Abstract
Non-breeding common ravens (Corvus corax) live in complex social groups with a high degree of fission–fusion dynamics. They form valuable relationships and alliances with some conspecifics, while taking coordinated action against others. In ravens, affiliates reconcile their conflicts, console each other after conflicts with a third party, and provide each other with social support — all behaviors that presumably reduce corticosterone levels and alleviate stress. However, how well an individual is socially integrated in a (sub)group might vary substantially. This raises the question whether the social integration of a raven affects its stress responses to fission–fusion dynamics. The present study aims to investigate this effect experimentally by separating single ravens (n = 16) individually from their group for four days and subsequently reintroducing them. To determine stress response patterns in the separated individuals we measured the amounts of immunoreactive corticosterone metabolites (CM) in droppings. We compared two enzyme immunoassays, which we validated by conducting an ACTH challenge, and finally decided to apply an 11-oxoetiocholanolone enzyme immunoassay. Additionally, we determined levels of social integration using focal observations. Our findings suggest that a strong social integration is related to low CM levels when the individuals are within the group and high levels during separations, implying that separation leads to stress in these birds. In contrast, poorly socially integrated ravens seem to exhibit the opposite pattern, indicating that to them group living is more stressful than being temporarily separated. We, therefore, conclude that the birds’ adrenocortical activity is modulated by their social integration.
Keywords: Social relationships, Fission–fusion dynamics, Social network, Separation, Stress, Glucocorticoids, Fecal corticosterone metabolites, Common raven, Corvus corax
Introduction
Social life does not only bring benefits, but also entails many challenges. Interactions between individuals can, therefore, depending on the circumstances, either alleviate or cause stress (DeVries et al., 2003). One of the mechanisms serving to diminish the effects of stressors is social support, which leads to less intense (physiological) responses to stressful situations (“buffering model” reviewed by Cohen and Wills, 1985). It is important to note, however, that social support is not directed towards random individuals, but rather specifically towards close affiliates or bonding partners. Hence, the quality of the social relationship (see Cords and Aureli, 2000; Fraser et al., 2008) determines the amount of social support given and received. Close social bonds are therefore very valuable and accordingly, one could assume that being separated from a social ally acts primarily as a psychological stressor and results in increased glucocorticoid levels. Indeed, Remage-Healey et al. (2003) showed that in zebra finches (Taeniopygia guttata) corticosterone, the major glucocorticoid in birds (Holmes and Phillips, 1976), is elevated due to pair mate separation (48 h) and returns to baseline levels upon reunion. The study also discovered that the presence of other conspecifics during separation from the bonded partner did not significantly attenuate the hypothalamic-pituitary-adrenal axis activation.
So far, however, little is known about animals’ adrenocortical activity in groups of affiliated, but not pair-bonded individuals with a high degree of fission–fusion dynamics, where long-lasting separations of affiliates may occur frequently. Unlike previous uses of the term “fission– fusion” in the context of a specific type of social system (i.e. “fission–fusion society”), it is now referred to in more dynamic terms defined by the degree of spatial and temporal cohesion of individuals in a group (Aureli et al., 2008). This means that over time groups can change in size and composition to varying extents (e.g. in Guiana dolphins, Sotalia guianensis, Lunardi and Ferreira, 2014; spider monkey, Ateles geoffroyi, Ramos-Fernández and Morales, 2014).
Non-breeding common ravens (Corvus corax) are highly gregarious and live in groups that exhibit high degrees of fission–fusion dynamics (Braun et al., 2012). Individuals usually remain in these groups until they gain sexual maturity and form long-term monogamous pairs that establish large breeding territories (size depends on the availability of food and breeding sites, Drack and Kotrschal, 1995), which they defend all year round (Heinrich, 1989). Non-breeding ravens often form small subgroups of individuals for socializing during the day (Braun et al., 2012), and join bigger non-breeder groups to be able to compete for food with territorial breeding pairs (Marzluff and Heinrich, 1991) or potentially dangerous predators, like gray wolves (Canis lupus, Stahler et al., 2002).
Additionally, raven non-breeder groups are structured by close social bonds, characterized by affiliative interactions and selective cooperation (Braun and Bugnyar, 2012; Heinrich, 2011). Affiliated birds reciprocate active agonistic support (Fraser and Bugnyar, 2012), console one another after conflicts with other individuals (Fraser and Bugnyar, 2010) and also reconcile conflicts with each other (Fraser and Bugnyar, 2011). Recent findings have further revealed that ravens are able to remember the relationship valence even for years (Boeckle and Bugnyar, 2012) and understand third-party relations without having to interact physically with the respective conspecifics (Massen et al., 2014a). All these findings suggest that in ravens social bonds are pivotal in respect to solving problems in daily social life. Whether or not individuals can rely on a bonding partner affects their status in the group and ability to secure resources (Braun and Bugnyar, 2012). It might also affect their response to challenges like being separated from and reunited with conspecifics, as it is typical in fission–fusion dynamics.
With the present study we aimed at investigating changes of adrenocortical activity in ravens during experimentally induced fission–fusion situations. By individually separating group members for four days and subsequently reintroducing them into their group and simultaneously measuring immunoreactive corticosterone metabolites (CM) in the birds’ droppings, we intend to investigate changes in the ravens’ stress levels throughout the experiment. Considering the positive effect of social bonds, we predicted that corticosterone secreted in ravens is increasing while they are individually separated and declining once they are released back into the group, and we hypothesize that the stress-induced changes in the adrenocortical activity depend on how well an individual is socially integrated in the group.
Material and methods
Subjects and housing
The study was conducted on 16 ravens (7 males, 9 females) at the Haidlhof Research Station in Bad Vöslau, Austria. With the exception of one female who hatched in the wild, all individuals were born and raised in captivity. In the study period the birds were 1½ to 2 years old, hence still sub-adult and sexually immature. Individuals were grouped into two mixed-sex non-breeder groups of 8 birds each, ensuring a species-specific social situation that resembles natural conditions at that early stage of a raven’s life. To facilitate individual identification all birds were marked with colored leg-rings.
The ravens were housed in an aviary complex, consisting of three main units (approx. 230 m2 each), which were connected by lattice fence runways. While the birds had permanent access to one main unit, all the others were at least temporary accessible, hence, the ravens were acquainted with all areas. Like all units, the ravens’ main aviary consisted of freely accessible indoor and outdoor compartments and was furnished with branches, trees, and shallow water basins. The ground was covered in equal manner with wood chips, stones and sand, offering plenty of opportunities to cache food and other items, which ravens do frequently. Their diet consisted of meat, chicken eggs, vegetables, fruits and yoghurt and was provided on a daily basis, while water was available ad libitum.
Social integration
We calculated a social integration score for each bird using data from the behavioral parameter “contact sit” (birds sitting within one body length to each other), which proved to be an appropriate indicator for close socio-positive relationships in a previous study (Schwab et al., 2008). For each bird the data was extracted from 5-minute focal observations of 12 randomly selected days (except for experiment days) distributed over the study period (group 1: Dec. 2011–May 2012; group 2: Dec. 2012–May 2013). From these data we constructed weighted, undirected social networks for both groups and calculated normalized Freeman degree values for each individual, using UCINET 6 (Borgatti et al., 2002). Weighted degree values consider the number of interaction partners of an individual as well as the number of interactions with these partners. To get comparable individual scores from both groups, weighted degree values were transformed into relative values (in %) measured against the individual with the highest value of the respective group, which then represented an individual’s relative social integration in its respective group (for details see Supplementary material Tab. S1).
Experimental procedure
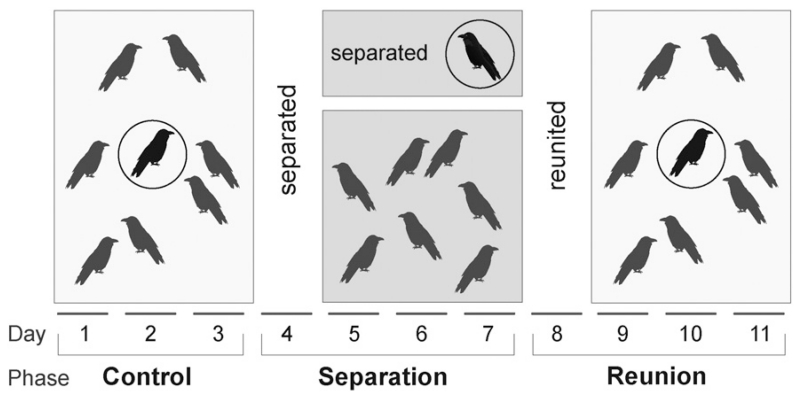
Each tested individual (hereafter “focal” individual) was subjected to an 11-day experiment, which started with a 3-day control phase during which the focal individual was housed in its everyday social group. On day 4 the focal raven was separated individually from his or her conspecifics for four days. On this day, around 9:30 am, the respective focal individual was isolated in a side compartment of the main aviary and caught with a net within only a few minutes. Afterwards the raven was transferred to a familiar compartment (80 m2) of another aviary unit that was situated approximately 20 m away from the group’s aviary. Hence, the bird was isolated visually, but not acoustically. During the separation phase the focal raven was presented with the same dietary conditions as the group to minimize environmental influences other than social aspects. On day 8, after the separation phase, the bird was allowed to move back to the group through a runway to avoid any further handling stress. This reunion event was followed by a 3-day reunion phase (Fig. 1).
Fig. 1.
Experimental design: Focal individual during the three phases (control, separation, reunion). Droppings were collected during the control phase as well as on days 5–7 and 9–11.
Droppings of each focal individual were collected each day during the entire experiment with the exception of day 4 when the focal bird was separated and day 8 when it was reunited, because the birds might have been stressed due to moving from one aviary to another (Fig. 1). This allowed us to determine not only the changes in adrenocortical activity in response to the separation itself, but also the metabolite level prior to separation and the adrenocortical response to the reunion (Fig. 1). For the sake of brevity, the corticosterone level prior to the separation is referred to as “control”, even though it is hard to rule out that unknown or uncontrolled stress factors were acting on the animals.
To be sure about the origin of a dropping the focal individual was visually tracked until it produced a sample, which was then immediately collected. The collection happened between 10:00 to 11:30 am to avoid measuring the corticosterone peak many bird species excrete in the early morning (Carere et al., 2003; Meier and Fivizzani, 1975). Immediately after the collection period samples were frozen at −20 °C (Möstl and Palme, 2002).
Between experiments there was a minimum of a two-week period in order to give the birds time to re-stabilize relationships in the group that were potentially disturbed due to the separation. Since hormone levels vary with age of the individual and season (Breuner, 2002; Stöwe et al., 2008), we tested the first group, of which all ravens hatched in 2010, between December 2011 and May 2012 and the second one, where all but two individuals hatched in 2011, between December 2012 and May 2013. All procedures were approved by the Austrian Federal Ministry of Science, Research and Economy (approval number: BMWFW-66.006/0016-WF/II/3b/2014).
Choosing the most appropriate enzyme immunoassay — ACTH challenge
To date the enzyme immunoassay used to measure corticosterone metabolites (CM) in ravens had been validated only biologically (Stöwe et al., 2008). To add a physiological validation and to test the potential suitability of another, more recently developed assay we performed an ACTH challenge on four ravens in September 2012. Two experienced researchers caught two males and two females out of their home aviary. Subsequently, a veterinarian conducted a health check on all birds before administering the ACTH (Synacthen: 250 μg/ml, Novartis) injection in the right brachial vein. Each individual received 0.5 ml of synthetic ACTH. Immediately after, the ravens were released in male–female pairs in two separate compartments of their home aviary and all droppings were collected for the following four hours. Droppings were also collected two hours prior to capturing to measure the basal corticosterone metabolite concentration on that day.
All samples were analyzed with two different enzyme immunoassays: (A) an 11-oxoetiocholanolone assay (using 11-oxoetiocholanolone as standard and antibodies raised in rabbits against 5β-androstane-3α-ol-11,17-dione-17-CMO: bovine serum albumin; sensitivity: 1 ng CM/g dropping, detailed assay description see Möstl et al., 2002), which turned out to be the most appropriate assay for ravens following the biological validation (Stöwe et al., 2008). This assay does not only cross-react with C19O3 steroids but also with C21O4 metabolites that possess a 3α-ol-11-oxo structure. The second assay (B) was a cortisone assay (using 4-pregnene-17α,21-diol-3,11,20-trione as standard and antibodies raised in rabbits against 4-pregnene-17α,21-diol-3,11,20-trione-21-HS: bovine serum albumin; sensitivity: 3 ng CM/g dropping, detailed assay description in Stöwe et al., 2013), which is suitable to measure CM excretion in several bird species e.g. northern wheatears, Oenanthe oenanthe (Eikenaar et al., 2014), chicken, Gallus gallus domesticus, and quail, Coturnix japonica (Rettenbacher et al., 2004; Stöwe et al., 2013). Another 11-oxoetiocholanolone EIA (detailed assay description see Palme and Möstl, 1997) was initially also taken into consideration, but already the immunogram showed that this assay is not appropriate for measuring CM in raven droppings.
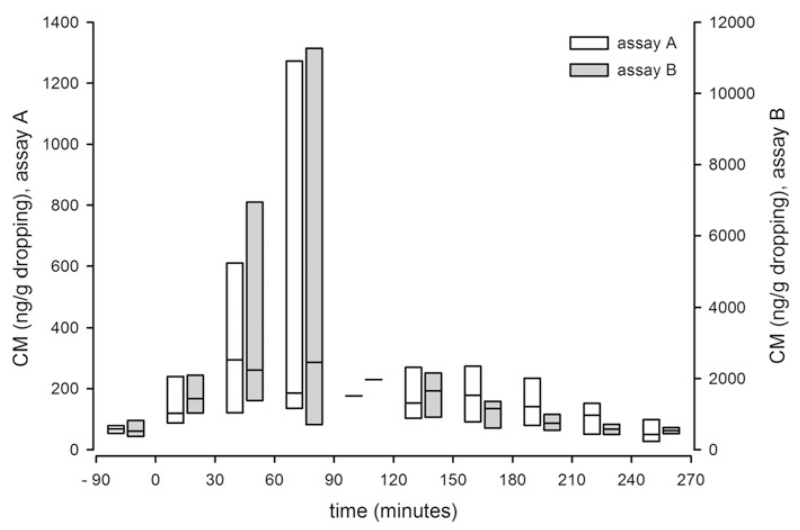
Even though assay A as well as B could detect a CM peak (Fig. 2), it seems that this peak is related to the short, but stressful event of catching rather than to the ACTH treatment itself, which in the bird treated last happened 45 min after catching. This time delay was caused by the thorough medical examination of each of the four birds prior to the injection. The time lag between the start of catching and the appearance of the CM peak ranges from 76 to 79 min with an 7.2 ± 3.7 fold CM increase in assay A, and from 76 to 91 min using assay B, which showed an 6.3 ± 2.3 fold surge. Although it appears that both assays are equally suitable for the measurement of CM, assay A was chosen for the further analysis, first, because it showed a somewhat higher increase (though assay B yielded higher absolute concentrations) above baseline after the challenge, and second, in order to facilitate comparability of the values to those of previous studies (e.g. Stöwe et al., 2008).
Fig. 2.
Concentrations of corticosterone metabolites (CM, ng/g dropping) before and after the ACTH administration (time 0) measured with assay A (white boxes, left axis) and assay B (gray boxes, right axis). Each box includes one value of each of the four individuals (2 males, 2 females), which is either a single value or the mean of all samples collected from one bird within 30 min. The boxes present the median (line) as well as the minimum and maximum value.
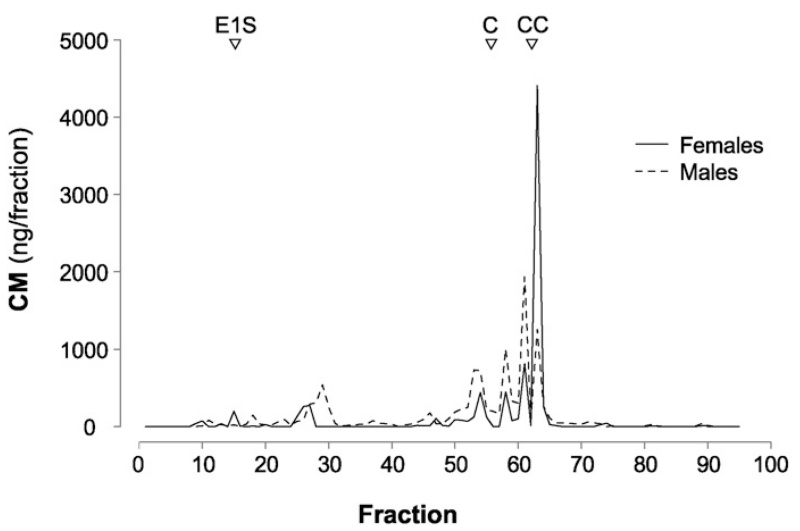
Since in some species sex differences in the biochemical structure of the CM excreted have been observed (reviewed in Goymann, 2005; Touma and Palme, 2005), a reversed-phase high-performance liquid chromatography (RP-HPLC, linear water/methanol gradient 20%– 100%) was conducted with a pool sample for each sex (sample preparation as described in Stöwe et al., 2013). Our results show that males and females excrete almost identical patterns of CM (Fig. 3; the difference in height of the peaks is most likely due to different CM amounts in the pool sample). Therefore, CM of males and females can be compared directly without any concerns.
Fig. 3.
Immunogram of ravens’ excretion patterns of corticosterone metabolites (CM). The continuous line represents females, the dotted line males. The triangles on the top are indicating the elution pattern of the following steroids: E1S = estrone sulfate, C = cortisol, and CC = corticosterone.
Extraction and analysis of immunoreactive corticosterone metabolites
Droppings were frozen at −20 °C until analysis. For the CM extraction 0.1 g of wet dropping and 1 ml 60% methanol were mixed, shaken for 30 min and centrifuged for 15 min at 1942 g (Palme et al., 2013). If droppings weighed less than 0.1 g, the amounts of methanol and distilled water were adjusted accordingly. The resulting extract was diluted with assay buffer (1 + 4) and analyzed with enzyme immunoassay A. All samples were analyzed in duplicates. The inter-assay coefficient of variance (CV) of the separations in 2012 and 2013 were 10.1% and 7.5%, respectively, while the intra-assay CV was 5.7%.
Statistical data analysis
To test whether CM levels during the experiment were influenced by the level of social integration, we used a general linear mixed model (GLMM; Baayen, 2008). Into this we included social integration, phase (control, separation, reunion) and their interaction as fixed effects and individual and test day (nested in individual) as random effects. The reason for including the interaction was that we expected the effect of social integration to depend on the phase. To control for the effect of sampling time (in relation to sunrise) we included the time elapsed since sunrise as an additional fixed effect into the model. To keep type I error rate close to the nominal level of 0.05 we included random slopes (Barr et al., 2013; Schielzeth and Forstmeier, 2009) of phase and sampling time within individual (after manually dummy coding it). We did not include correlations between random intercepts and random slopes to avoid the model getting too complex (according to Barr et al., 2013 neglecting random slopes does not appear to compromise type I error rates). To get an overall test of the effect of the level of social integration (as a main effect or as an interaction with phase) we compared the full model with a null model comprising only phase, sampling time and the same random effects as the full model (Forstmeier and Schielzeth, 2011) using a likelihood ratio test (Dobson, 2002). We also tested the effect of the interaction by dropping it from the model and comparing the full and the reduced model using a likelihood ratio test.
Prior to running the model we z-transformed social integration to a mean of zero and a standard deviation of one (Schielzeth, 2010). We also log transformed CM levels to achieve normally distributed and homogeneous residuals (ascertained by visual inspection of a qq plot of the residuals and residuals plotted against fitted values). The models were fitted in R (R Core Team, 2014) using the function lmer of the package lme4 (Bates et al., 2014). The model was fitted using Maximum Likelihood (lmer argument REML set to FALSE) on a data set including a total of 218 CM measures made at 121 test days and from 16 individuals.
Results
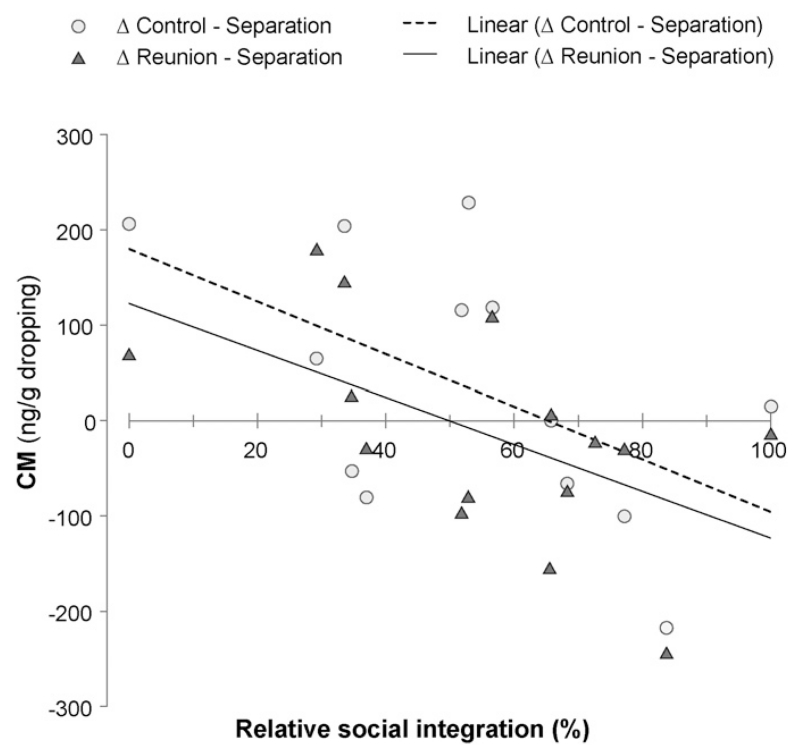
Overall, there was a clear effect of the ravens’ social integration on their adrenocortical activity (full-null model comparison: χ2 = 13.51, df = 3, P = 0.004). More specifically, individuals having low scores of social integration were showing elevated CM levels during the control and reunion phase, but we found no strong effects of social integration during the separation phase, meaning that in this phase all individuals showed similar CM levels (test of the interaction to: χ2 = 8.32, df = 2, P = 0.016; Fig. 4). Sampling time did not have a significant effect on the ravens’ adrenocortical activity. For further details on the GLMM see Supplementary material Tab. S2 and Fig. S1.
Fig. 4.
Differences (Δ) in the mean CM levels of individuals (n = 16) plotted against their relative social integration (%) between the separation phase (set to 0 as a reference point) and the other experimental phases (within the group): control (light gray circles), reunion (dark gray triangles). Individuals with CM values above 0 have higher CM levels when in the group (control, reunion) compared to when alone (separation). CM values below 0 indicate that these birds excrete higher amounts of CM during the separation phase than during the control phase or upon reunion. The levels at 0% integration include values of two individuals.
Note that the CM levels of some individuals, in particular in the middle of the social integration range, do not follow the clear pattern of having either higher or lower CM levels during the separation than during the phases in which the bird was within the group.
Discussion
This study demonstrates that ravens do show changes in CM levels due to being separated from their group as well as due to being reunited. Interestingly, the predicted pattern of CM levels in the course of the experiment – an elevation of CM following the separation and a decrease upon the reunion – was more likely to be found in socially well-integrated than in poorly integrated individuals. Poorly integrated ravens seemed to be less stressed during the separation than during the control and reunion phase, excreting less CM while being socially separated and more when they were within the group.
These results indicate that the adrenocortical activity of better integrated subjects is more likely to be attenuated when they are in the group, because they have more bonding partners with which they shared more positive interactions, while ravens that are poorly integrated are more stressed because they might lack social support and are therefore also prone to experience conflicts (see Fraser and Bugnyar, 2012). While separated, however, the latter do not have to compete for food, nor can they fall victim to agonistic interactions. Being alone, thus, appears to be stressful for well-integrated individuals, but more tolerable, or maybe even pleasant for ravens of poor social integration. It is important, though, to note that the focal individual was only isolated visually, but not acoustically. Given this setting, the separated bird could probably have communicated with the ones remaining in the group and, hence, could have known that the others are still in proximity (see Boeckle and Bugnyar, 2012).
The ravens were maintained on the same diet throughout the whole study. Ravens cache food, which makes it very difficult to assess how much they are actually ingesting. Therefore, we cannot exclude changes in food consumption during isolation, which could have affected amounts of droppings excreted and thus CM concentrations measured. However, as body weight did not correlate with an individual’s social integration score (Pearson correlation coefficient: males r = 0.14; females r = −0.16), we do not think that social integration had an effect on the total food intake. Moreover, if poorly integrated birds ate more of their highly preferred food (meat) when alone, total dropping weight should have been lowered due to the better digestibility of meat. Consequently, CM concentrations should be higher, but we observed the opposite patterns in socially less integrated individuals. Therefore, we consider it unlikely that food intake accounts for the differences in CM levels measured during the three experimental conditions.
The adrenocortical activity of some individuals, in particular in the middle of the social integration range, do not seem to follow the clear pattern of having either higher or lower CM levels during the separation than during the phases in which the bird was within the group. Massen et al. (2014b) could show that ravens intervene more often in affiliative interactions of loosely bonded individuals than of strongly bonded ones, presumably in order to prevent others from forming alliances. Accordingly, it might well be that ravens in the middle of the social integration range, which can be seen as an approximate equivalent to the loosely bonded birds in Massen et al.’s study, experience interventions in their affiliative interactions more frequently and therefore do not exhibit consistently higher or lower stress levels when in the group, compared to when separated (Fig. 4).
In recent years it was hypothesized that enhanced cognition, which involves behavioral flexibility, might provide alternative stress coping strategies to neuroendocrine stress responses. Lendvai et al. (2013), for example, found that within long-lived bird species, large brains may serve as compensatory mechanism, allowing the animals to avoid or anticipate stressors and reduce corticosterone levels. Although ravens were not taken into account in this analysis, these findings might also apply to them, as they too are a long living and large-brained bird species. Individual differences in the ability to cope with stressful situation, i.e. coping styles, in terms of cognition could have affected the birds’ physiological stress responses and thereby also the results we observed. Individuals with a proactive coping style exhibit active, fast and bold behavioral reactions and low adrenocortical stress responses, whereas birds of reactive coping styles show passive, slow and shy behavioral and strong adrenocortical responses (e.g. in great tits, Parus major, Stöwe et al., 2010; Cockrem, 2007). The coping style of an individual not only influences its physiological stress response, but also the way it deals with the presence/absence of social allies (e.g. in ravens,Stöwe et al., 2006, Stöwe and Kotrschal, 2007; great tits, Stöwe et al., 2009).
That the ravens’ adrenocortical activity depends on social context is comparable with findings of other studies. For instance, in greylag goose (Anser anser) secondary families (consisting of last year’s offspring, now subadults, rejoining their parents, if the latter fail to reproduce, Lorenz, 1988) adult as well as subadult females profit from passive social support and therefore exhibit reduced adrenocortical responses during socially stressful situations (Scheiber et al., 2009). Another, more recent work discovered that also in a cichlid fish (Oreochromis mossambicus) the effect of a social isolation on the separated individual’s cortisol level is modulated by the previous social context (Galhardo and Oliveira, 2014). In this study it was found that individuals from groups with a stable hierarchy were more stressed during separation than their conspecifics from groups with an unstable hierarchy, irrespective of their social status (Galhardo and Oliveira, 2014).
The socio-ecological implications of our findings could likely be related to the emergence of social niches. In wild ravens, Loretto et al. (2015) could observe individual differences in the birds’ movement patterns, which might have resulted from various social aspects, such as the number of social allies and the aggression rate within the group. Accordingly, it might well be that poorly integrated individuals join groups less frequently or for a shorter period than well-integrated ones. Individuals may therefore rely on different socio-ecological strategies, which in turn might have an effect on their survival. Whether this holds true has yet to be shown in further studies on wild raven populations. Moreover, future research, including separations of whole sub-groups, is needed to better understand the endocrine influences of fission–fusion dynamics on the remaining group.
Supplementary Material
Acknowledgments
We want to thank Martina Schiestl and Tanja Hampel for helping out with collecting droppings, and Elisa Pschernig, Samy El Makarem and Maja Jovanovic for their help with the steroid analysis. We are grateful to Riccardo Hofer and Matthias Loretto for their technical support, Jorg Massen for his scientific advice and our anonymous reviewers for their constructive comments. Our special thanks go to Roger Mundry, who contributed a major part to the statistics. Finally, we acknowledge the FWF (Austrian Science Fund, projects Y366-B17 and W1234 to Thomas Bugnyar). Christine Schwab has been supported by the Vienna Science and Technology Fund (WWTF) through project CS11-008. Martina Stocker is currently funded by the FWF Stand-Alone-Project P 26806.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yhbeh.2015.11.009.
References
- Aureli F, Schaffner CM, Boesch C, Bearder SK, Call J, Chapman CA, Connor R, Di Fiore A, Dunbar RIM, Henzi SP, Holekamp K, Korstjens AH, Layton R, Lee P, Lehmann J, Manson JH, Ramos-Fernandez G, Strier KB, Van Schaik CP. Fission–fusion dynamics new research frameworks. Curr. Anthropol. 2008;49:627–654. [Google Scholar]
- Baayen RH. Analyzing Linguistic Data: A Practical Introduction to Statistics Using R. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 2013;68:255–278. doi: 10.1016/j.jml.2012.11.001. http://dx.doi.org/10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker BM, Walker S. {lme4}: Linear Mixed-effects Models Using Eigen and S4. R Package Version 1. 2014:1–7. [Google Scholar]
- Boeckle M, Bugnyar T. Long-term memory for affiliates in ravens. Curr. Biol. 2012;22:801–806. doi: 10.1016/j.cub.2012.03.023. http://dx.doi.org/10.1016/j.cub.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti SP, Everett MG, Freeman LC. Ucinet for Windows: Software for Social Network Analysis. Analytic Technologies; Harvard, MA: 2002. [Google Scholar]
- Braun A, Bugnyar T. Social bonds and rank acquisition in raven nonbreeder aggregations. Anim. Behav. 2012;84:1507–1515. doi: 10.1016/j.anbehav.2012.09.024. http://dx.doi.org/10.1016/j.anbehav.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Walsdorff T, Fraser ON, Bugnyar T. Socialized sub-groups in a temporary stable Raven flock? J. Ornithol. 2012;153:97–104. doi: 10.1007/s10336-011-0810-2. http://dx.doi.org/10.1007/s10336-011-0810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner C. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. http://dx.doi.org/10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- Carere C, Groothuis TGG, Möstl E, Daan S, Koolhaas JM. Fecal corticosteroids in a territorial bird selected for different personalities: daily rhythm and the response to social stress. Horm. Behav. 2003;43:540–548. doi: 10.1016/s0018-506x(03)00065-5. http://dx.doi.org/10.1016/s0018-506X(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Cockrem JF. Stress, corticosterone responses and avian personalities. J. Ornithol. 2007;148:169–178. http://dx.doi.org/10.1007/s10336-007-0175-8. [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol. Bull. 1985;98:310–357. http://dx.doi.org/10.1037/0033-2909.98.2.310. [PubMed] [Google Scholar]
- Cords M, Aureli F. Reconciliation and relationship qualities. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. University of California Press; Berkeley: 2000. pp. 177–198. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing. Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. http://dx.doi.org/10.1016/S0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Dobson AJ. An Introduction to Generalized Linear Models. Chapman & Hall/CRC; Boca Raton: 2002. [Google Scholar]
- Drack G, Kotrschal K. Aktivitätsmuster und Spiel von freilebenden Kolkraben Corvus corax im inneren Almtal/Oberösterreich. Monticola. 1995;7:159–174. [Google Scholar]
- Eikenaar C, Bairlein F, Stöwe M, Jenni-Eiermann S. Corticosterone, food intake and refueling in a long-distance migrant. Horm. Behav. 2014;65:480–487. doi: 10.1016/j.yhbeh.2014.03.015. http://dx.doi.org/10.1016/j.yhbeh.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Forstmeier W, Schielzeth H. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 2011;65:47–55. doi: 10.1007/s00265-010-1038-5. http://dx.doi.org/10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Bugnyar T. Do ravens show consolation? Responses to distressed others. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010605. http://dx.doi.org/10.1371/journal.pone.0010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Bugnyar T. Ravens reconcile after aggressive conflicts with valuable partners. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018118. http://dx.doi.org/10.1371/journal.pone.0018118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Bugnyar T. Reciprocity of agonistic support in ravens. Anim. Behav. 2012;83:171–177. doi: 10.1016/j.anbehav.2011.10.023. http://dx.doi.org/10.1016/j.anbehav.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Schino G, Aureli F. Components of relationship quality in chimpan-zees. Ethology. 2008;114:834–843. http://dx.doi.org/10.1111/j.1439-0310.2008.01527.x. [Google Scholar]
- Galhardo L, Oliveira RF. The effects of social isolation on steroid hormone levels are modulated by previous social status and context in a cichlid fish. Horm. Behav. 2014;65:1–5. doi: 10.1016/j.yhbeh.2013.10.010. http://dx.doi.org/10.1016/j.yhbeh.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Goymann W. Noninvasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann. N. Y. Acad. Sci. 2005;1046:35–53. doi: 10.1196/annals.1343.005. http://dx.doi.org/10.1196/annals.1343.005. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Ravens in Winter. Summit Books of Simon & Schuster; New York: 1989. [Google Scholar]
- Heinrich B. Conflict, cooperation, and cognition in the common raven. In: Brockmann HJ, Roper TJ, Naguib M, Mitani JC, Simmons LW, editors. Advances in the Study of Behavior. Academic Press; 2011. pp. 189–237. http://dx.doi.org/10.1016/B978-0-12-380896-7.00004-6189. [Google Scholar]
- Holmes W, Phillips J. The adrenal cortex of birds. In: Chester-Jones I, Henderson I, editors. General, Comparative, and Clinical Endocrinology of the Adrenal Cortex. Academic Press; London: 1976. pp. 293–420. [Google Scholar]
- Lendvai Á, Bókony V, Angelier F, Chastel O, Sol D. Do smart birds stress less? An interspecific relationship between brain size and corticosterone levels. Proc. R. Soc. B Biol. Sci. 2013;280 doi: 10.1098/rspb.2013.1734. http://dx.doi.org/10.1098/rspb.2013.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K. Hier bin ich - wo bist Du? Ethologie der Graugans. München; Piper Verlag: 1988. [Google Scholar]
- Loretto MC, Schuster R, Bugnyar T. GPS tracking of Non-breeding ravens reveals the importance of anthropogenic food sources during their dispersal in the Eastern Alps. Curr. Zool. 2015 doi: 10.1093/cz/zow016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunardi DG, Ferreira RG. Fission–fusion dynamics of Guiana dolphin (Sotalia guianensis) groups at Pipa Bay, Rio Grande do Norte, Brazil. Mar. Mammal Sci. 2014;30:1401–1416. http://dx.doi.org/10.1111/mms.12121. [Google Scholar]
- Marzluff JM, Heinrich B. Foraging by common ravens in the presence and absence of territory holders: an experimental analysis of social foraging. Anim. Behav. 1991;42:755–770. http://dx.doi.org/10.1016/S0003-3472(05)80121-6. [Google Scholar]
- Massen JJM, Pašukonis A, Schmidt J, Bugnyar T. Ravens notice dominance reversals among conspecifics within and outside their social group. Nat. Commun. 2014a;5:3679. doi: 10.1038/ncomms4679. http://dx.doi.org/10.1038/ncomms4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massen JJM, Szipl G, Spreafico M, Bugnyar T. Ravens intervene in others’ bonding attempts. Curr. Biol. 2014b;24:2733–2736. doi: 10.1016/j.cub.2014.09.073. http://dx.doi.org/10.1016/j.cub.2014.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier AH, Fivizzani AJ. Changes in the daily rhythm of plasma corticosterone concentration related to seasonal conditions in the white-throated sparrow, Zonotrichia albicollis. Proc. Soc. Exp. Biol. Med. 1975;150:356–362. doi: 10.3181/00379727-150-39035. http://dx.doi.org/10.3181/00379727-150-39035. [DOI] [PubMed] [Google Scholar]
- Möstl E, Palme R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002;23:67–74. doi: 10.1016/s0739-7240(02)00146-7. http://dx.doi.org/10.1016/s0739-7240(02)00146-7. [DOI] [PubMed] [Google Scholar]
- Möstl E, Maggs JL, Schrötter G, Besenfelder U, Palme R. Measurement of cortisol metabolites in faeces of ruminants. Vet. Res. Commun. 2002;26:127–139. doi: 10.1023/a:1014095618125. http://dx.doi.org/10.1023/A:1014095618125. [DOI] [PubMed] [Google Scholar]
- Palme R, Möstl E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Int. J. Mamm. Biol. 1997;62(Suppl. 2):192–197. [Google Scholar]
- Palme R, Touma C, Arias N, Dominchin MF, Lepschy M. Steroid extraction: get the best out of faecal samples. Wiener Tierärztliche Monatsschrift - Vet. Med. Austria. 2013;100:238–246. [Google Scholar]
- Ramos-Fernández G, Morales JM. Unraveling fission–fusion dynamics: how subgroup properties and dyadic interactions influence individual decisions. Behav. Ecol. Sociobiol. 2014;68:1225–1235. http://dx.doi.org/10.1007/s00265-014-1733-8. [Google Scholar]
- Remage-Healey L, Adkins-Regan E, Romero LM. Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm. Behav. 2003;43:108–114. doi: 10.1016/s0018-506x(02)00012-0. http://dx.doi.org/10.1016/s0018-506x(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Rettenbacher S, Möstl E, Hackl R, Ghareeb K, Palme R. Measurement of corticosterone metabolites in chicken droppings. Br. Poult. Sci. 2004;45:704–711. doi: 10.1080/00071660400006156. http://dx.doi.org/10.1080/00071660400006156. [DOI] [PubMed] [Google Scholar]
- Scheiber IBR, Kotrschal K, Weiss BM. Benefits of family reunions: social support in secondary greylag goose families. Horm. Behav. 2009;55:133–138. doi: 10.1016/j.yhbeh.2008.09.006. http://dx.doi.org/10.1016/j.yhbeh.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 2010;1:103–113. http://dx.doi.org/10.1111/j.2041-210X.2010.00012.x. [Google Scholar]
- Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 2009;20:416–420. doi: 10.1093/beheco/arn145. http://dx.doi.org/10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab C, Bugnyar T, Schloegl C, Kotrschal K. Enhanced social learning between siblings in common ravens, Corvus corax. Anim. Behav. 2008;75:501–508. doi: 10.1016/j.anbehav.2007.06.006. http://dx.doi.org/10.1016/j.anbehav.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahler D, Heinrich B, Smith D. Common ravens, Corvus corax, preferentially associate with grey wolves, Canis lupus, as a foraging strategy in winter. Anim. Behav. 2002;64:283–290. http://dx.doi.org/10.1006/anbe.2002.3047. [Google Scholar]
- Stöwe M, Kotrschal K. Behavioural phenotypes may determine whether social context facilitates or delays novel object exploration in ravens (Corvus corax) J. Ornithol. 2007;148:179–184. http://dx.doi.org/10.1007/s10336-007-0145-1. [Google Scholar]
- Stöwe M, Bugnyar T, Loretto MC, Schloegl C, Range F, Kotrschal K. Novel object exploration in ravens (Corvus corax): effects of social relationships. Behav. Process. 2006;73:68–75. doi: 10.1016/j.beproc.2006.03.015. http://dx.doi.org/10.1016/j.beproc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Stöwe M, Bugnyar T, Schloegl C, Heinrich B, Kotrschal K, Möstl E. Corticosterone excretion patterns and affiliative behavior over development in ravens (Corvus corax) Horm. Behav. 2008;53:208–216. doi: 10.1016/j.yhbeh.2007.09.021. http://dx.doi.org/10.1016/j.yhbeh.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöwe M, Drent P, Möstl E. Social context and within pair behaviour may modulate hormonal stress response in great tits (Parus major) In: Heatherton AT, Walcott VA, editors. Handbook of Social Interactions in the 21st Century. Nova Science Publishers, Inc.; 2009. pp. 159–178. [Google Scholar]
- Stöwe M, Rosivall B, Drent PJ, Möstl E. Selection for fast and slow exploration affects baseline and stress-induced corticosterone excretion in Great tit nestlings, Parus major. Horm. Behav. 2010;58:864–871. doi: 10.1016/j.yhbeh.2010.08.011. http://dx.doi.org/10.1016/j.yhbeh.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Stöwe M, Rettenbacher S, Busso JM, Grasse A, Mahr K, Vogl W, Winkler H, Möstl E. Patterns of excreted glucocorticoid metabolites change during development — analytical and physiological implications. Wiener Tierärztliche Monatsschrift - Vet. Med. Austria. 2013;100:295–313. [Google Scholar]
- Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann. N. Y. Acad. Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. http://dx.doi.org/10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.