Abstract
Epigenetic mechanisms that regulate endothelial cell (EC) gene expression are now emerging. DNA methylation is the most stable epigenetic mark that confers persisting changes in gene expression. Not only is DNA methylation important in rendering cell identity by regulating cell type-specific gene expression throughout differentiation, but it is becoming clear that DNA methylation also plays a key role in maintaining EC homeostasis and in vascular disease development. Disturbed blood flow (d-flow) causes atherosclerosis while stable flow (s-flow) protects against it by differentially regulating gene expression in ECs. Recently, we and others have shown that flow-dependent gene expression and atherosclerosis development are regulated by mechanisms dependent on DNA methyltransferases (DNMT1 and 3A). D-flow upregulates DNMT expression both in vitro and in vivo which leads to genome-wide DNA methylation alterations and global gene expression changes in a DNMT-dependent manner. These studies revealed several mechanosensitive genes, such as HoxA5, Klf3, and Klf4, whose promoters were hypermethylated by d-flow, but rescued by DNMT inhibitors such as 5Aza-2-deoxycytidine. These findings provide new insight into the mechanism by which flow controls epigenomic DNA methylation patterns, which in turn alters endothelial gene expression, regulates vascular biology, and induces atherosclerosis.
Keywords: Epigenetics, DNA methylation, DNA Methyltransferases, gene expression, flow, shear stress, endothelial cells, atherosclerosis
Introduction
The discovery of disease master regulators that control a large network of genes is of high interest to vascular biologists because such biomolecules would serve as excellent therapeutic targets for disease treatment and prevention. Potentially important master regulators include epigenetic modifiers and transcription factors that participate in pre-transcriptional regulation, microRNAs involved in post-transcriptional regulation, and post-translational modifiers. Epigenetic modifications such as DNA methylation, histone modifications, and chromatin remodeling complexes, alter the genomic DNA structure and accessibility 1. Putative master regulators of vascular biology, including chromatin structure remodelers, epigenetic DNA modifications (cytosine modifications including DNA methylation, and histone modifications), and microRNAs have recently been the subject of extensive research to determine their role in large-scale gene network regulation in endothelial cell biology and cardiovascular disease.
1. The role of flow in atherosclerosis and endothelial cell function
Blood flow generates shear stress on the vascular walls. Unidirectional, laminar shear stress (LS), or stable flow (s-flow) is crucial for normal vascular function, whereas disturbed flow (d-flow), characterized by low and reversing oscillatory shear stress (OS), causes endothelial dysfunction and atherosclerosis 2–6. ECs have dramatically altered gene expression patterns when exposed to d-flow versus s-flow 7–12. Atherosclerosis preferentially develops in areas of d-flow, where the dysfunctional EC phenotype initiates and perpetuates plaque accumulation 13–15.
2. Flow-mediated gene expression
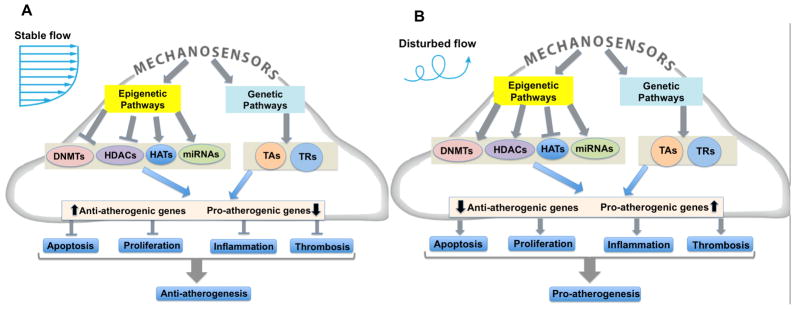
Shear stress regulates endothelial gene expression in vitro and in vivo 5, 16–21. ECs have numerous mechanoreceptors that sense changes in shear stress direction and magnitude which initiates a complex cascade of signaling events that alters global gene expression and regulates endothelial structure and function 22–24. The net effect of s-flow is to upregulate “atheroprotective” genes and downregulate “pro-atherogenic” genes, while the net effect of d-flow is the opposite: upregulation of pro-atherogenic genes and suppression atheroprotective genes (Figure 1). However, the mechanism by which d-flow causes changes in EC gene expression is still incomplete.
Figure 1. Flow regulates both genetic and epigenetic pathways involved in gene expression control – Working model.
(A) Stable flow (s-flow) downregulates DNA Methyltransferases (DNMTs) and histone deactylases (HDACs), and upregulates histone acetyltranferases (HATs). MicroRNAs (miRNAs) and transcription factors (both transcriptional activators (TAs) and transcriptional repressors (TRs) are also regulated by flow and demonstrate molecule-specific, coordinated responses. The interplay of these events leads to orchestrated transcription of a subset of anti-atherogenic genes and also prevents the expression of pro-atherogenic genes. (B) Under d-flow conditions, the opposite responses occur: HATs are downregulated and HDACs and DNMTs are overexpressed, leading to repression of a subset of anti-atherogenic genes and increased expression of a subset of pro-atherogenic genes. This mechanosensitive reprograming of gene expression by epigenomic DNA methylation leads to atherosclerosis development.
3. Epigenetics introduction
Epigenetics is defined as the modification of genetic information in a sequence-independent manner and it may be mediated by altering DNA conformation. Genomic DNA in an open, relaxed conformation (euchromatin) is associated with acetylated histones, unmethylated DNA, and active transcription, whereas condensed heterochromatin is associated with repressive marks such as trimethylated-histone 3 lysine 9 (H3K9), trimethylated-histone 3 lysine 27 (H3K27), and DNA methylation. Heterochromatin generally contains repeat elements, imprinted, and transcriptionally silent genes, and its compaction predominantly occurs during development and differentiation 25–29.
Shear stress has been found to mediate chromatin remodeling and histone modifications and this is thought to play a role in shear-induced gene expression changes 30, 31. LS activates histone acetyltransferase (HAT) activity, deactivates histone deacetylases (HDACs), and induces histone H3/H4 acetylation and H3 phosphorylation in cultured ECs, and this regulates the key EC transcription factors Kruppel-like Factors 2 and 4 (Klf2, Klf4), and endothelial nitric oxide synthase (eNOS), via myocyte enhancer factor-2 (MEF2) 30, 32, 33. Conversely, OS causes HDAC overexpression, and HDAC inhibition prevents OS-induced EC proliferation and EC inflammation in vivo and in vitro 34–36.
DNA methylation involves the addition of a methyl group to the 5′ carbon of a cytosine base pair (5-methylcytosine, 5mC), which occurs most frequently in the context of a CG dinucleotide 37, 38. CpG islands are dense regions of CG sites with higher than expected CG content (generally defined as > 50% with an observed vs. expected ratio of >0.6, over a distance of at least 200 base pairs) 39. Approximately 40% of human genes are associated with CpG islands, which are normally unmethylated 40–43, but transcriptionally repressed regions are generally highly methylated 41. CG sites are otherwise sparse throughout the genome due to a high rate of C to T mutation caused by spontaneous deamination of methylated cytosine, and only functionally important CG sites are likely to be evolutionarily protected from this mutation 44–47.
DNA Methyltransferases (DNMTs) catalyze the addition of the methyl group to cytosine. DNMT1 is thought to preferentially methylate hemi-methylated DNA. Thus, the complementary strand of a CG dinucleotide, which is a mirroring GC dinucleotide, usually carries the same methylation markings as its CG partner. Although DNMT1 is classically referred to as a maintenance methylase, it also has de novo methylation capabilities 48, 49. Both DNMT1 overexpression and DNMT1 deficiency are embryonic lethal, and DNMT1 deletion causes genome-wide hypomethylation while DNMT1 overexpression causes genome-wide hypermethylation 50–53. DNMT3a and DNMT3b are classically referred to as de novo methyltransferases that preferentially add methyl groups to fully unmethylated DNA. DNMT3A and DNMT3B establish global DNA methylation patterns during embryogenesis and gametogenesis 54.
CG methylation in a gene promoter, close to the transcription start site, generally suppresses gene expression 55–57. Promoter methylation is thought to inhibit gene transcription in two ways: first, by physically impeding transcription factor binding to the gene promoter, and second, by binding to methyl-CpG-binding domain proteins which recruit repressive machinery such as histone and chromatin modifiers to the locus that cause chromatin compaction 58. The detailed crosstalk between DNA methylation and histone modifications remains a topic of extensive research 59, 60. However, the role of DNA methylation in the gene body is less straightforward 61–63.
4. DNA Methylation in endothelial cell development and disease
Global DNA methylation patterns change dramatically over the course of cell differentiation and the stable epigenetic marks of terminally differentiated cells govern their gene expression and function 64. EC biology differs across vascular beds based on the functional requirements in a given anatomical region. Differences in EC subtype gene expression are regulated, in part, by epigenetic DNA methylation and histone modifications 65. Similar differences may also be extended to endothelial dysfunction and atherosclerosis.
Aberrant epigenetic patterns of DNA methylation and histone modifications are hallmarks of diseases such as cancer. Although atherosclerosis shares many phenotypic characteristics with cancer, including hyperproliferation, migration, and inflammation, whether those abnormal epigenetic changes also occur in atherosclerosis is unclear.
Drugs that inhibit DNA methyltransferases have proven to be promising treatment options for many cancers. 5Aza-2′-deoxycytidine (5Aza) is a well-studied DNMT1 preferential inhibitor 66. 5Aza is a nucleoside analog that works by trapping DNMT1 in a covalent complex with DNA, resulting in DNMT1 degradation. 5Aza, also known as Decitabine, is used as a chemotherapeutic agent for acute myeloid leukemia and myelodysplastic syndromes 67–71. However, its cytotoxicity limits its therapeutic capacity.
5. Flow-dependent DNA methylation in endothelial biology and atherosclerosis
Recently, three groups working independently and using different model systems converged on a seminal finding that DNA methyltransferases are regulated by shear stress and that they regulate flow-mediated endothelial gene expression and function as well as atherosclerosis.
Jiang et al. showed that flow regulates endothelial Klf4 transcription by DNMT3A-mediated DNA methylation using porcine aorta and cultured human aortic endothelial cells 72. Klf4 is a key mediator of endothelial function and has been well documented to maintain an anti-inflammatory, quiescent EC state in unidirectional flow conditions. They showed that d-flow increases expression of DNMT3A, which in turn induces Klf4 promoter hypermethylation thereby decreasing MEF2 binding to the promoter and ultimately suppressing Klf4 transcription, in a manner reversible by the DNMT inhibitors 5Aza and RG108. Moreover, RG108 treatment reversed the d-flow-induced loss of downstream Klf4 targets eNOS and thrombomodulin (THBD), and the d-flow-induced overexpression of the monocyte chemoattractant protein-1 (MCP-1). They validated that d-flow induces MEF2 binding site hypermethylation in the Klf4 promoter in vivo using endothelium isolated from swine aorta. This work uncovered a novel epigenetic regulatory mechanism for the key EC gene Klf4.
Our group aimed to discover whether d-flow could cause DNA methylation changes that would alter gene expression globally and promote atherosclerosis. Dunn et al. performed genome-wide studies of DNA methylation and gene expression using reduced representation bisulfite sequencing (RRBS) and microarray, respectively 73. We found that d-flow regulates genome-wide DNA methylation patterns in a DNMT-dependent manner. Using partial carotid ligation surgery in a murine model to induce d-flow, we found that DNMT1 expression is upregulated by d-flow in ECs both in vivo and in vitro. Further, 5Aza or DNMT1 siRNA markedly reduced OS-induced endothelial inflammation in human umbilical endothelial cells (HUVECs). Moreover, 5Aza treatment drastically prevented atherosclerosis development in two different models of murine atherosclerosis – the partial carotid ligation and the standard high-fat diet model. More recently, Cao et al. have shown a similar anti-atherogenic effect of 5-Aza using LDLR−/− mice 74.
Zhou et al. also reported that d-flow causes DNMT1 overexpression 75. Using HUVECs, they found that OS increases DNMT1 expression, DNMT1 nuclear translocation, and 5mC content in a 5Aza-inhibitable manner. They also used a rat partial carotid ligation model and showed that d-flow induced DNMT1 protein expression and increased 5mC content.
We further carried out additional studies to determine genome-wide DNA methylome changes and gene expression using the partial carotid ligation model with or without the 5Aza treatment. These two DNA methylome and transcriptome datasets were analyzed by systems biological approaches. Our initial analysis focused on finding genes that are hypermethylated in their promoter regions and have downregulated gene expression by d-flow in a 5Aza-reversible manner. This analysis revealed 11 genes, HoxA5, Klf3, Tmem184b, Adamtsl5, Cmkrl1, Pkp4, Acvrl1, Dok4, Spry2, Zfp46, and F2rl1 (Table 1). Interestingly, 5 of them (HoxA5, Klf3, Cmklr1, Acvrl1, and Spry2) contain C-AMP response elements (CRE) in their promoters. HoxA5 and Klf3 encode transcription factors and thus the methylation status of these loci could serve as a mechanosensitive master switch in gene expression. The biological function of those genes is currently under investigation.
Table 1.
The subset of flow-sensitive genes described here have dramatic d-flow induced promoter hypermethylation corresponding to suppressed gene expression, both of which can be reversed by 5Aza treatment.
| Gene ID | Type | Known Function |
|---|---|---|
| Acvrl1 | serine/threonine kinase receptor | Type I cell-surface TGFβ receptor that may be involved in hemorrhagic telangiectasia, pulmonary arteriovenous malformations, and severe pulmonary arterial hypertension |
| Adamtsl5 | ECM-binding protein | Involved in fibrillin and heparin binding and metalloendopeptidase activity |
| Cmkrl1 | G-protein-coupled receptor | Leukotriene B4 (inflammatory cytokine) receptor that can activate the transcription factor PPARα. May play a role in cardiac muscle contraction and lipid metabolism, and is implicated in pancreatic cancer. |
| Dok4 | Scaffolding protein | Docking platform for assembly of multimolecular signaling complexes. May be involved in T-cell immune regulation, phospholipid binding, and insulin receptor binding. |
| F2rl1 | G-protein-coupled receptor | Proteolytically-activated receptor that modulates inflammatory responses. |
| HoxA5 | Transcription Factor | Differentiation, development, cancer, vascular biology |
| Klf3 | Transcription Factor | Predominantly acts transcriptional repressor. Involved in erythroid cell maturation and adipogenesis |
| Pkp4 | Junctional protein | Armadillo-like protein that plays a role as a regulator of Rho activity during cytokinesis. May play a role in desmosomal junctional plaque organization and cadherin function. |
| Spry2 | Receptor tyrosine kinase signaling protein | Bimodal regulator of epidermal growth factor receptor/MAPk signaling. May function as an antagonist of fibroblast growth factor pathways and may negatively modulate respiratory organogenesis. |
| Tmem184b | Transmembrane Protein | Mostly unknown, but may activate mitogen- activated protein kinase (MAPk) signaling |
| Zfp46 | Transcription Factor | Mostly unknown, but may be a transcriptional repressor. |
Together, these results demonstrated that d-flow controls epigenomic DNA methylation patterns in a DNMT-dependent manner, which in turn alters endothelial gene expression and induces atherosclerosis 73. This study generated new knowledge about the epigenetic EC flow response and uncovered novel therapeutic gene targets for further study.
Genome-wide DNA methylation studies can be used as a discovery platform to reveal novel flow-sensitive genes. Our study was the first to report that HoxA5 and Klf3 are mechanosensitive transcription factors. Homeobox (Hox) genes are highly conserved, known to be important in development and dysregulated in cancer, and are controlled by DNA methylation 76–81. Hox family members have been implicated in vascular remodeling, angiogenesis, and disease by orchestrating changes in gene expression, the extracellular matrix, and integrins 82. HoxA5 is known to regulate various endothelial functions such as migration, angiogenesis, and inflammation 83–89. Klf3, unlike the other two well-known known mechanosensitive transcriptional activators Klf2 and Klf4, is known to repress transcription 90–92. Interestingly, Klf3 is suppressed in acute myeloid leukemia (AML), and 5Aza is used to treat this disease. AML is also treated with all-trans retinoic acid, which was shown to rescue Klf3 expression by an unknown mechanism 93, 94. It would be interesting to determine whether the therapeutic effects of 5Aza and all-trans retinoic acid on AML are mediated, at least in part, by rescuing Klf3 promoter hypermethylation.
Atherosclerosis develops due to dysfunction of multiple cell types, including ECs, smooth muscle cells (SMCs), immune cells, and fibroblasts, and the anti-atherogenic effects of 5Aza have been shown to be mediated by not only ECs, but also by immune cells 73 (114).
6. Relationship between histone modification and DNA methylation and epigenetic crosstalk
Flow mediated gene expression is regulated by both genetic (sequence-dependent transcription factor binding) as well as epigenetic (histone modifications and DNA methylation) mechanisms (Figure 1). Although it has not been directly demonstrated under different flow conditions in endothelial cells, it is well established that epigenetic mechanisms involving histone modifications (e.g., acetylation, deacetylation, methylation, phosphorylation) and DNA methylation interact and influence one another, affecting overall gene expression activity. It is also likely that interaction of these epigenetic and genetic mechanisms leads to an orchestrated net effect of s-flow (or LS) and d-flow (or OS) on mechanosensitive gene expression in ECs. The importance and extent of these interactions remains to be determined. Our working model is that DNA methylation in the promoter regions by d-flow leads to chronic repression of anti-atherogenic genes (epigenetic mechanisms), while pro-atherogenic gene expression is mediated by regulation of transcription factors (genetic pathways). In contrast, s-flow rescues (de-represses) expression of anti-atherogenic genes by decreasing DNA methylation in the promoter regions via reduced DNMT activity and altered histone modifications. S-flow also regulates genetic pathways by regulating transcription factor binding to specific gene promoter sites, leading to increased anti-atherogenic genes and decreased pro-atherogenic genes.
Most of the DNA CpG sites are methylated during development by DNMT3a and DNMT3b with CpG islands being subject to differential methylation affected by various conditions. There have been numerous reports describing the interaction between histone modifications such as acetylation and methylation (ex: by G9a, or SET domain containing histone methytransferases) with DNA methylation and repression of transcription. DNMT3A and DNMT3B share an unmodified-histone binding site. H3K4 methylation is associated with decreased DNA methytion in multiple cell types, whereas H3K9 methylation and histone deacytelation are associated with increased DNA methylation which leads to gene repression by forming heterochromatin 95. DNMT3A and DNMT3B also harbor domains that read H3K36me3 which is found in the bodies of certain genes and it is thus speculated that they play a role in gene body methylation which is abundant but not very well understood 95. Moreover, DNMT1 also recognizes H3K23 monoubiquitination which seems to play a role in maintenance of DNA methylation 95.
Emerging evidence indicates that DNA methylation can also affect histone modifications via interactions of DNMTs with histone methyltransferases and deacytelases. For example, CpG methylation enhances UHRF1 (Ubiquitin-like containing PHD and RING finger domains 1, a DNMT1 recruiter) and perturbs KDM2A (Lysine-specific demethylase 2A, a H3K36 demythelase enzyme) binding to H3K9me3-containing nucleosomes 96. Also, DNA methylation causes H3K9 demethylation, which is a marker of repressive chromatin, by interaction of G9a and DNMT1. DNA methylation also inhibits H3K4 methylation which is a marker of active transcription 96. This is supported by the fact that unmethylated DNA is mainly assembled in nucleosomes that contain acetylated histones, while methylated DNA is assembled in nucleosomes containing acetylated H3 and H4 which leads to more compact chromatin. Moreover, there have been reports that methylcytosine-binding proteins, such as meCP2 or mbD2 might help recruit HDACs and thus lead to histone acetylation 96.
It is becoming clear that an interplay exists among methylation, miRNAs and histone acetylation. Hypermethylation of multiple miRNA promoter sites (ex: miR-1, 26a and 137) has been reported in multiple cancer types. Moreover, HDACs (HDAC1-9 and 11) are direct targets of miRNAs, while HDACs have shown to regulate expression of miRNAs (e.g., miR-15-a/16-1, 183, 224, 449a). In ECs, Klf4 and Klf2 are two key flow-sensitive genes that are co-regulated by miR-92, and regulate expresion of multiple microRNAs as well 97. As we described above, Klf4 is also controlled by flow-dependent DNA methylation 72. This opens up the interesting possibility of cooperation between flow-mediated epigenetic events.
Perspectives
Emerging studies clearly demonstrate that d-flow regulates epigenomic DNA methylation by upregulated DNMTs in ECs, which in turn alters endothelial gene expression and function, ultimately contributing to atherosclerosis. The advent of new technologies to resolve genome-level methylation at the nucleotide scale combined with advanced systems biology approaches will continue to play a key role better understanding the effects of DNA methylation in gene network regulation, endothelial biology, and vascular disease. Currently, DNA methylation studies mainly focus on studying 5mC, but other modifications such as 5-hydroxymethylcytosine (5hC), 5-formylcytosine (5fC) and 5-carboxycytosine (5cC) are also likely to be important. In addition, demethylating enzymes such as Ten-eleven-translocation (TET) may also play an important role in regulating gene expression in this disease.
Experimental methods to interrogate the epigenome of flow-regulated ECs involve the use of either in vivo-derived or in vitro cultured cells. In vivo methods have a clear benefit due to biologically artificial conditions of cell culture, which are known to have epigenetic consequences. For example, ECs in vivo are constantly exposed to flow, whereas cultured ECs must be exposed to static, no-flow conditions during the culture process and non-adherent conditions during passaging. These intermediate mechanical conditions can have dramatic effects on long-term epigenetic state. Gene expression studies by microarray using cultured ECs demonstrate that in vitro biological responses are often quite different from events that occur in vivo98. This raises an important consideration, especially persisting epigenetic changes, for the limitations of in vitro studies and points to the major benefit of models that utilize in vivo-derived genomic DNA with no intermediate cell culture steps.
There are several methods available to interrogate genome-wide DNA methylation patterns. The most comprehensive is whole genome bisulfite sequencing, but its high cost and the large amount of data generated, including poorly understood intergenic “desert” regions, currently makes whole genome sequencing a less desirable method. Alternative methods with higher efficiency and practicality to resolve the endothelial DNA methylome include methylation microarrays and reduced representation bisulfite sequencing (RRBS) methods. Methylation microarrays are limited by the probe set and only examine regions defined by previous knowledge (such as promoter- and CpG island-confined CpG sites). RRBS is currently a gold standard for genome-scale methylation studies at the nucleotide resolution, although incomplete CG coverage is an inherent limitation of RRBS due to the MspI restriction enzyme digest, PCR, and sequencing steps99. Both methylation microarrays and RRBS produce partial genomic coverage, which may result in overlooking potentially important methylation sites. Compared to currently existing technologies, RRBS is a much more comprehensive but cost-effective method to interrogate genome-wide DNA methylation.
While most work in this realm has been mainly focused on CpG methylation in the promoter region of genes, DNA methylation in other locales, including coding or noncoding gene regulatory regions, and at other motifs such as CpH (where H is A/T/C), are known to be important for controlling gene expression, alternative transcripts, chromatin conformation and genome stabilization 61, 62, 100, 101. Additionally, while DNA methylation at the 5′ carbon of a cytosine base pair is the most well studied epigenetic covalent DNA modification, several others are known to exist and were recently implicated to be important in gene expression regulation, although their mechanisms are very unclear. These modifications include 5-hydroxymethylcytosine (5hC), 5-formylcytosine (5fC) and 5-carboxycytosine (5cC)102. Emerging methods of detection will enable future study of these cytosine modifications in endothelial biology and pathogenesis 103.
Much remains to be determined about the nature of DNA methylation changes in response to flow; How does d-flow regulate DNMT expression and what controls flow-dependent DNA demethylation? What are the functional consequences of DNA methylation at the genome-wide and individual gene levels? How does DNA methylation at a specific promoter site affect transcription of the flow-sensitive genes? It is also important to compare and translate mouse data to clinical data, with important limitations to experimental models, especially since human atherosclerosis develops over decades and is multifactorial in nature. For example, the study of epigenetic factors involved in atherosclerosis would expectedly become more complicated in humans due to differences in life habits such as smoking, sedentary life styles as well as other comorbidities such as hypertension, diabetes and obesity. There have been conflicting reports showing global hypomethylation in human atherosclerotic plaques 104, while other studies in contrast showed hypermethylation across many genomic loci in diseased aortas 105, indicating that more studies are needed. Given the known side effects of using DNMT inhibitors such as 5Aza as therapeutics, future studies are needed to identify additional downstream target genes that may be more effective therapeutic points of intervention with reduced undesirable effects.
Significance
These emerging findings surrounding the endothelial cell epigenetic response to flow provide new insight into the mechanism by which flow controls epigenomic DNA methylation, which in turn regulates endothelial gene expression, vascular biology, and ultimately atherogenesis. Based on our recent mouse DNA methylome study, it is tempting to speculate that physical inactivity increases atherosclerosis risk partly because it is associated with d-flow, which leads to chronic repression of anti-atherogenic genes by the epigenetic DNA methylation-dependent mechanism. In contrast, the well-known anti-atherogenic effect of aerobic exercise may be due in part mediated by de-repression of those anti-atherogenic genes caused by DNA methylation. The discovery of endothelial cell disease master regulators that control a large network of genes, such as DNA Methyltransferases, and their specific target genes is important to enable further development of therapeutics for vascular disease treatment and prevention.
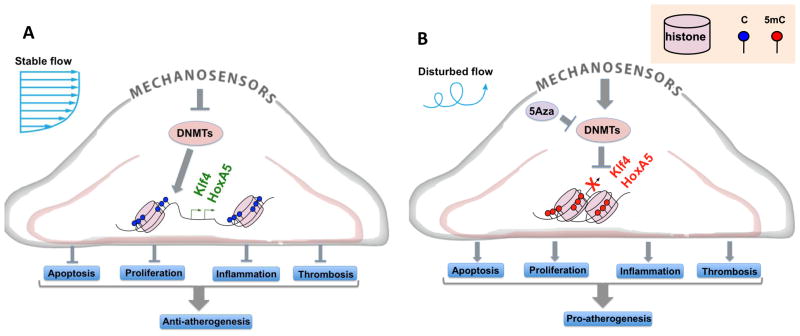
Figure 2.
(A) Stable flow (s-flow) downregulates expression of DNA Methyltransferases (DNMTs) which allows the promoter of anti-atherogenic genes such as Klf4 and HoxA5 to remain demethylated, enabling their expression. (B) Disturbed flow (d-flow) upregulates DNMT expression, leading to hypermethylation of the promoter of anti-atherogenic genes such as Klf4 and HoxA5, repressing their expression.
Acknowledgments
All authors, JD, ST and HJ have significantly contributed to writing of this article and approved it.
Sources of funding: This work was supported by funding from National Institutes of Health grants HL119798, HL113451, HL095070 and HL124879 to H. Jo. JD is a National Science Foundation pre-doctoral fellow.
Footnotes
Disclosures: No conflict of interest to disclosure.
References
- 1.Robertson KD. DNA methylation and chromatin - unraveling the tangled web. Oncogene. 2002;21:5361–5379. doi: 10.1038/sj.onc.1205609. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(sdi1/cip1/waf1) Circ Res. 2000;86:185–190. doi: 10.1161/01.res.86.2.185. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 6.He XH, Wu GF, Zhang Y, et al. effect of chronic enhanced external counterpulastion on gene expression profiles of arterial endothelial cells of pigs fed with high-cholesterol diet. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2008;28:1195–1197. [PubMed] [Google Scholar]
- 7.White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrevoets AJ, Newby AC. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol. 2011;226:2841–2848. doi: 10.1002/jcp.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Annals of the New York Academy of Sciences. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- 9.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan SS, Avati Nanjundappa RP, Branch JR, Taylor WR, Quyyumi AA, Jo H, McDaniel MC, Suo J, Giddens D, Samady H. Shear stress and plaque development. Expert review of cardiovascular therapy. 2010;8:545–556. doi: 10.1586/erc.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear stress induces expression of vascular endothelial growth factor receptor flk-1/kdr through the ct-rich sp1 binding site. Arterioscler Thromb Vasc Biol. 2002;22:907–913. doi: 10.1161/01.atv.0000018300.43492.83. [DOI] [PubMed] [Google Scholar]
- 12.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: Cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, Timmins LH, Quyyumi AA, Giddens DP. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 15.Pedrigi RM, de Silva R, Bovens SM, Mehta VV, Petretto E, Krams R. Thin-cap fibroatheroma rupture is associated with a fine interplay of shear and wall stress. Arterioscler Thromb Vasc Biol. 2014;34:2224–2231. doi: 10.1161/ATVBAHA.114.303426. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Cardena G, Comander JI, Blackman BR, Anderson KR, Gimbrone MA. Mechanosensitive endothelial gene expression profiles: Scripts for the role of hemodynamics in atherogenesis? Ann N Y Acad Sci. 2001;947:1–6. [PubMed] [Google Scholar]
- 17.Chien S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 18.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr, Gimbrone MA., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skogsberg J, Lundstrom J, Kovacs A, Nilsson R, Noori P, Maleki S, Kohler M, Hamsten A, Tegner J, Bjorkegren J. Transcriptional profiling uncovers a network of cholesterol-responsive atherosclerosis target genes. PLoS Genet. 2008;4:e1000036. doi: 10.1371/journal.pgen.1000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiological genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Tarbell JM, Shi ZD, Dunn J, Jo H. Fluid mechanics, arterial disease, and gene expression. Annu Rev Fluid Mech. 2014;46:591–614. doi: 10.1146/annurev-fluid-010313-141309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwak BR, Back M, Bochaton-Piallat ML, et al. Biomechanical factors in atherosclerosis: Mechanisms and clinical implications. European heart journal. 2014;35:3013–3020. 3020a–3020d. doi: 10.1093/eurheartj/ehu353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 25.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 26.Ansel KM, Lee DU, Rao A. An epigenetic view of helper t cell differentiation. Nature immunology. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 27.de Laat W, Grosveld F. Spatial organization of gene expression: The active chromatin hub. Chromosome research : an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2003;11:447–459. doi: 10.1023/a:1024922626726. [DOI] [PubMed] [Google Scholar]
- 28.Fahrner JA, Baylin SB. Heterochromatin: Stable and unstable invasions at home and abroad. Genes & development. 2003;17:1805–1812. doi: 10.1101/gad.1123303. [DOI] [PubMed] [Google Scholar]
- 29.Ragoczy T, Telling A, Sawado T, Groudine M, Kosak ST. A genetic analysis of chromosome territory looping: Diverse roles for distal regulatory elements. Chromosome research : an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2003;11:513–525. doi: 10.1023/a:1024939130361. [DOI] [PubMed] [Google Scholar]
- 30.Illi B, Nanni S, Scopece A, Farsetti A, Biglioli P, Capogrossi MC, Gaetano C. Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ Res. 2003;93:155–161. doi: 10.1161/01.RES.0000080933.82105.29. [DOI] [PubMed] [Google Scholar]
- 31.Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, Capogrossi MC, Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96:501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Bacanamwo M, Harrison DG. Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric-oxide synthase mrna transcription. J Biol Chem. 2008;283:16293–16298. doi: 10.1074/jbc.M801803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of hdac5 and mediates expression of klf2 and enos. Blood. 2010;115:2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee DY, Lee CI, Lin TE, Lim SH, Zhou J, Tseng YC, Chien S, Chiu JJ. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci U S A. 2012;109:1967–1972. doi: 10.1073/pnas.1121214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue K, Kobayashi M, Yano K, Miura M, Izumi A, Mataki C, Doi T, Hamakubo T, Reid PC, Hume DA, Yoshida M, Aird WC, Kodama T, Minami T. Histone deacetylase inhibitor reduces monocyte adhesion to endothelium through the suppression of vascular cell adhesion molecule-1 expression. Arterioscler Thromb Vasc Biol. 2006;26:2652–2659. doi: 10.1161/01.ATV.0000247247.89787.e7. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Mahmud SA, Bitterman PB, Huo Y, Slungaard A. Histone deacetylase inhibitors suppress tf-kappab-dependent agonist-driven tissue factor expression in endothelial cells and monocytes. J Biol Chem. 2007;282:28408–28418. doi: 10.1074/jbc.M703586200. [DOI] [PubMed] [Google Scholar]
- 37.Jeltsch A. Beyond watson and crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem. 2002;3:274–293. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Eckhardt F, Lewin J, Cortese R, et al. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet. 2006;38:1378–1385. doi: 10.1038/ng1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardiner-Garden M, Frommer M. Cpg islands in vertebrate genomes. Journal of molecular biology. 1987;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 40.Bestor TH, Gundersen G, Kolsto AB, Prydz H. Cpg islands in mammalian gene promoters are inherently resistant to de novo methylation. Genetic analysis, techniques and applications. 1992;9:48–53. doi: 10.1016/1050-3862(92)90030-9. [DOI] [PubMed] [Google Scholar]
- 41.Takai D, Jones PA. Comprehensive analysis of cpg islands in human chromosomes 21 and 22. Proc Natl Acad Sci U S A. 2002;99:3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strichman-Almashanu LZ, Lee RS, Onyango PO, Perlman E, Flam F, Frieman MB, Feinberg AP. A genome-wide screen for normally methylated human cpg islands that can identify novel imprinted genes. Genome Res. 2002;12:543–554. doi: 10.1101/gr.224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rollins RA, Haghighi F, Edwards JR, Das R, Zhang MQ, Ju J, Bestor TH. Large-scale structure of genomic methylation patterns. Genome Res. 2006;16:157–163. doi: 10.1101/gr.4362006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Chen SS. The tendency to recreate ancestral cg dinucleotides in the human genome. BMC evolutionary biology. 2011;11:3. doi: 10.1186/1471-2148-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan BK, Miller JH. Mutagenic deamination of cytosine residues in DNA. Nature. 1980;287:560–561. doi: 10.1038/287560a0. [DOI] [PubMed] [Google Scholar]
- 46.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 47.Rideout WM, 3rd, Coetzee GA, Olumi AF, Jones PA. 5-methylcytosine as an endogenous mutagen in the human ldl receptor and p53 genes. Science. 1990;249:1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- 48.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh CL. The de novo methylation activity of dnmt3a is distinctly different than that of dnmt1. BMC Biochem. 2005;6:6. doi: 10.1186/1471-2091-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki M. Rna interference-mediated knockdown of DNA methyltransferase 1 leads to promoter demethylation and gene re-expression in human lung and breast cancer cells. Cancer Res. 2004;64:3137–3143. doi: 10.1158/0008-5472.can-03-3046. [DOI] [PubMed] [Google Scholar]
- 51.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 52.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 53.Biniszkiewicz D, Gribnau J, Ramsahoye B, Gaudet F, Eggan K, Humpherys D, Mastrangelo MA, Jun Z, Walter J, Jaenisch R. Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 2002;22:2124–2135. doi: 10.1128/MCB.22.7.2124-2135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okano M, Li E. Genetic analyses of DNA methyltransferase genes in mouse model system. The Journal of nutrition. 2002;132:2462S–2465S. doi: 10.1093/jn/132.8.2462S. [DOI] [PubMed] [Google Scholar]
- 55.Song F, Smith JF, Kimura MT, Morrow AD, Matsuyama T, Nagase H, Held WA. Association of tissue-specific differentially methylated regions (tdms) with differential gene expression. Proc Natl Acad Sci U S A. 2005;102:3336–3341. doi: 10.1073/pnas.0408436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grunau C, Hindermann W, Rosenthal A. Large-scale methylation analysis of human genomic DNA reveals tissue-specific differences between the methylation profiles of genes and pseudogenes. Hum Mol Genet. 2000;9:2651–2663. doi: 10.1093/hmg/9.18.2651. [DOI] [PubMed] [Google Scholar]
- 57.Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schubeler D. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- 58.Clouaire T, de Las Heras JI, Merusi C, Stancheva I. Recruitment of mbd1 to target genes requires sequence-specific interaction of the mbd domain with methylated DNA. Nucleic Acids Res. 2010;38:4620–4634. doi: 10.1093/nar/gkq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashimoto H, Vertino PM, Cheng X. Molecular coupling of DNA methylation and histone methylation. Epigenomics. 2010;2:657–669. doi: 10.2217/epi.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007;27:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maunakea AK, Nagarajan RP, Bilenky M, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jjingo D, Conley AB, Yi SV, Lunyak VV, Jordan IK. On the presence and role of human gene-body DNA methylation. Oncotarget. 2012;3:462–474. doi: 10.18632/oncotarget.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, Cross MK, Williams BA, Stamatoyannopoulos JA, Crawford GE, Absher DM, Wold BJ, Myers RM. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013;23:555–567. doi: 10.1101/gr.147942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jamal A, Man HS, Marsden PA. Gene regulation in the vascular endothelium: Why epigenetics is important for the kidney. Seminars in nephrology. 2012;32:176–184. doi: 10.1016/j.semnephrol.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T, Jacob ST. 5-aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the ken box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol. 2005;25:4727–4741. doi: 10.1128/MCB.25.11.4727-4741.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Mossman D, Kim KT, Scott RJ. Demethylation by 5-aza-2′-deoxycytidine in colorectal cancer cells targets genomic DNA whilst promoter cpg island methylation persists. BMC Cancer. 2010;10:366. doi: 10.1186/1471-2407-10-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z, Zhang L, Ding F, Li J, Guo M, Li W, Wang Y, Yu Z, Zhan Q, Wu M. 5-aza-2′-deoxycytidine induces retinoic acid receptor-beta(2) demethylation and growth inhibition in esophageal squamous carcinoma cells. Cancer Lett. 2005;230:271–283. doi: 10.1016/j.canlet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 69.Christman JK. 5-azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 70.Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S, Talpaz M, Garcia-Manero G, Kantarjian HM. Phase ii study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 71.Lemaire M, Chabot GG, Raynal NJ, Momparler LF, Hurtubise A, Bernstein ML, Momparler RL. Importance of dose-schedule of 5-aza-2′-deoxycytidine for epigenetic therapy of cancer. BMC Cancer. 2008;8:128. doi: 10.1186/1471-2407-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang YZ, Jimenez JM, Ou K, McCormick ME, Zhang LD, Davies PF. Hemodynamic disturbed flow induces differential DNA methylation of endothelial kruppel-like factor 4 (klf4) promoter in vitro and in vivo. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.303883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124:3187–3199. doi: 10.1172/JCI74792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao Q, Wang X, Jia L, Mondal AK, Diallo A, Hawkins GA, Das SK, Parks JS, Yu L, Shi H, Shi H, Xue B. Inhibiting DNA methylation by 5-aza-2′-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology. 2014 doi: 10.1210/en.2014-1595. en20141595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou J, Li YS, Wang KC, Chien S. Epigenetic mechanism in regulation of endothelial function by disturbed flow: Induction of DNA hypermethylation by dnmt1. Cell Mol Bioeng. 2014;7:218–224. doi: 10.1007/s12195-014-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanzer A, Amemiya CT, Kim CB, Stadler PF. Evolution of micrornas located within hox gene clusters. Journal of experimental zoology. Part B, Molecular and developmental evolution. 2005;304:75–85. doi: 10.1002/jez.b.21021. [DOI] [PubMed] [Google Scholar]
- 77.Illingworth R, Kerr A, Desousa D, Jorgensen H, Ellis P, Stalker J, Jackson D, Clee C, Plumb R, Rogers J, Humphray S, Cox T, Langford C, Bird A. A novel cpg island set identifies tissue-specific methylation at developmental gene loci. PLoS biology. 2008;6:e22. doi: 10.1371/journal.pbio.0060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rossig L, Urbich C, Bruhl T, et al. Histone deacetylase activity is essential for the expression of hoxa9 and for endothelial commitment of progenitor cells. The Journal of experimental medicine. 2005;201:1825–1835. doi: 10.1084/jem.20042097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han L, Witmer PD, Casey E, Valle D, Sukumar S. DNA methylation regulates microrna expression. Cancer biology & therapy. 2007;6:1284–1288. doi: 10.4161/cbt.6.8.4486. [DOI] [PubMed] [Google Scholar]
- 80.Pei L, Choi JH, Liu J, et al. Genome-wide DNA methylation analysis reveals novel epigenetic changes in chronic lymphocytic leukemia. Epigenetics. 2012;7:567–578. doi: 10.4161/epi.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rauch TA, Wu X, Zhong X, Riggs AD, Pfeifer GP. A human b cell methylome at 100-base pair resolution. Proc Natl Acad Sci U S A. 2009;106:671–678. doi: 10.1073/pnas.0812399106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorski DH, Walsh K. The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res. 2000;87:865–872. doi: 10.1161/01.res.87.10.865. [DOI] [PubMed] [Google Scholar]
- 83.Lee JY, Park KS, Cho EJ, Joo HK, Lee SK, Lee SD, Park JB, Chang SJ, Jeon BH. Human hoxa5 homeodomain enhances protein transduction and its application to vascular inflammation. Biochem Biophys Res Commun. 2011;410:312–316. doi: 10.1016/j.bbrc.2011.05.139. [DOI] [PubMed] [Google Scholar]
- 84.Myers C, Charboneau A, Cheung I, Hanks D, Boudreau N. Sustained expression of homeobox d10 inhibits angiogenesis. Am J Pathol. 2002;161:2099–2109. doi: 10.1016/S0002-9440(10)64488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role for hox a5 in regulating angiogenesis and vascular patterning. Lymphatic research and biology. 2005;3:240–252. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- 86.Arderiu G, Cuevas I, Chen A, Carrio M, East L, Boudreau NJ. Hoxa5 stabilizes adherens junctions via increased akt1. Cell adhesion & migration. 2007;1:185–195. doi: 10.4161/cam.1.4.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y, Cuevas IC, Gabriel RA, Su H, Nishimura S, Gao P, Fields A, Hao Q, Young WL, Yang GY, Boudreau NJ. Restoring transcription factor hoxa5 expression inhibits the growth of experimental hemangiomas in the brain. Journal of neuropathology and experimental neurology. 2009;68:626–632. doi: 10.1097/NEN.0b013e3181a491ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y, Gorski DH. Regulation of angiogenesis through a microrna (mir-130a) that down-regulates antiangiogenic homeobox genes gax and hoxa5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H, Rubin E, Zhang H, Chung S, Jie CC, Garrett E, Biswal S, Sukumar S. Identification of transcriptional targets of hoxa5. J Biol Chem. 2005;280:19373–19380. doi: 10.1074/jbc.M413528200. [DOI] [PubMed] [Google Scholar]
- 90.Pearson RC, Funnell AP, Crossley M. The mammalian zinc finger transcription factor kruppel-like factor 3 (klf3/bklf) IUBMB life. 2011;63:86–93. doi: 10.1002/iub.422. [DOI] [PubMed] [Google Scholar]
- 91.Villarreal G, Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, Garcia-Cardena G. Defining the regulation of klf4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun. 2010;391:984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr, Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (klf2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 93.Miftakhova R, Sandberg T, Hedblom A, Nevzorova T, Persson JL, Bredberg A. DNA methylation in atra-treated leukemia cell lines lacking a pml-rar chromosome translocation. Anticancer research. 2012;32:4715–4722. [PubMed] [Google Scholar]
- 94.Humbert M, Halter V, Shan D, Laedrach J, Leibundgut EO, Baerlocher GM, Tobler A, Fey MF, Tschan MP. Deregulated expression of kruppel-like factors in acute myeloid leukemia. Leukemia research. 2011;35:909–913. doi: 10.1016/j.leukres.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Cedar H, Bergman Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 96.Rothbart SB, Strahl BD. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 2014;1839:627–643. doi: 10.1016/j.bbagrm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fang Y, Davies PF. Site-specific microrna-92a regulation of kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hongcang Gu CB, Mikkelsen Tarjei S, Natalie Jäger ZDS, Tomazou Eleni, Gnirke Andreas, Lander Eric S, Meissner A. Genome-scale dna methylation mapping of clinical samples at single-nucleotide resolution. Nat Methods. 2010:7. doi: 10.1038/nmeth.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lorincz MC, Dickerson DR, Schmitt M, Groudine M. Intragenic DNA methylation alters chromatin structure and elongation efficiency in mammalian cells. Nature structural & molecular biology. 2004;11:1068–1075. doi: 10.1038/nsmb840. [DOI] [PubMed] [Google Scholar]
- 101.Guo JU, Su Y, Shin JH, et al. Distribution, recognition and regulation of non-cpg methylation in the adult mammalian brain. Nature neuroscience. 2014;17:215–222. doi: 10.1038/nn.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng J, Shao N, Szulwach KE, et al. Role of tet1 and 5-hydroxymethylcytosine in cocaine action. Nature neuroscience. 2015;18:536–544. doi: 10.1038/nn.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kriaucionis S, Tahiliani M. Expanding the epigenetic landscape: Novel modifications of cytosine in genomic DNA. Cold Spring Harbor perspectives in biology. 2014;6:a018630. doi: 10.1101/cshperspect.a018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aavik E, Lumivuori H, Leppanen O, Wirth T, Hakkinen SK, Brasen JH, Beschorner U, Zeller T, Braspenning M, van Criekinge W, Makinen K, Yla-Herttuala S. Global DNA methylation analysis of human atherosclerotic plaques reveals extensive genomic hypomethylation and reactivation at imprinted locus 14q32 involving induction of a mirna cluster. European heart journal. 2014 doi: 10.1093/eurheartj/ehu437. [DOI] [PubMed] [Google Scholar]
- 105.Zaina S, Heyn H, Carmona FJ, Varol N, Sayols S, Condom E, Ramirez-Ruz J, Gomez A, Goncalves I, Moran S, Esteller M. DNA methylation map of human atherosclerosis. Circulation. Cardiovascular genetics. 2014;7:692–700. doi: 10.1161/CIRCGENETICS.113.000441. [DOI] [PubMed] [Google Scholar]