA); Myc-Tag (9B11) at 1:1,000 dilution (catalog no. 2276, Cell Signaling, Danvers, MA); β-actin at 1:500 dilution (catalog no. sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA); and TOC1 at 1:500 dilution (a gift from Dr. Lester Binder's laboratory)]. All antibodies were diluted in 5% BSA or 5% milk according to the manufacturer's instructions, and membranes were incubated overnight at 4°C, probed with species-appropriate horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution) for 1 h at room temperature, and developed using SuperSignal West Femto chemiluminescent substrate (catalog no. 34096, Thermo Scientific, Rockford, IL).
Densitometry analysis.
Densitometry was performed using ImageJ 1.47; a region of interest was applied over the detected protein bands, and the digital intensity of the signal in the appropriate channel was determined. The same-size region of interest was used for each band within a given blot. The protein intensity of the bands was quantified by densitometry and calculated as an area (arbitrary units) or as a ratio to the corresponding β-actin loading control (catalog no. sc-47778, Santa Cruz Biotechnology). Where appropriate, all values were statistically compared with baseline conditions.
Immunofluorescence.
PMVECs were fixed with 2% paraformaldehyde for 30 min at room temperature and then blocked for 45 min with 100 mM glycine. Cells were rinsed in PBS, treated with TOC1 antibody (1:2,000 dilution) for 1 h, rinsed again in PBS, and then incubated with FITC-anti-mouse IgM (1:250 dilution) for 60 min. After a final rinse in buffer containing Hoechst (catalog no. B1155, Sigma, St. Louis, MO), the cells were mounted in 1:1 PBS-glycerol and observed with a high-resolution digital fluorescence imaging system based on a Nikon Eclipse TE2000-U inverted microscope. The imaging system is fitted with two automated 10-position filter wheels for both excitation and emission (model Lambda 10-2, Sutter Instruments), an automated dichroic filter cube changer (Nikon), and a xyz-axis mechanized stage (Prior Scientific). Specifications for the ×60 objective are as follows: Nikon ×60 A/1.40 0.1 Plan-Apochromat DIC H WD 0.21 ∞/0.21. Images were captured using a high-resolution 12-bit digital camera (Orca-100 ER IEEE1394, Hamamatsu) at 20–25°C. PMVECs were grown on glass coverslips and immersed in immersion oil (catalog no. 16212, Cargille Laboratories, Cedar Grove, NJ) at the time of image acquisition. Software was MetaMorph 7.8 (Molecular Devices). The fluorochrome green fluorescent protein with excitation spectrum of 480–510 nm was used.
Isolated lung and assessment of endothelial permeability.
Animals were anesthetized using pentobarbital sodium (Nembutal; 65 mg/kg body wt). Once a surgical plane was achieved, as defined by the absence of a withdrawal reflex following toe and tail pinch, animals were intubated and ventilated, a sternotomy was performed, and pulmonary artery and left ventricular catheters were placed. Blood was taken by heart puncture from the right ventricle. Heart and lungs were removed en bloc and suspended in a humidified chamber, where mechanical ventilation and blood flow were established. Rat lungs were perfused at constant flow (40 ml·min−1·kg body wt−1) with buffer (in mmol/l: 119.0 NaCl, 4.7 KCl, 1.17 MgSO4, 1.0 Na2HPO4, 1.18 KH2PO4, 2.2 NaHCO3, and 5.5 glucose) containing 4% autologous BSA and 6% whole blood in a 50-ml total volume and physiological (2.2 mmol/l) CaCl2 at pH 7.35 at 38°C. After the lungs were perfused for 15 min to reach an equilibrated status, 1 ml of control or ExoU supernatant (concentrated 10-fold) was slowly infused into the lung circulation by injection into the inflowing perfusate and circulated for 2 h; then the filtration coefficient (Kf) was measured as previously described (3) using zone 3 conditions. Kf was calculated as the rate of weight gain obtained 13–15 min after a 10-cmH2O increase in pulmonary venous pressure, normalized per 100 g of predicted wet lung weight. Kf, the product of specific endothelial permeability and surface area for exchange, is a sensitive measure of lung endothelial permeability when surface area is fully recruited.
Image acquisition and processing.
Phase-contrast microscope images were acquired using a Nikon Eclipse TS100 microscope. Images were captured using a Nikon Digital Sight DS-5M camera (model no. 121099) at 20–25°C. PMVECs were grown and retained in DMEM or HBSS at the time of image acquisition. Specifications for the objectives are as follows: Nikon 4×/0.10 WD 30 ∞/− for the ×4 objective, Nikon 10×/0.25 Ph1 ADL WD 6.2 ∞/1.2 for the ×10 objective, and Nikon LWD 20×/0.40 Ph1 ADL WD 3.0 ∞/1.2 for the ×20 objective. Software was Nikon Digital Sight DS-L1. After image acquisition, brightness, contrast, setting to gray scale, and crop processing were achieved using Microsoft Powerpoint Mac 2008. Gamma settings were not altered at any point.
Statistical analysis.
Statistical analyses of isolated perfused lung permeability (Kf) and Matrigel-enclosed network data were determined as follows. Statistical differences between groups were assessed using Student's t-test in GraphPad Prism 5 software. Statistical analysis of immunoblot densitometry values was determined by one-way ANOVA and Dunnett's multiple-comparison test to compare values with baseline conditions, where appropriate. Statistical significance was determined if the analysis reached 95% confidence.
Ethical considerations.
All animal studies were approved by the University of South Alabama Institutional Animal Care and Use Committee.
RESULTS
PMVECs express an endothelial form of the microtubule-associated protein tau.
Neuronal and nonneuronal forms of tau are described elsewhere (11, 66), although expression of an endothelial cell tau is not widely acknowledged. Our previous studies using P. aeruginosa ExoY indicate that it induces a microtubule-dependent permeability defect. ExoY generates cyclic nucleotides that cause phosphorylation of an endothelial form of the microtubule-associated protein tau. This endothelial cell tau is recognized by several tau antibodies at 37 kDa, including Tau-5 and a pS214-tau antibody (41). To rigorously substantiate expression of an endothelial tau, we prepared microtubule pellets as described above (see Preparation of microtubule pellets). Microtubules were assembled from PMVEC extracts using taxol and then pelleted through a sucrose cushion. The proteins in the microtubule pellet were separated by one-dimensional SDS-PAGE, the band reactive to the anti-tau antibody was digested with trypsin (Fig. 1A), and the digest was subjected to mass spectrometry analysis. Two peptides were assigned to rat brain tau in the database analysis of the mass spectrometry results (Fig. 1B).
Fig. 1.
Pulmonary endothelium possesses the microtubule-associated protein tau. A taxol-stabilized microtubule preparation was analyzed by gel electrophoresis and then by mass spectrometry. A: whole cell lysate was harvested from pulmonary microvascular endothelial cells (PMVECs), and microtubules were stabilized using taxol. This preparation was subjected to gel electrophoresis and Coomassie stain. Protein bands were excised and subjected to trypsin digestion and mass spectrometry analysis. Blue arrows indicate the specific bands that were excised (∼50 and ∼39 kDa). B: 2 exclusive unique peptide sequences for the microtubule-associated protein tau in pulmonary endothelium (blue) and the sequence that has been cloned by RT-PCR (gray) between the 2 unique peptide sequences.
PCR primers were generated on the basis of the obtained peptide sequences, and RT-PCR was performed using RNA obtained from rat PMVECs. A RT-PCR product of the expected size was obtained, and the product was sequenced. Cloning of the RT-PCR product revealed 100% identity with the sequence of rat brain tau (data not shown), confirming that the 37-kDa band is indeed an endothelial form of tau.
ExoY+ infection produces injurious supernatant that contains TOC1-immunoreactive proteins.
ExoY generates a cyclic nucleotide signature that activates protein kinases A and G, resulting in hyperphosphorylation and insolubility of the endothelial tau (41). In neural disease, tau hyperphosphorylation and insolubility lead to its oligomerization, which is a hallmark of neurodegenerative tauopathies (8, 48). However, in previous studies we were unable to detect ExoY-induced tau oligomerization in endothelial cell lysates, an effect that was attributed to phosphorylation at Ser214, because tau phosphorylation at this site has been shown to inhibit aggregation (57). Recently, Ward et al. (68, 69) developed TOC1, an antibody that recognizes oligomerized tau with high specificity and sensitivity. In addition, new evidence indicates that hyperphosphorylated, insoluble tau may be released from cells and either accumulate in the extracellular space or be endocytosed by adjacent cells (52). The idea that endothelial tau could be released from cells in a high-molecular-weight, injurious form represents a mechanism of host-pathogen interaction not previously considered.
To test the hypothesis that ExoY induces an injurious agent that can be transferred to naïve endothelial cells, we infected PMVECs with ExoY+, a strain of P. aeruginosa that injects only ExoY derivatives (MOI of 20:1). During an 8-h time course, we examined cell shape and measured TOC1 immunoreactivity. ExoY+ infection caused progressive interendothelial cell gap formation over the time course of the infection (Fig. 2A). Endothelial cell gaps visible by light microscopy were first noted after 3 h, which is consistent with previous accounts (39, 41, 55). PMVECs rounded and lost cell adhesions, leaving only membranous strands as a means of cell-cell communication. In the early stages of infection, cell gaps formed in discrete cellular regions, and these gaps expanded into cavitary-like lesions. However, as the infection progressed, cell borders were uniformly disrupted throughout the monolayer. These findings are consistent with the idea that ExoY functions as an edema factor (55).
Fig. 2.
ExoY+ infection decreases intracellular endothelial tau abundance while producing injurious supernatant containing high-molecular-weight tau. PMVECS were inoculated with the ExoY+ bacterial strain in Hanks' balanced salt solution (HBSS) for 8 h at a multiplicity of infection (MOI) of 20:1. A: beginning at 3 h after inoculation with ExoY+, gap formation increased in a time-dependent manner. B: culture medium was collected at 1-h intervals from 0 (baseline) to 8 h. After removal of bacteria, PMVECs were lysed, and both fractions were prepared for immunoblotting. ExoY+ infection caused a decrease in intracellular tau by 1 h that continued until 8 h. MW, molecular weight. C: at 7–8 h postinfection, supernatant from PMVECs infected with ExoY+ contained extracellular monomeric and high-molecular-weight tau. D: filtered supernatant collected 8 h postinfection (ExoYK81M or ExoY+) was transferred to naïve PMVECs, and images of injurious effects were captured 6 h posttransfer. E: PMVECs (40,000 cells per well) were seeded in Matrigel for 24 h to allow network formation. After 24 h, filtered supernatant collected 8 h postinfection was mixed in a 1:1 ratio with serum-containing culture medium and added to naïve PMVECs in Matrigel. Images of injurious effects were captured 24 h posttransfer. Images in A and D are each representative of ≥3 separate experiments. Images in E are representative of 2 separate experiments (cells treated with supernatant from 2 separate experiments). Images in A and D were captured at ×20 magnification, with scale bar = 10 μm. Arrows indicate interendothelial cell gaps. Images in E were captured at ×4 magnification, with scale bar = 100 μm. Blots in B and C are representative of 3 separate experiments. Densitometric values for tau oligomer complex (TOC1)/actin in B are means ± SE from 4 separate blots. Values for pan tau/actin are means ± SE from 3 separate blots (2 for baseline condition). One-way ANOVA and Dunnett's multiple-comparison tests were conducted to assess statistical significance for comparison of infection time points with baseline values. *P < 0.05 in B. No statistical analyses were conducted in C, as no bands were detected at baseline in any experiment. *P < 0.05 (by Student's t-test) in E.
We then examined whether ExoY alters TOC1 immunoreactivity in cellular lysates. As noted previously (41), an ∼37-kDa immunoreactive band was visible using the Tau-5 antibody. An additional ∼74-kDa immunoreactive band was resolved using the TOC1 antibody. As shown in Fig. 2B, immunodetection of the 37-kDa band was reduced over the time course of the ExoY+ infection, to the point where neither the 37- nor the 74-kDa band could be resolved in cellular lysates. Consequently, as the 37-kDa band began to decrease in the lysate, the 74-kDa band began to increase. Evaluation of the supernatant fractions revealed a high-molecular-mass, 74-kDa, TOC1-immunoreactive band by 7 h (Fig. 2C). Thus, ExoY+ induces a time-dependent release of a TOC1-immunoreactive protein from PMVECs that is resolved in the supernatant fraction.
We next assessed whether the supernatant from infected PMVECs could be injurious to uninfected control cells, which we refer to as “naïve endothelial cells.” PMVECs were infected for 7–8 h with ExoY+ or ExoYK81M, and supernatant was collected and filtered. Filtering (0.22-μm filter) of the supernatant removed cellular debris and/or remnant bacteria, which was verified by measurement of bacterial growth on lysogeny broth-agar plates (data not shown). Filtered supernatant was directly added to naïve confluent PMVECs for 6 h. In separate experiments, naïve PMVECs were allowed to form networks on Matrigel for 24 h; then injurious supernatant was mixed (1:1) with fresh culture medium and added to the Matrigel for an additional 24 h. PMVEC exposure to supernatant from ExoY+-infected cells not only injured the endothelial monolayer, it greatly reduced endothelial network stability in the Matrigel assay (Fig. 2, D and E). Hence, filtered supernatant containing high-molecular-weight TOC1-immunoreactive tau is injurious to naïve PMVECs.
PA103 infection causes interendothelial gaps, accumulation of extracellular high-molecular-weight TOC1-immunoreactive tau, and injurious supernatant.
Arachidonic acid induces tau oligomerization (30, 70). Although addition of free arachidonic acid to purified tau is sufficient to induce oligomer formation in biochemical assays, an in vivo arachidonic acid stimulus that causes tau oligomerization has not been identified. ExoU possesses phospholipase A2 activity, which can lead to production of arachidonic acid (45, 51, 53). Therefore, we tested whether ExoU intoxication is sufficient to cause release of the high-molecular-weight endothelial tau.
First, PMVECs were infected with PA103 (MOI of 20:1), and interendothelial cell gaps and TOC1 immunoreactivity were monitored. This bacterial strain intoxicates cells with ExoT and ExoU. PMVEC gaps formed within 1 h postinfection and expanded throughout the course of the infection (Fig. 3A); experiments were terminated at 4–5 h postinfection, because cells were unable to tolerate longer periods of infection. Compared with the ExoY+ strain, PA103 caused very rapid deterioration of the cell monolayer. This rapid response is likely due to the presence of ExoT and ExoU, and not the bacterial strain per se, since the ExoY+ strain is generated from PA103. Although gaps formed in response to both bacterial strains, the cell phenotype following PA103 infection was remarkably different from that following ExoY+ infection. In response to PA103, cell membranes were visibly injured, and cell nuclei were sunken and prominent.
Fig. 3.
PA103 infection decreases intracellular tau abundance while producing injurious supernatant containing high-molecular-weight tau. PMVECS were inoculated with PA103 in HBSS for 5 h at a MOI of 20:1. A: beginning as early as 1 h after inoculation with PA103, gap formation increased in a time-dependent manner. B: culture medium was collected at 1-h intervals from 0 (baseline) to 5 h. After removal of bacteria, PMVECs were lysed, and both fractions (culture medium and lysate) were prepared for immunoblotting. PA103 infection caused a decrease in intracellular monomeric tau by 3 h that continued until 5 h. PA103 infection also caused an increase in intracellular high-molecular-weight tau by 1 h that continued until 5 h. C: at 4 and 5 h postinfection, supernatant from PMVECs infected with PA103 contained extracellular monomeric and high-molecular-weight tau. D: PMVECs were infected with 3 separate strains of Pseudomonas aeruginosa (ΔPcrV contains ExoU and ExoT but cannot inject the exoenzymes, ΔUΔT forms the type 3 secretion system but does not possess exoenzymes, and PA103 injects ExoU and ExoT enzymes) for 5 h at a MOI of 20:1. Supernatant and lysates were collected 5 h postinfection (control supernatant was generated by HBSS on PMVECs for 5 h without infection) and prepared for immunoblotting. Only supernatant from PMVECs infected with PA103 contained high-molecular-weight tau. E: filtered supernatant collected 4.5 h postinfection (ΔUΔT or PA103) was transferred to naïve PMVECs, and images of injurious effects were captured 23 h posttransfer. F: PMVECs (40,000 cells per well) were seeded in Matrigel for 6 h to allow network formation. After 6 h, filtered supernatant collected 4.5 h postinfection was mixed in a 1:1 ratio with serum-containing culture medium and added to naïve PMVECs in Matrigel. Images of injurious effects on network stability were captured 24 h posttransfer. Images in A, E, and F are each representative of ≥3 separate experiments. Images in A could not be captured at the 5-h time point, because the cell monolayer was no longer intact. Images in A and E were captured at ×20 magnification, with scale bar = 10 μm. Arrowheads indicate interendothelial cell gaps. Images in F were captured at ×4 magnification, with scale bar = 100 μm. Blots in B–D are representative of ≥2 separate experiments. Densitometric values for TOC1/actin in B are means ± SE from 5 separate blots (4 for baseline condition). Values for pan tau/actin are means ± SE from 4 separate blots (3 for baseline condition). One-way ANOVA and Dunnett's multiple-comparison test were conducted to assess statistical significance in comparison of infection time points with baseline values. *P < 0.05. No statistical analyses were conducted in C, as no bands were detected at baseline in any experiment. *P < 0.05 (by Student's t-test) in F.
We collected whole cell lysate and supernatant and subjected both fractions to immunoblot analysis using Tau-5 and TOC1 antibodies. As in our previous experiments, 37- and 74-kDa immunoreactive bands were detected (Fig. 3B). We found that although interendothelial gaps formed by 1 h (Fig. 3A), intracellular monomeric tau abundance did not decrease noticeably until 3–4 h postinfection (Fig. 3B, middle). We noticed that a resident population of intracellular higher-molecular-weight TOC1-immunoreactive bands began to increase by 1 h postinfection (Fig. 3B, top). To complement this decrease in intracellular monomeric tau, we detected monomeric tau and high-molecular-weight TOC1-immunoreactive tau outside the cell at 3–4 h postinfection (Fig. 3C). Hence, PA103 infection induces release of high-molecular-weight TOC1-immunoreactive tau, which can be detected outside PMVECs.
To verify that production of high-molecular-weight TOC1-immunoreactive tau was indeed a product of P. aeruginosa T3SS effector proteins, we tested additional bacterial strains. First, we incubated PMVECs for 5 h in HBSS alone to mimic the infection process in the absence of bacteria; this treatment did not generate injurious supernatant. We then infected PMVEC for 5 h with two separate control strains of P. aeruginosa, ΔPcrV and ΔUΔT: ΔPcrV synthesizes exoenzymes but is incapable of forming a functional translocon for injection into host cells, whereas ΔUΔT forms the T3SS but does not possess exoenzymes. Among these treatment groups, only PA103 infection (used as our positive control) was able to produce extracellular high-molecular-weight TOC1-immunoreactive tau, even though ΔUΔT caused an apparent increase in intracellular high-molecular-weight tau (Fig. 3D).
To test whether the supernatant containing high-molecular-weight TOC1-immunoreactive tau was injurious to naïve endothelial cells, we employed a strategy similar to that employed for the studies performed previously with ExoY. At 4 h after ΔUΔT or PA103 infection, we collected filtered supernatant and treated confluent monolayers, as well as endothelial networks, in Matrigel. Supernatant from PA103-infected PMVECs caused significant monolayer injury as well as disruption of endothelial networks (Fig. 3, E and F).
Creation of a doxycycline-inducible ExoU mammalian expression system that allows for tight regulation of ExoU expression.
While PA103 is a clinically relevant bacterial strain that expresses ExoU (35), P. aeruginosa utilizes additional virulence mechanisms, and so the specific importance of ExoU cannot be determined using this bacterium. To examine whether ExoU is sufficient to produce interendothelial cell gaps and induce high-molecular-weight TOC1-immunoreactive tau, we generated a novel two-tier ExoU expression system to control expression of toxic genes in mammalian cells, as previously reported for ExoY (41). A gene encoding the L618 ExoU mutant was engineered into PMVECs. This construct was inducible by doxycycline and contained a myc sequence and FKBP12 protein stabilization domain on the COOH terminus (ExoU-myc-FKBP). Upon expression, the L618 ExoU mutant possesses ∼30% of the usual ExoU activity, which allows for prolonged ExoU expression without such rapid deleterious effects (56).
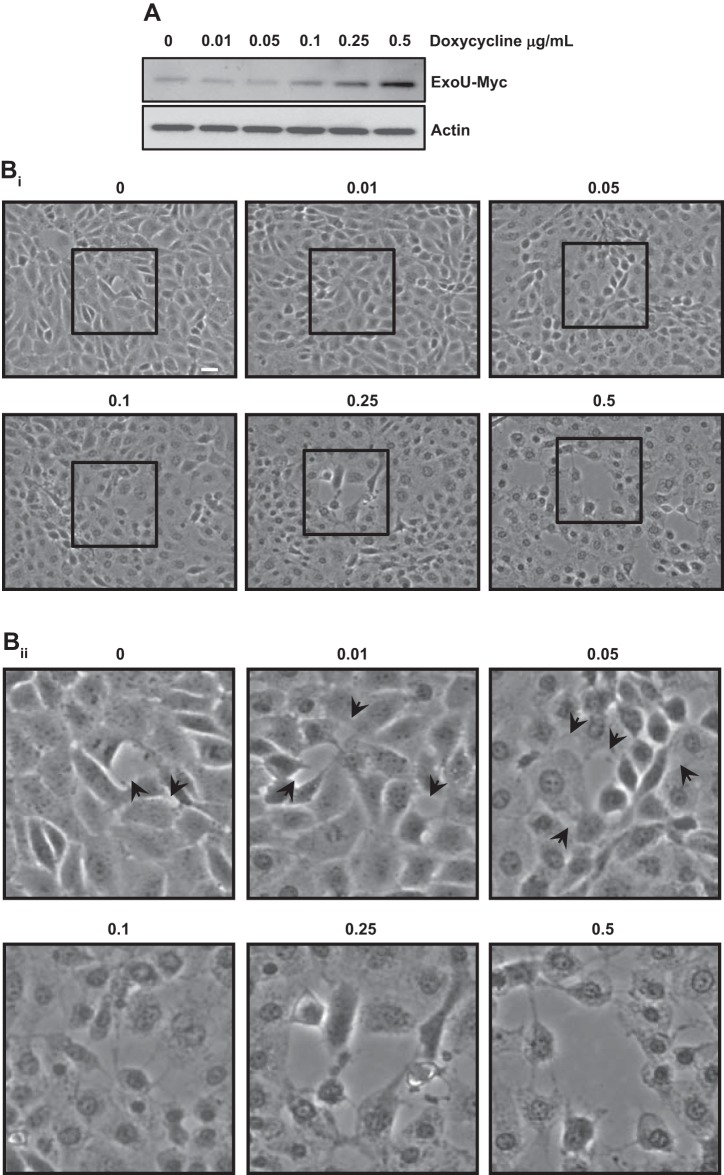
To test for inducible expression of ExoU-myc-FKBP, cells were incubated with 2 μM Shield1 for 2 h in the presence of increasing concentrations of doxycycline. As shown in Fig. 4A, Shield1 was sufficient to induce a discernable ExoU-myc-immunoreactive band detected by a myc antibody, which was increased further by doxycycline treatments. In parallel studies, we examined whether induction of ExoU generated interendothelial cell gaps (Fig. 4, Bi and Bii). Consistent with this idea, small interendothelial cells gaps were seen in Shield1-treated cells. Addition of increasing concentrations of doxycycline generated a concentration-dependent increase in gap formation. Cell morphology generally resembled that in Fig. 3A following PA103 infection, where cell membranes appeared injured and nuclei were sunken and prominent. On the basis of these results, all subsequent experiments were conducted with 2 μM Shield1 and a doxycycline concentration of 0.1 μg/ml.
Fig. 4.
ExoU is sufficient to promote interendothelial gap formation in PMVECs. ExoU expression in PMVECs was induced by increasing concentrations of doxycycline (0–0.5 μg/ml), and the protein was stabilized by the small molecule Shield1 (2 μM) over a period of 2 h. A: after 2 h of ExoU induction, cells were lysed and prepared for immunoblotting. ExoU-myc expression increases in a dose-dependent manner. Bi and Bii: gap formation number and size increased in a dose- and time-dependent manner after addition of doxycyline and Shield1. Blots in A are representative of 3 separate experiments. Images in Bi and Bii are each representative of ≥3 separate experiments. Area outlined by black box in Bi is shown at higher magnification in Bii.
ExoU is sufficient to impair PMVEC barrier integrity while producing injurious supernatant containing high-molecular-weight TOC1-immunoreactive tau.
We next used our doxycycline-inducible ExoU mammalian expression system to test the effects of ExoU on interendothelial gap formation and high-molecular-weight TOC1-immunoreactive tau over a 6-h time course. ExoU induced interendothelial cell gaps within 2 h that progressively increased in size throughout a 6-h time course (Fig. 5A). Although gaps began to form by 2 h, cells were generally healthy. By 3 h, however, gaps were uniformly visible throughout the monolayer, and cell membranes were unhealthy and appeared porous. This injury progressed throughout the time course to 6 h, at which time the cells could no longer withstand the ExoU expression.
Fig. 5.
ExoU impairs PMVEC barrier integrity within 2 h of induction. ExoU expression was induced in PMVECs for 6 h by 0.1 μg/ml doxycycline and 2 μM Shield1. A: gap formation was assessed at each time point (1-h intervals), and images were captured. Gap formation starts at 2 h after ExoU induction and continues through the 6-h time point. B: at each time point, supernatant was harvested, cells were lysed, and both fractions were prepared for immunoblotting. There is a slight decrease in intracellular tau abundance by 2 h after ExoU induction (middle blot), paralleled by a decrease in higher-molecular-weight extracellular TOC1-immunoreactive bands (top blot). C: supernatant (concentrated 10-fold) was assessed for the presence of monomeric and high-molecular-weight tau using the pan tau and TOC1 antibodies, respectively. Extracellular monomeric and high-molecular-weight tau were detected by 2 h after ExoU induction and continued to increase for up to 6 h. D: filtered supernatant (control supernatant was generated by HBSS on PMVECs for 6 h without ExoU induction) collected 1 and 6 h after ExoU induction was transferred to naïve PMVECs, and images of injurious effects were captured 6 h posttransfer. Images in A and D were captured at ×20 magnification, with scale bar = 10 μm. Images in A and D are each representative of ≥3 separate experiments. Blots in B and C are representative of 3 separate experiments. E: injurious supernatant promoted TOC1-immunoreactive fluorescence in naïve PMVECs as denoted by white arrowheads (top row). After supernatant treatment, naïve PMVECS were fixed for immunofluorescence and probed using the TOC1 antibody. Control supernatant did not stimulate production of TOC1-immunoreactive fluorescence in naïve PMVECs. Bottom row of images represents Hoechst staining of PMVEC nuclei. Images in E were captured at ×60 at 5 h after supernatant transfer. Scale bar = 10 μm. F: injurious supernatant is sufficient to induce permeability defects in isolated-perfused lung. ExoU expression was induced in PMVECs for 6 h by 0.1 μg/ml doxycycline and 2 μM Shield1. Control supernatant was generated by HBSS on PMVECs for 6 h without ExoU induction. After 6 h with or without ExoU induction, supernatant was collected, filtered, and concentrated 10-fold. Rat lungs were perfused at constant flow (40 ml·min−1·kg body wt−1) with buffer containing 4% autologous BSA and 6% whole blood in a 50-ml total volume of physiological buffer. After lungs were perfused for 15 min to reach an equilibrated status, 1 ml of control or ExoU supernatant (concentrated 10-fold) was slowly infused into the lung circulation by injection into the inflowing perfusate and circulated for 2 h; then filtration coefficient (Kf) was measured as an indicator of permeability. Addition of injurious supernatant causes a statistically significant increase (∼2-fold) in permeability (Kf). *P < 0.05.
The tau-banding pattern was assessed throughout the time course of the experiment. As in our previous studies, 37- and 74-kDa immunoreactive bands were seen in cellular lysates. However, within 2 h of ExoU induction, the tau bands were significantly reduced, and by 3 h they were undetectable (Fig. 5B). Assessment of the cellular supernatant revealed a 74-kDa immunoreactive band within 2 h that increased in abundance throughout the time course (Fig. 5C). These data demonstrate that ExoU is sufficient to induce the release of high-molecular-weight TOC1-immunoreactive tau into the extracellular space.
To test whether the supernatant containing high-molecular-weight TOC1-immunoreactive tau produced by ExoU-expressing PMVECS was injurious to naïve endothelial cells, we employed a strategy similar to that used for the studies performed previously with ExoY+ and PA103. At 1 and 6 h after ExoU induction, we collected filtered supernatant and treated confluent monolayers. Supernatant caused significant injury to the naïve PMVEC monolayer by 6 h after transfer (Fig. 5D). We found that supernatant from 1 h after ExoU induction was unable to exert injurious effects on naïve PMVECs, supporting the assertion that high-molecular-weight TOC1-immunoreactive tau is contributing to the injury.
Transmissible supernatant injury cannot be reversed by tau antibodies.
Monoclonal antibodies targeting tau oligomers have been reported to possess therapeutic potential in various proteinopathy models (50). We therefore tested whether three independent tau antibodies, TOC1, Tau-5, and TauC3 antibodies, confer protection against the injurious supernatant. We treated the ExoU-induced supernatant with the TOC1 antibody prior to transfer of the supernatant to naïve PMVECs. Supernatant-induced cellular injury was not prohibited by three separate TOC1 antibody concentrations (range 10–50 μg/ml; data not shown). We then tested the Tau-5 and TauC3 antibodies, neither of which conferred protection (data not shown). Thus it is unlikely that these antibodies have therapeutic potential in infectious forms of proteinopathy (25, 50).
We hypothesized that the antibodies may not prevent endothelial uptake of tau, despite avid binding and, hence, may be unable to protect against cellular injury. We therefore attempted to capture the tau-antibody complex using protein A agarose beads after addition of TOC1 antibody to the supernatant prior to addition of supernatant to naïve PMVECs. In this case, injurious supernatant was incubated with antibody-conjugated beads, beads were retrieved, and the remaining supernatant was added to naïve cells. Our results indicate that antibody capture provided no protection against the injury (data not shown). Interestingly, use of the protein A beads seemed to enhance the injurious effects. Hence, we used an SDS-PAGE approach to probe for tau species bound to the agarose beads. We were able to detect tau monomers (37-kDa band) in all conditions where protein A beads were used, regardless of the presence or absence of TOC1 antibody (data not shown). However, we were unable to detect any of the 74-kDa TOC1-immunoreactive bands (data not shown), suggesting that our antibody approaches were unsuccessful in capturing the potentially injurious tau species; therefore, this antibody capture approach was not pursued further.
If we were unable to bead-capture the injurious tau species or if we removed a cytoprotective species, then the remaining injurious supernatant should transfer high-molecular-weight endothelial tau to naïve cells or send a signal to stimulate formation of tau clusters. To address this possibility, injurious supernatant was isolated and incubated with antibody-labeled beads. Beads were separated, and the remaining supernatant was incubated on naïve PMVECs for 5 h. Then the cells were fixed and immunocytochemistry experiments were performed. We detected TOC1-immunoreactive fluorescence enriched in dense clusters (Fig. 5E), suggesting that our inability to capture high-molecular-weight TOC1-immunoreactive tau from the supernatant resulted in its uptake or that the injurious supernatant enhanced formation of high-molecular-weight tau in naïve PMVECs.
We next sought to determine whether injurious supernatant is sufficient to cause lung injury in situ. To address this issue, ExoU expression was induced in PMVECs for 6 h, and supernatant was collected; control supernatant was generated using uninduced cells. After 6 h of ExoU induction, supernatant was collected, filtered, and concentrated 10-fold. Rat lungs were perfused at constant flow (40 ml·min−1·kg body wt−1) with buffer containing 4% autologous BSA and 6% whole blood in a 50-ml total volume of physiological buffer. After the lungs were perfused for 15 min to reach equilibrium, 1 ml of control or ExoU-induced supernatant (concentrated 10-fold) was slowly infused into the lung circulation by injection into the pulmonary artery catheter. Supernatant was allowed to recirculate for 2 h, and Kf was measured as an indication of permeability. Whereas control supernatant had no effect on Kf, ExoU-induced supernatant caused an approximately twofold increase in permeability (Fig. 5F). Thus, even a small amount of concentrated supernatant (1 ml diluted into 50 ml) is injurious to the pulmonary circulation.
High-molecular-weight tau is sufficient to cause damage to naïve PMVECs.
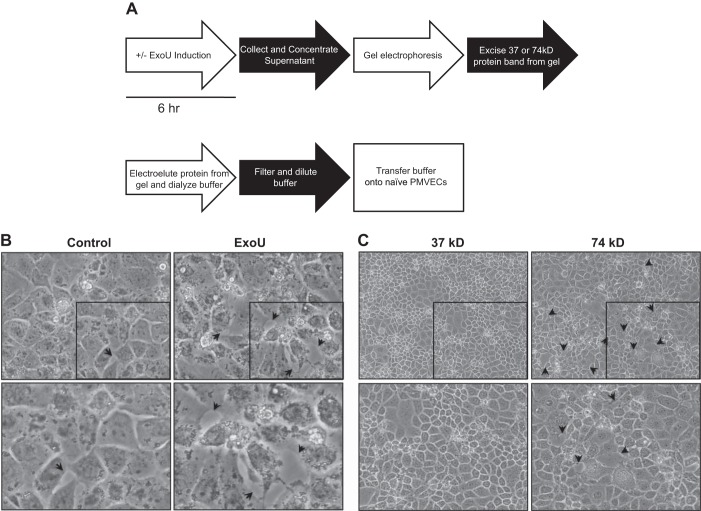
It is likely that bacterial infection causes release of multiple proteins from host cells. In this case, many proteins could contribute to disease propagation. In recognizing this possibility, we wanted to assess whether the high-molecular-weight TOC1-immunoreactive species is sufficient to cause injury to naïve endothelium. We therefore developed a novel electrophoresis-electroelution approach to enrich for high-molecular-weight TOC1-immunoreactive species. As schematically shown in Fig. 6A and described above (see Electroelution experiments), we isolated the 74-kDa protein(s) from control and ExoU supernatant and transferred the protein(s) to naïve PMVECs. After 24 h, we microscopically assessed the injury. Control supernatant produced only limited interendothelial gaps, whereas cells treated with ExoU supernatant enriched for the 74-kDa endothelial tau displayed cellular injury similar to that observed during the initial infection (Fig. 6B). To further assess whether it was specifically the 74-kDa protein(s) from the ExoU supernatant that contributed to cellular injury, we isolated the 37- and 74-kDa protein(s) from ExoU supernatant and transferred the protein(s) to naïve PMVECs. We found that only the cells treated with the 74-kDa protein(s), and not those treated with the 37-kDa protein(s), became injured, as evidenced by interendothelial gaps (Fig. 6C). Thus, transfer of the high-molecular-weight endothelial tau to naïve cells is sufficient to cause injury.
Fig. 6.
High-molecular-weight TOC1-immunoreactive protein is sufficient to damage PMVECS. A: schematic outlining electrophoresis-electroelution procedure. ExoU expression was induced in PMVECs for 6 h by 0.1 μg/ml doxycycline and 2 μM Shield1. Control supernatant was generated by HBSS on PMVECs for 6 h without ExoU induction. After 6 h with or without ExoU induction, supernatant was collected, filtered, and concentrated 10-fold. After concentration, supernatant was resolved on 4–12% Bis-Tris polyacrylamide gels. After electrophoresis, the 74-kDa protein band was excised from the gels, and gel pieces were minced. Proteins were then eluted out of the gel into PBS buffer in the dialysis bag. Buffer was collected and centrifuged to pellet remaining gel pieces, transferred to a fresh dialysis bag, dialyzed at 4°C for 24–36 h in HBSS, diluted with HBSS and filter-sterilized, and further diluted 1:1 with sterile HBSS before transfer to naïve PMVECs. B: images of injurious effects of 74-kDa protein were captured at ×40 magnification 24 h after supernatant transfer. Arrowheads indicate areas of interendothelial gap formation. Images at bottom are magnified portions (denoted by black box) of images at top. C: 74-kDa protein(s) are sufficient to cause interendothelial cell gaps. Following the procedure outlined in A, ExoU expression was induced for 6 h. After electrophoresis, 37- and 74-kDa proteins were excised separately and, after completion of electroelution, dialysis, and dilution, transferred to naïve PMVECs. Images of injurious effects of 74-kDa protein were captured at ×20 magnification 24 h after supernatant transfer. Arrowheads indicate areas of interendothelial gap formation. Area outlined by black box (top) is shown at higher magnification at bottom.
DISCUSSION
P. aeruginosa is a leading cause of pneumonia that can progress to sepsis and acute lung injury. This organism disrupts the alveolar-capillary barrier, at least in part by injecting exoenzymes into the host cell's cytoplasm through the bacterial T3SS (60). Of the four known exoenzymes, ExoY is among the most commonly expressed (17), while ExoU is the most toxic. Our previous studies addressed ExoY's mechanism of action in the endothelium and the pulmonary microvasculature (5, 41, 55). Our laboratory and others have determined that ExoY is a purine and pyrimidine cyclase that synthesizes cytosolic cAMP, cGMP, and cUMP (39, 41, 59, 72). The cAMP signal, in particular, results in endothelial cell tau hyperphosphorylation and insolubility, which result in dissociation of tau from microtubules, leading to their breakdown (5, 46, 55). Such microtubule breakdown induces interendothelial cell gap formation, causes pulmonary edema, and, importantly, hinders repair following injury (63).
Although we have established that ExoY+ hinders vascular repair following infection (63), the mechanism of this effect remains unclear. Here, we questioned whether the ExoY+-induced phosphorylated, insoluble tau accumulates outside the cell in a high-molecular-weight form, characteristic of such chronic neurodegenerative tauopathies as Alzheimer's disease (8, 48). In our previous work utilizing the Tau-5 antibody to detect total tau and the phosphorylated (Ser214) tau antibody to detect phosphorylated tau, we found no evidence of a high-molecular-weight endothelial tau. However, by extending the infection time course and examining both cellular lysates and supernatant in our present studies, we found that ExoY+ decreases the abundance of intracellular tau protein and promotes accumulation of extracelluar high-molecular-weight TOC1-immunoreactive tau. This finding is striking because of recent reports illustrating that extracellular tau oligomers can be transferred to adjacent cells, causing cytotoxicity (28, 33) and providing a putative molecular mechanism for end-organ dysfunction following lung infection. Previously, a relevant (patho)physiological stimulus for tau oligomerization has remained elusive, despite suggestions of infectious causes (6, 26, 31). Here, we report that ExoY is a pathophysiologically relevant stimulus for the accumulation of high-molecular-weight (TOC1-immunoreactive) extracellular tau that is transmissible to naïve cells, leading to cellular injury. Future studies are required to determine whether the high-molecular-weight tau identified in the present study represents an oligomeric form of the 37-kDa endothelial tau.
Whereas ExoY is a purine and pyrimidine cyclase, ExoU is a phospholipase. ExoU generates arachidonic acid in mammalian cells (51), and since arachidonic acid promotes tau aggregation (30, 70), we examined whether ExoU induces extracellular accumulation of high-molecular-weight endothelial tau. The PA103 strain caused interendothelial cell gap formation with some degree of apparent cell injury, and these changes in cell shape were accompanied by decreased intracellular tau protein and accumulation of extracellular high-molecular-weight endothelial tau. PA103 possesses ExoU and ExoT and utilizes additional virulence mechanisms. We therefore tested the function of ExoU in isolation from the bacterium. Using an approach pioneered for ExoY expression (41), we engineered endothelial cells for the inducible expression of an L618 ExoU mutant. The L618 ExoU mutant possesses ∼30% of the wild-type enzymatic activity, which allows for improved temporal resolution of its biological effects. As with the PA103 infection, L618 ExoU induced interendothelial cell gaps and caused a time-dependent decrease in intracellular tau protein that was accompanied by accumulation of extracellular high-molecular-weight endothelial tau. Although previous studies have utilized free arachidonic acid to initiate tau oligomerization in chemical assays (30, 70), a (patho)physiologically relevant arachidonic acid stimulus for tau oligomerization has not been identified. Our results indicate that the phospholipase A2 enzyme ExoU is sufficient to cause extracellular accumulation of high-molecular-weight endothelial tau; future studies are required to determine whether this form is oligomerized tau.
There is no consistent standard for the molecular mass of tau oligomers; in fact, reports of tau oligomer molecular weights vary greatly, even in studies utilizing the TOC1 antibody (69). Such variability has largely been attributed to the tau isoform expressed in the cell or organ evaluated (13), the degree and site(s) of posttranslational modification, including phosphorylation (27), and the susceptibility of the tau oligomer to proteolytic cleavage (20) (see Ref. 38 and references therein for discussion of each topic). Each of these issues is likely to be extremely important in determining the fate or biological activity of the high-molecular-weight endothelial tau, as some oligomers show significant cytotoxicity, whereas others are less detrimental.
In our studies, a loss of intracellular tau was paralleled by the appearance of extracellular tau. This finding brings into question how tau is released from cells. Until recently, it was widely accepted that neuronal tau was passively released from dead or dying neurons in conditions such as Alzheimer's disease (9, 64). An emerging hypothesis suggests that tau is actively secreted before cell death (see Ref. 23 and references therein). However, tau lacks any discernible secretion signal sequence. Without a secretion signal sequence, the manner in which tau is released from cells has been difficult to understand. Evaluation of how other aggregation-prone proteins, such as prion protein, α-synuclein, and β-amyloid, are released from cells in neurodegenerative disease provides possible clues. Studies focusing on the release of misprocessed proteins determined that exosome-mediated release is a common secretion mechanism for aggregation-prone proteins, including tau (1, 40, 52). Consistent with these studies, we find the 37-kDa tau species, as well as high-molecular-weight TOC1-immunoreactive (74-kDa) tau species, in the extracellular space. It is possible that dissociation from microtubules, hyperphosphorylation, and oligomerization are sufficient stimuli for endothelial tau secretion, yet even if this is the case, the physical nature of the released tau is unresolved, as it is not clear whether the high-molecular-weight endothelial tau is soluble in the supernatant or persists in microparticles. In preliminary studies, the ExoU-induced high-molecular-weight TOC1-immunoreactive tau is not present in supernatant microparticles (Morrow and Bauer, unpublished results).
Increasing evidence suggests that tau oligomers can impair normal cellular function by propagating in a prion-like manner (28, 33). We do not know whether the ExoY- and ExoU-induced high-molecular-weight endothelial tau propagates among cells in this way. In subsequent studies, it will be critical to determine whether the extracellular high-molecular-weight endothelial tau enters naïve cells. It will also be important to determine whether its uptake impairs endothelial cell function, most notably the ability to migrate, proliferate, and undergo an appropriate angiogenic response. Indeed, P. aeruginosa produces numerous heat-stable extracellular proteins. Tau may be a contributor to a larger complex of proteins that cause a “proteinopathy.” Proteinopathies have been described in neurodegenerative diseases such as Parkinson's disease, chronic traumatic encephalopathy, and Alzheimer's disease, as well as several others (see Ref. 65 and references therein for review). Proteins involved in these neurodegenerative diseases usually include tau, α-synuclein, β-amyloid, prion protein, and fyn kinase, in various combinations (15, 18, 49). In fact, others have identified some of these proteins in the same exosomal compartment in phosphorylated tau mutant or tau-overexpressing cells, suggesting that a common synergistic mechanism plays a role in disease states (10, 18, 52). Discerning proteins in a larger molecular complex and their associated roles will be crucial to understanding the lingering deleterious effects of infection in patients who have cleared the bacterium, yet still display organ dysfunction.
The appearance of extracellular high-molecular-weight endothelial tau occurs much more rapidly in response to ExoU than ExoY intoxication, yet the ExoY-dependent supernatant is similarly transmissible and, in some cases, more injurious. This finding is of critical clinical importance, because, unlike ExoU, ExoY is not thought to be as highly virulent, suggesting that necrotic death and release of intracellular contents are unlikely to account for the transmissible proteinopathy. Moreover, ExoY is present in most Pseudomonas isolates. Our studies support the assertion that ExoU elicits greater cellular injury than does ExoY during the initial infection period. However, virulence during the initial infection must be considered separately from the ensuing sequela of events. It is during this latter period that ExoY's actions are likely to be the most debilitating, hindering migration, proliferation, and tissue repair after infection (63). Our data would support the idea that extracellular high-molecular-weight endothelial tau is a target for therapy (25, 50). However, following infection, none of the antibodies tested provided protection against the injurious supernatant, likely because of the antibodies' inability to capture and remove the potentially injurious high-molecular-weight tau species from the supernatant or the antibodies' success in removing some cytoprotective species. It is likely that tau may be one of many cytotoxic species in the injurious supernatant. Thus it will be important to develop an approach to produce large quantities of this cytotoxic supernatant to enable isolation and purification of the cytotoxic agent(s) for systematic and rigorous testing.
In summary, we report that P. aeruginosa ExoY and ExoU effectors target microtubules as part of their virulence arsenal to impair pulmonary vascular function. We provide the first concrete evidence of pathophysiological stimuli, ExoY and ExoU, that induce extracellular accumulation of a high-molecular-weight endothelial tau that is transmissible and causes cellular injury. These observations may provide mechanistic insight into why, even after Pseudomonas infections are successfully treated, organ dysfunction progresses and some patients fail to recover.
GRANTS
This work was supported by American Heart Association Postdoctoral Fellowship 14POST18080004 (K. A. Morrow) and National Institutes of Health Grants HL-60024 and HL-66299 (T. Stevens, R. Balczon, and M. Alexeyev), HL-07612 and HL-107122 (C. D. Ochoa), and 1RO1 RR-031286 (M. Alexeyev).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.M. and T.S. developed the concept and designed the research; K.A.M., C.D.O., R.B., C.Z., L.C., M.A., and K.M.S. performed the experiments; K.A.M., R.B., C.Z., and D.W.F. analyzed the data; K.A.M., R.B., C.Z., D.W.F., and T.S. interpreted the results of the experiments; K.A.M. and R.B. prepared the figures; K.A.M. drafted the manuscript; K.A.M., C.D.O., R.B., M.A., D.W.F., and T.S. edited and revised the manuscript; K.A.M., C.D.O., R.B., C.Z., L.C., M.A., K.M.S., D.W.F., and T.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Linn Ayers, Mita Patel, Dr. Abu-Bakr Al-Mehdi, and Anna Buford for their contribution to the development of this work. We also thank Dr. Lester Binder (deceased) and Dr. Nicholas Kanaan for guidance and for providing the TOC1 antibody.
REFERENCES
- 1.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64: 783–790, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Alexeyev MF, Fayzulin R, Shokolenko IN, Pastukh V. A retro-lentiviral system for doxycycline-inducible gene expression and gene knockdown in cells with limited proliferative capacity. Mol Biol Rep 37: 1987–1991, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol 286: L445–L451, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Balczon R, Overstreet KA, Zinkowski RP, Haynes A, Appel M. The identification, purification, and characterization of a pancreatic beta-cell form of the microtubule adenosine triphosphatase kinesin. Endocrinology 131: 331–336, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Balczon R, Prasain N, Ochoa C, Prater J, Zhu B, Alexeyev M, Sayner S, Frank DW, Stevens T. Pseudomonas aeruginosa exotoxin Y-mediated tau hyperphosphorylation impairs microtubule assembly in pulmonary microvascular endothelial cells. PLos One 8: e74343, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balin BJ, Little CS, Hammond CJ, Appelt DM, Whittum-Hudson JA, Gerard HC, Hudson AP. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer's disease. J Alzheimers Dis 13: 371–380, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126: 995–1004, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchard M, Suchowersky O. Tauopathies: one disease or many? Can J Neurol Sci 38: 547–556, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 82: 239–259, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic interactions between Aβ, τ, and α-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 30: 7281–7289, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross DC, Munoz JP, Hernandez P, Maccioni RB. Nuclear and cytoplasmic tau proteins from human nonneuronal cells share common structural and functional features with brain tau. J Cell Biochem 78: 305–317, 2000. [PubMed] [Google Scholar]
- 12.Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109: 1019–1029, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Crowther RA, Goedert M. Abnormal tau-containing filaments in neurodegenerative diseases. J Struct Biol 130: 271–279, 2000. [DOI] [PubMed] [Google Scholar]
- 14.El-Solh AA, Hattemer A, Hauser AR, Alhajhusain A, Vora H. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med 40: 1157–1163, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30: 6838–6851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol 12: 61–66, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147: 2659–2669, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci USA 101: 9683–9688, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845–12852, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci USA 100: 10032–10037, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimley JS, Chen DA, Banaszynski LA, Wandless TJ. Synthesis and analysis of stabilizing ligands for FKBP-derived destabilizing domains. Bioorg Med Chem Lett 18: 759–761, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakki M, Limaye AP, Kim HW, Kirby KA, Corey L, Boeckh M. Invasive Pseudomonas aeruginosa infections: high rate of recurrence and mortality after hematopoietic cell transplantation. Bone Marrow Transplant 39: 687–693, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hall GF, Saman S. Death or secretion? The demise of a plausible assumption about CSF-τ in Alzheimer disease? Commun Integr Biol 5: 623–626, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7: 654–665, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes BB, Diamond MI. Prion-like properties of tau protein: the importance of extracellular tau as a therapeutic target. J Biol Chem 289: 19855–19861, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer's disease. Neurobiol Aging 25: 619–627, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Jeganathan S, Hascher A, Chinnathambi S, Biernat J, Mandelkow EM, Mandelkow E. Proline-directed pseudo-phosphorylation at AT8 and PHF1 epitopes induces a compaction of the paperclip folding of tau and generates a pathological (MC-1) conformation. J Biol Chem 283: 32066–32076, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of tau aggregation by fibrillar species. J Biol Chem 287: 19440–19451, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004. [DOI] [PubMed] [Google Scholar]
- 30.King ME, Gamblin TC, Kuret J, Binder LI. Differential assembly of human tau isoforms in the presence of arachidonic acid. J Neurochem 74: 1749–1757, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita J. Pathogens as a cause of Alzheimer's disease. Neurobiol Aging 25: 639–640, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lange M, Hamahata A, Enkhbaatar P, Esechie A, Connelly R, Nakano Y, Jonkam C, Cox RA, Traber LD, Herndon DN, Traber DL. Assessment of vascular permeability in an ovine model of acute lung injury and pneumonia-induced Pseudomonas aeruginosa sepsis. Crit Care Med 36: 1284–1289, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muoz MJ, Jackson GR, Kayed R. Preparation and characterization of neurotoxic tau oligomers. Biochemistry 49: 10039–10041, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Le Berre R, Nguyen S, Nowak E, Kipnis E, Pierre M, Quenee L, Ader F, Lancel S, Courcol R, Guery BP, Faure K, Pyopneumagen G. Relative contribution of three main virulence factors in Pseudomonas aeruginosa pneumonia. Crit Care Med 39: 2113–2120, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Liu PV. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis 116: 481–489, 1966. [DOI] [PubMed] [Google Scholar]
- 36.Lorimer D, Raymond A, Walchli J, Mixon M, Barrow A, Wallace E, Grice R, Burgin A, Stewart L. Gene composer: database software for protein construct design, codon engineering, and gene synthesis. BMC Biotechnol 9: 36, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 49: 1306–1311, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci 15: 4671–4713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow KA, Seifert R, Kaever V, Britain AL, Sayner SL, Ochoa CD, Cioffi EA, Frank DW, Rich TC, Stevens T. Heterogeneity of pulmonary endothelial cyclic nucleotide response to Pseudomonas aeruginosa ExoY infection. Am J Physiol Lung Cell Mol Physiol 309: L1199–L1207, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak P, Prcina M, Kontsekova E. Tauons and prions: infamous cousins? J Alzheimers Dis 26: 413–430, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J Biol Chem 287: 25407–25418, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochoa CD, Stevens T, Balczon R. Cold exposure reveals two populations of microtubules in pulmonary endothelia. Am J Physiol Lung Cell Mol Physiol 300: L132–L138, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ofori-Acquah SF, King J, Voelkel N, Schaphorst KL, Stevens T. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res 75: 391–402, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker JC, Stevens T, Randall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 291: L30–L37, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Phillips RM, Six DA, Dennis EA, Ghosh P. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J Biol Chem 278: 41326–41332, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol 297: L73–L83, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res 77: 53–63, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 362: 329–344, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA 103: 11172–11177, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasool S, Martinez-Coria H, Wu JW, LaFerla F, Glabe CG. Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Aβ deposition and tau pathology in 3xTg-AD mice. J Neurochem 126: 473–482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saliba AM, de Assis MC, Nishi R, Raymond B, Marques Ede A, Lopes UG, Touqui L, Plotkowski MC. Implications of oxidative stress in the cytotoxicity of Pseudomonas aeruginosa ExoU. Microbes Infect 8: 450–459, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287: 3842–3849, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol 53: 1279–1290, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 98: 675–681, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95: 196–203, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Schmalzer KM, Benson MA, Frank DW. Activation of ExoU phospholipase activity requires specific C-terminal regions. J Bacteriol 192: 1801–1812, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38: 3549–3558, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Segovia JA, Tsai SY, Chang TH, Shil NK, Weintraub ST, Short JD, Bose S. Nedd8 regulates inflammasome-dependent caspase-1 activation. Mol Cell Biol 35: 582–597, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seifert RH, Wolter S, Reinecke D, Burhenne H, Kaever V, Munder A, Tummler BS, Grundmann M, Kostenis E, Frank DW, Beckert U. Pseudomonas aeruginosa ExoY, a cyclic GMP- and cyclic UMP-generating nucleotidyl cyclase. BMC Pharmacol Toxicol 14 Suppl 1: O1–P80, 2013.24517645 [Google Scholar]
- 60.Shafikhani SH, Morales C, Engel J. The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell Microbiol 10: 994–1007, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407: 762–764, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc 5: 783–791, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens TC, Ochoa CD, Morrow KA, Robson MJ, Prasain N, Zhou C, Alvarez DF, Frank DW, Balczon R, Stevens T. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am J Physiol Lung Cell Mol Physiol 306: L915–L924, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su JH, Deng G, Cotman CW. Transneuronal degeneration in the spread of Alzheimer's disease pathology: immunohistochemical evidence for the transmission of tau hyperphosphorylation. Neurobiol Dis 4: 365–375, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Tartaglia MC, Hazrati LN, Davis KD, Green RE, Wennberg R, Mikulis D, Ezerins LJ, Keightley M, Tator C. Chronic traumatic encephalopathy and other neurodegenerative proteinopathies. Front Hum Neurosci 8: 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thurston VC, Zinkowski RP, Binder LI. Tau as a nucleolar protein in human nonneural cells in vitro and in vivo. Chromosoma 105: 20–30, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Vallee RB, Collins CA. Purification of microtubules and microtubule-associated proteins from sea urchin eggs and cultured mammalian cells using taxol and use of exogenous taxol-stabilized brain microtubules for purifying microtubule-associated proteins. Methods Enzymol 134: 116–127, 1986. [DOI] [PubMed] [Google Scholar]
- 68.Ward SM, Himmelstein DS, Lancia JK, Fu Y, Patterson KR, Binder LI. TOC1: characterization of a selective oligomeric tau antibody. J Alzheimers Dis 37: 593–602, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward SM, Himmelstein DS, Ren Y, Fu Y, Yu XW, Roberts K, Binder LI, Sahara N. TOC1: a valuable tool in assessing disease progression in the rTg4510 mouse model of tauopathy. Neurobiol Dis 67C: 37–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson DM, Binder LI. Free fatty acids stimulate the polymerization of tau and amyloid-β peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer's disease. Am J Pathol 150: 2181–2195, 1997. [PMC free article] [PubMed] [Google Scholar]
- 71.Wong-Beringer A, Wiener-Kronish J, Lynch S, Flanagan J. Comparison of type III secretion system virulence among fluoroquinolone-susceptible and -resistant clinical isolates of Pseudomonas aeruginosa. Clin Microbiol Infect 14: 330–336, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 95: 13899–13904, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]