Hemoglobin variants and increases in circulating fetal hemoglobin (HbF), defined as HbF>2%, have been reported to interfere with some assay methods for HbA1c (1–3). In view of the relatively common occurrence of such increases in HbF (~ 1.5% of the US population), and the fact that individuals with increased HbF are usually asymptomatic, it is important to know whether HbA1c methods show interference and if so, at what level of HbF. In this report, we evaluate the presence of HbF interference with several HPLC methods.
This study was approved by the Institutional Review Board of the University of Utah and Johns Hopkins Medical Institutional Review Board where the samples originated. The following commercial HPLC methods were evaluated: G7 and G8 Variant Mode (Tosoh Bioscience), D-10™ HbA1c Program (D-10), VARIANT™ II HbA1c Program (VII NU), VARIANT™ II TURBO HbA1c Program (VII Turbo), and VARIANT™ II TURBO HbA1c Kit – 2.0 (VII Turbo 2.0) (Bio-Rad Laboratories) and ultra2™ A1c boronate affinity (Trinity Biotech). HbF level was estimated from the G7 or G8 %HbF.
A small number of samples with and without increased HbF (n=24) were analyzed by both the IFCC-CE method (4) and the G7 in order to verify that the G7 did not have interference from increased HbF (3). From 88 to 123 samples (depending upon the method) were analyzed by each of the commercial HPLC methods. Results that were considered acceptable based on manufacturer guidelines for each method were compared to the G7. A multiple regression model (Y=α+βX+γZ+e, where Y = test method %HbA1c, X = G7 %HbA1c, Z = %HbF, e= random error) was used to determine if the relationship between HbA1c results obtained from each test method and the IFCC-CE (in the case of the G7) or G7 method were significantly (P<0.05) affected by HbF. Methods shown to have a statistically significant interference were then evaluated for clinical importance by dividing the samples into three groups, 0–5%, >5%–15%, and >15% HbF, and by conducting a Deming regression comparison of results between each method and the G7. We then calculated the bias between the groups attributable to the presence of increased HbF; a relative percentage difference for a given method between-groups of >7% at a G7 HbA1c level of 6% or 9% was defined as clinically important.
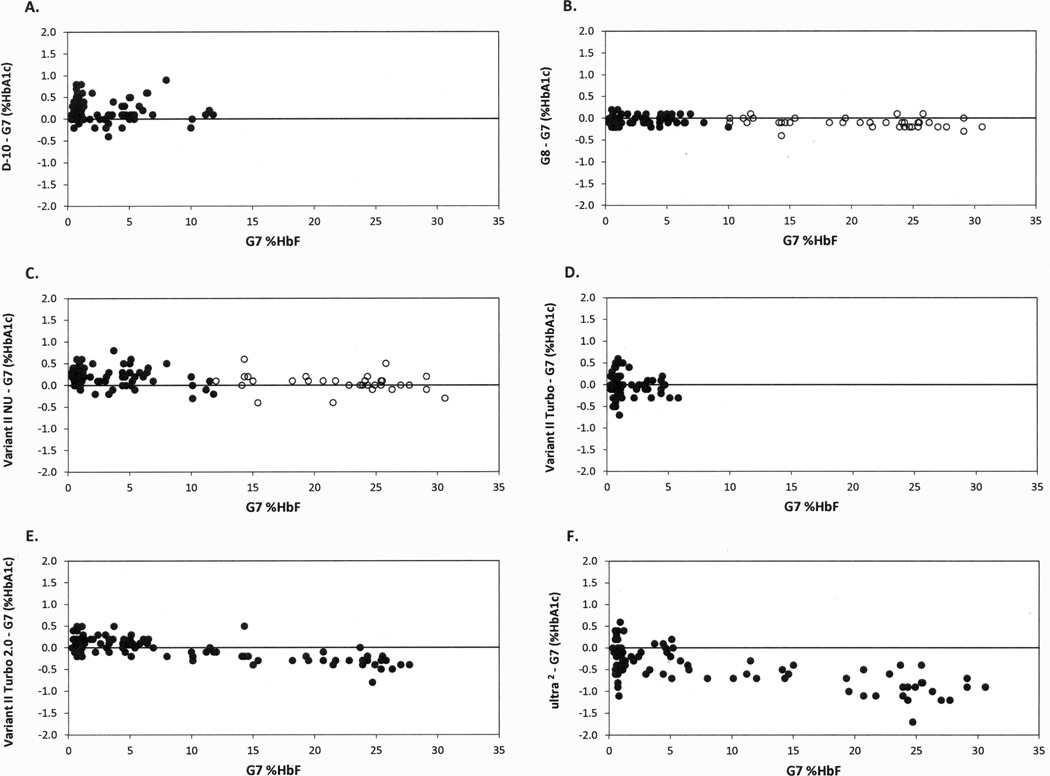
HbA1c results showed excellent agreement between the Tosoh G7 and the IFCC-CE for samples with normal HbF (r2=0.99; y=1.048x−0.172) and for those with HbF >2% (r2=0.99; y=1.038X−0.007). There was no statistically significant difference in the relationships between the IFCC-CE and G7 (P=0.16). Figure 1 shows the bias in percentage HbA1c for six assay methods vs. percentage HbF (by G7) for samples within the manufacturer recommendations and, in two cases, additional results outside the manufacturer’s limits. For the D-10, using only those samples considered to be within the manufacturer specifications (D-10 HbF ≤10%), there was no statistically significant effect of increasing HbF (P=0.4664). For the G8 there was no statistically significant effect of HbF on HbA1c results (P=0.540), within the manufacturer’s claim of 15% HbF. In addition, when all samples were included (up to approximately 30% HbF), the effect of HbF was not found clinically important. For the VII NU, although there was a marginal statistically significant effect of HbF on HbA1c results (VII NU HbF≤10% HbF; P=0.0329), this interference was not clinically important. When all levels of HbF were included there was still no clinically important effect of HbF. For the VII Turbo no statistically significant effect of HbF was found (VII Turbo HbF ≤5%, P=0.2312). For the VII Turbo 2.0, although there was a statistically significant effect of HbF on HbA1c results (VII Turbo 2.0 HbF ≤25%, P<0.0001), the differences were not clinically important. The current results for the Trinity Biotech HPLC support previous findings; the differences are both statistically significant (P<0.0001) and clinically important for samples with increased HbF owing to lower glycation of HbF (1). However, for samples with HbF <15% the effect of HbF on HbA1c results was not clinically important.
Figure 1.
Difference in % HbA1c between each test method (A–F) and the Tosoh G7 for samples vs. %HbF, including samples within the manufacturer’s acceptable ranges (●) as well as results outside the manufacturer’s HbF limits (○) for two methods.
Our results for HbA1c results from the ion-exchange HPLC methods evaluated thus far show that if the manufacturer’s instructions are followed then clinically appropriate results will be reported. Results for the boronate affinity method showed statistically significant and clinically important effects from increased HbF >15%. There are no manufacturer claims for HbF interference for this method. Moreover, as with immunoassay methods for which the same HbF interference applies (1), there is no indication in the reported result that an increased HbF level is present (unlike with ion-exchange HPLC); therefore, artificially low results will be reported for samples with HbF>15%. Physicians and laboratory professionals need to be aware of potential interference from increased HbF levels that could adversely affect HbA1c results.
Acknowledgments
The authors thank Dr. Thomas Kickler and Joanne Wilson at Johns Hopkins Hospital for providing some of the samples for this study and Bio-Rad Laboratories for providing the HbA1c analyses for their methods.
Abbreviations
- HbA1c
Hemoglobin A1c
- IFCC
International Federation for Clinical Chemistry
- CE
Capillary Electrophoresis
- NGSP
National Glycohemoglobin Standardization Program
References
- 1.Rohlfing CL, Connolly SM, England JD, Hanson SE, Moellering CM, Bachelder JR, et al. The effect of elevated fetal hemoglobin on hemoglobin A1c results: five common hemoglobin A1c methods compared with the IFCC reference method. Am J Clin Path. 2008;129:811–814. doi: 10.1309/YFVTUD0GHJF7D16H. [DOI] [PubMed] [Google Scholar]
- 2.Mongia SK, Little RR, Rohlfing CL, Hanson S, Roberts RF, Owen WE, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Path. 2008;130:136–140. doi: 10.1309/1YU0D34VJKNUCGT1. [DOI] [PubMed] [Google Scholar]
- 3.Little RR, Rohlfing CL, Hanson S, Connolly S, Higgins T, Weykamp CW, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54:1277–1282. doi: 10.1373/clinchem.2008.103580. [DOI] [PubMed] [Google Scholar]
- 4.Jeppsson JO, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]