Abstract
Background
Deficits characteristic of Attention Deficit/Hyperactivity Disorder (ADHD), including poor attention and inhibitory control, are at least partially alleviated by factors that increase engagement of attention, suggesting a hypodopaminergic reward deficit. Lapses of attention are associated with attenuated deactivation of the Default Mode Network (DMN), a distributed brain system normally deactivated during tasks requiring attention to the external world. Task-related DMN deactivation has been shown to be attenuated in ADHD relative to controls. We hypothesised that motivational incentives to balance speed against restraint would increase task engagement during an inhibitory control task, enhancing DMN deactivation in ADHD. We also hypothesised that methylphenidate, an indirect dopamine agonist, would tend to normalise abnormal patterns of DMN deactivation.
Method
We obtained functional magnetic resonance images from eighteen methylphenidate-responsive children with ADHD (DSM-IV combined subtype) and 18 pairwise-matched typically developing children aged 9-15 years while they performed a paced Go/No-go task. We manipulated motivational incentive to balance response speed against inhibitory control, and tested children with ADHD both on and off methylphenidate.
Results
When children with ADHD were off-methylphenidate and task incentive was low, event-related DMN deactivation was significantly attenuated compared to controls, but the two groups did not differ under high motivational incentives. The modulation of DMN deactivation by incentive in the children with ADHD, off- methylphenidate, was statistically significant, and significantly greater than in typically developing children. When children with ADHD were on-methylphenidate, motivational modulation of event-related DMN deactivation was abolished, and no attenuation relative to their typically developing peers was apparent in either motivational condition.
Conclusions
During an inhibitory control task, children with ADHD exhibit a raised motivational threshold at which task-relevant stimuli become sufficiently salient to deactivate the DMN. Treatment with methylphenidate normalises this threshold, rendering their pattern of task-related DMN deactivation indistinguishable from that of typically developing children.
Keywords: Attention Deficit/Hyperactivity Disorder, Default Mode Network, Inhibitory Control, Motivation, Methylphenidate
Children with Attention Deficit/Hyperactivity Disorder (ADHD) may exhibit striking deficits in both attention and inhibitory control (Barkley, 1997; Sagvolden & Sergeant, 1998), although, intriguingly, their performance and behavior may approach that of their peers when a task is novel, stimulating or rewarding (Borger & van der Meere, 2000; Luman, Oosterlaan, & Sergeant, 2005; Slusarek, Velling, Bunk, & Eggers, 2001). This has led to the hypothesis that a motivational, possibly hypodopaminergic, dysfunction may underpin the disorder (Johansen, Aase, Meyer, & Sagvolden, 2002; Sergeant, 2000). Wilkison et al. (1995) found that methylphenidate, a dopamine re-uptake inhibitor, increased the value of reward in boys with ADHD, while Volkow et al. (2004; 2008) found that in healthy adults, methylphenidate enhanced the salience of a rewarded task, increased levels of extra-cellular dopamine, and induced reductions in glucose metabolism within the Default Mode Network (DMN).
The DMN is a distributed brain system, comprising medial pre-frontal cortex and medial and lateral parietal regions. It is anticorrelated with attentional networks activated by goal-directed behaviour, and is thought to reflect intrinsic brain activity, hence the term “default-mode” (Raichle et al., 2001). It is active during self-referential mental activity (Gusnard, Akbudak, Shulman, & Raichle, 2001) and mind-wandering (Mason et al., 2007), and deactivated both tonically (Fransson, 2006) and phasically (Singh & Fawcett, 2008) by tasks requiring attention to the external world. During rest, the “task-negative” DMN alternates spontaneously with activation in “task-positive” networks (Fox et al., 2005), while during tasks, deactivation of DMN appears to be modulated by task demands, greater deactivation being associated with greater difficulty, memory load, stimulus rate, and task engagement (Greicius & Menon, 2004; McKiernan, D'Angelo, Kaufman, & Binder, 2006; McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003; Singh, et al., 2008), and decreased deactivation with errors (Li, Yan, Bergquist, & Sinha, 2007).
This evidence for DMN deactivation during task engagement suggests that brain systems subserving attention do not merely involve up-regulation of brain areas implicated in processing external stimuli, but also down-regulation of intrinsic brain activity. Moreover, phasic dopamine release appears crucial to task-stimulus salience and thus reward-mediated processing (Caron & Wightman, 2009). If task-related down-regulation of the DMN is modulated by dopaminergic reward circuitry, a dopaminergic deficit might be expected to result in attenuated DMN deactivation during unengaging tasks, while an indirect dopamine agonist such as methylphenidate might be expected to enhance both task salience and DMN deactivation.
Sonuga-Barke and Castellanos (2007) have hypothesised that the sustained-attention deficits of ADHD may arise from altered modulation of DMN coherence, leading, under sub-optimal conditions, to intrusive DMN activity and lapses of attention. Two resting-state studies support this hypothesis: Castellanos et al (2008) report disrupted functional connectivity between the anterior cingulate cortex and regions of the DMN in adults with ADHD, while Uddin et al (2008) found reduced network homogeneity. Furthermore, Peterson et al (2009) found attenuated task-related DMN deactivation in ADHD during an inhibitory control task, normalised by methylphenidate, while Fassbender et al (2009) found that children with ADHD showed attenuated deactivation with increased working memory load in frontal DMN regions. Moreover, in the latter study, those with greatest reaction time variability, an index of distractibility, showed least deactivation.
Previous work (Liddle et al., 2009) suggests that when a strong incentive to inhibit is pitted against a stringent penalty for late responses, typically-developing children calibrate the balance between motor restraint and the drive to go, timing their responses to fall within a temporal “sweet spot” in which the probability of success is maximised. We hypothesized that if dopaminergic reward circuits are compromised in ADHD, not only might this impair the calibration process, but also raise the motivational threshold required to render task-stimuli sufficiently salient to induce consistent phasic DMN deactivation.
We therefore conducted a functional magnetic resonance imaging (fMRI) study in which methylphenidate-responsive children with ADHD and their typically developing peers performed a stringently-paced inhibitory control task. We manipulated motivational incentives and, in children with ADHD, stimulant medication status in order to address the following questions:
Do children with ADHD show attenuated event-related DMN deactivation compared to typically developing children during an inhibitory control task?
Is DMN deactivation modulated by incentive in either ADHD or typical development, and if so, does the degree of modulation differ?
Does stimulant medication normalise patterns of event-related DMN deactivation in children with ADHD relative to controls?
Methods
Subjects
Twenty-four right-handed children aged 9 to 15 years with a clinical diagnosis of ADHD (DSM-IV combined subtype), and responsive to methylphenidate (daily dose: mean = 1.01 mg/kg; SD=0.45) were recruited from child psychiatry and community paediatric clinics, and pairwise-matched with 24 typically developing volunteers for age (± 6 months), sex and socioeconomic status (SES). All participants were assessed using: the Conners’ Parent and Teacher Rating Scales-Revised (Long form) (Conners, 1996); the Strengths and Difficulties Questionnaire (SDQ) (Goodman, 2001); the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999); and the Digit Span item from the Wechsler Intelligence Scale for Children (WISC-III) (Wechsler, 1992). Diagnosis of ADHD was confirmed by consensus diagnostic conference, where two experienced child psychiatrists reviewed participants’ medical records and assessments, including the Development and Well Being Assessment (DAWBA) (Goodman, Ford, Richards, Gatward, & Meltzer, 2000) and the Social Communication Questionnaire (SCQ) (Rutter et al., 2003). All scales were completed by parents and teachers, with the exception of the SCQ (parents only).
Exclusion criteria were: Full Scale IQ (FSIQ) < 70; psychosis; bipolar disorder; major depression; Tourette Syndrome; Autistic Spectrum Disorder; major head trauma; epilepsy; co-prescription of antipsychotics or serotonin re-uptake inhibitors. Additional exclusion criteria for typically developing participants were: known or suspected major psychiatric disorders; a positive screen for ADHD (Score >5 on the SDQ Hyperactivity Subscale, or T score >60 on the Conners’ Parent Rating Scale-Revised). Six pairs were subsequently excluded from the study owing to excessive movement in the scanner, leaving eighteen pairs (1 female) for analysis. Of the children with ADHD remaining in the sample, 3 (16.7%) met criteria for an anxiety disorder, 8 (44.4%) met criteria for Oppositional Defiant Disorder (ODD) and 5 (27.8%) met criteria for Conduct Disorder (CD).
Local NHS Research & Development and ethical approval was obtained, and after complete description of the study to the subjects, written informed consent and verbal assent was obtained from parents and children, respectively.
Task
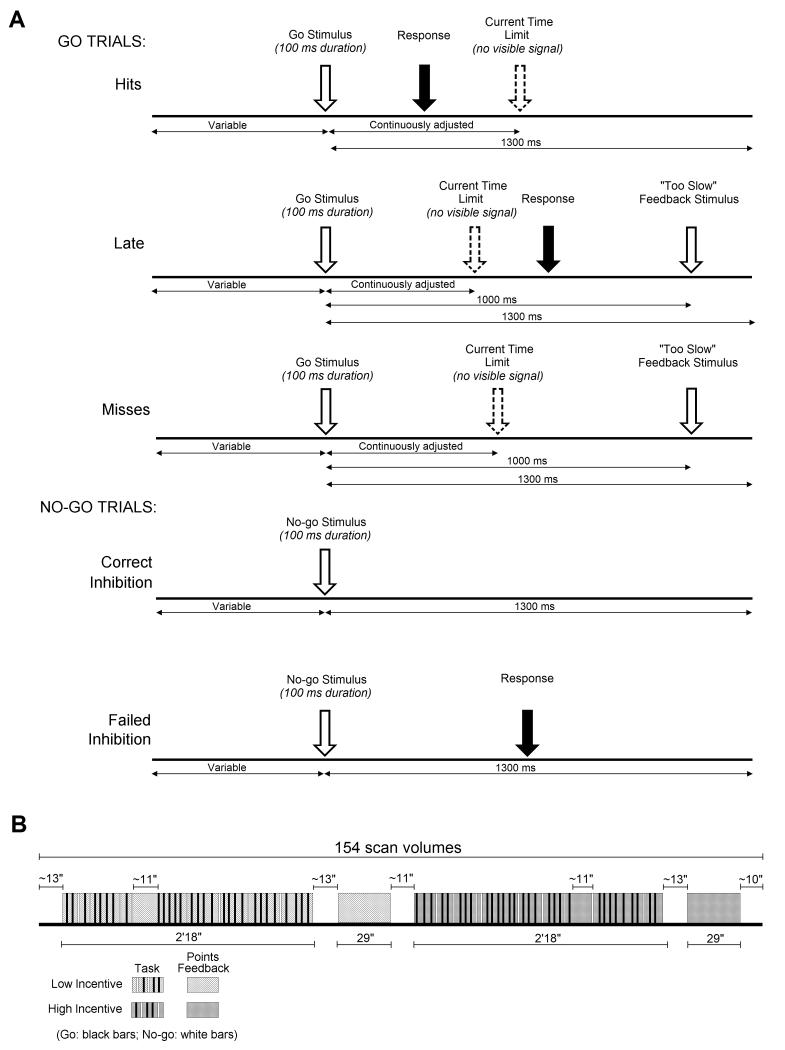
A visual Go/No-go task was presented as a point-scoring space game in blocks of 40 trials, viewed through a periscopic mirror. Go (75%) and No-go (25%) stimuli (duration 100ms) consisted of cartoon alien figures. Participants were instructed to “catch” the Go aliens (by pressing a button on a fiber optic response device held in the right hand), but to avoid the No-go “pet aliens” (by withholding the button press). Inter-stimulus interval (ISI) was randomly jittered between 2.8 and 3.8 seconds, with one ISI of 11.1 (± .3) seconds in each block to enable modeling of on-task baseline activation.
In a No-go task, in which the participant must try both to respond quickly to Go trials and to withhold responses on No-go trials, speed must be balanced against restraint, the balance being open to experimental manipulation either by instruction (Band, Ridderinkhof, & van der Molen, 2003) or incentive (Liddle, et al., 2009). It was the incentive to balance these two requirements that we sought to manipulate in our study. We therefore designed two motivational conditions (Low and High Incentive). In both conditions, a single point was awarded on Go trials for a timely response, and deducted for a late or missed response (indicated by a “late” signal 1000ms post-stimulus). However, whereas in the Low Incentive condition, on No-go trials, a single point was also awarded for a correctly inhibited response and deducted for a failed inhibition, in the High Incentive condition, this reward/penalty was raised to five points. Given the frequency ratio (3:1) of Go to No-go trials, this meant that balancing speed against restraint was more strongly reinforced in the High Incentive condition (where the rarer No-Go trials were worth five times as much as the more frequent Go trials) than in the Low Incentive condition (where the rarer No-go trials were worth only as much as the more frequent Go trials).
To maintain the pressure to respond and to promote a comparable number of successful and failed inhibition trials across subjects and groups, the time limit for Go trials was dynamically adjusted within each condition. A tracking algorithm decreased the time limit by 25 ms following a successful inhibition, and increased it by 25 ms (maximum = 900ms) following an inhibition failure. Initial values and lower bounds for the time limit were individually calibrated during 20 Go trials undertaken at the beginning of the scanning session. The task is illustrated schematically in Figure 1.
Figure 1.
On each day, in each of four scanning runs, two task blocks were presented, one in each motivational condition, in random order. Task blocks were followed by feedback animations (29 seconds) detailing points won and lost, separated by short rest periods (11-13 seconds). At the end of each run, if inhibition rate had been below 50%, on-screen instructions encouraged participants to watch out for the No-go aliens. If inhibition had been above 50%, instructions encouraged participants to try harder to catch the Go aliens.
Procedure
After an initial visit in which they performed a practice version of the task (repeated before each scanning session), all participants attended on two days, approximately one week apart (median = 7 days, interquartile distance = 5.75 days). Before one of these days (counter-balanced), the children with ADHD were withdrawn from methylphenidate for a minimum of 36 hours, continuing to take any other regular medication.
Scan acquisition
Echo planar imaging (EPI) blood oxygenation level dependent (BOLD-sensitive) T2*-weighted images (repetition time: 2.55 seconds; echo time: 60ms; voxel size: 3.92×3.92×3.92mm) were acquired in 30 axial slices on a 1.5 T Philips Achieva scannera using an 8 channel Sense head coil. An anatomical T1-weighted scan was also collected at the beginning of the first day’s scanning.
Image Preprocessing
Using SPM5b, functional volumes were slice-time corrected to the middle (15th) slice; realigned and unwarped to minimise movement-by-susceptibility artefact distortion; spatially normalised to the participant’s segmented, normalised structural image; and spatially smoothed with an 8 mm Full Width Half Maximum (FWHM) Gaussian kernel. Volumes with movement of more than 1 mm were replaced, using ArtRepair (Mazaika, Whitfield-Gabrieli, & Reiss, 2007), with a “repaired” volume (interpolated values). Participants in whom the proportion of repaired volumes exceeded 27% were excluded (N=6), together with their matched pair, from further analysis.
Analysis
Data from the children with ADHD on their off-methylphenidate and on-methylphenidate days were compared with those from their paired control on their equivalent day (Day 1 or 2); One ADHD participant (female) failed to attend her on-methylphenidate day, and one control participant failed to attend the equivalent day to that on which his paired ADHD participant was on-medication.
Behavioural Data
Inhibition rates (proportion of No-go trials correctly inhibited) and miss rates (proportion of Go trials for which no response was recorded) were normalised using a p-to-z transform. The difference between these two z-values generated a d' score, indexing the capacity to balance restraint against the drive to respond, while the mean generated a criterion score, indexing the degree of bias-to-inhibit. In addition, the lower bound for each subject’s time limit was subtracted from the median value for the time-limit in each motivational condition to give the median time-window available to that subject within which to make a response.
fMRI : within subjects
Statistical models were designed using SPM5b. Stimulus-onsets were modeled as events. In each motivational condition, event-types consisted of the three Go trial-types (Hits: a response made under the current time-limit; Late; a response made after the current time-limit: Missed: no recorded response) and the two No-go trial-types (Successful and Failed). Task Blocks in each motivational condition were modeled as epochs, as were the periods during which the Feedback Animations were displayed. Events and epochs were convolved with a canonical hemodynamic response and a temporal derivative. Eight nuisance regressors (six sets of realignment parameters, and the mean signal from white matter and cerebro-spinal fluid voxels respectively) were included in the model. The model was then estimated using ArtRepair (repaired scans down-weighted by a factor of 100).
fMRI: between-subjects
We adopted a Region-of-Interest (ROI) approach to the between-subjects analyses. This choice was made because our a priori hypotheses concerned interactions between three factors (diagnosis, motivation, and medication) affecting a specific regional network, namely, the DMN. Testing for those interactions within pre-specified regions within the DMN enabled us to conduct unambiguous follow-up tests for simpler interactions and main effects within identical regions, as well as obviating the risk of Type II error incurred by the heavy correction for multiple comparisons required in a voxel-based analysis.
We defined our ROIs as probabilistic masks using a DMN image derived from Independent Components Analysis (ICA) in 42 resting adult subjects, (Franco, Pritchard, Calhoun, & Mayer, 2009). While there is evidence from resting state functional connectivity studies that long-range connectivity within the DMN has a developmental trajectory that continues into the mid-twenties (Fair et al., 2009), there is also ICA evidence that a DMN network comprising the major adult regions (medial prefrontal cortex, posterior cingulate cortex, inferior parietal lobule, lateral temporal cortex) is present by two years of age (Gao et al., 2009). Moreover, Fassbender et al (2009) found significant task-related DMN deactivation in a group of healthy children with a mean age of 10.6 years. We therefore considered the choice of an adult mask justified. However, we also used voxel-based analysis to confirm that task-stimuli elicited reliable suppression of voxels within the DMN in our healthy control children (Supplementary Figure 1).
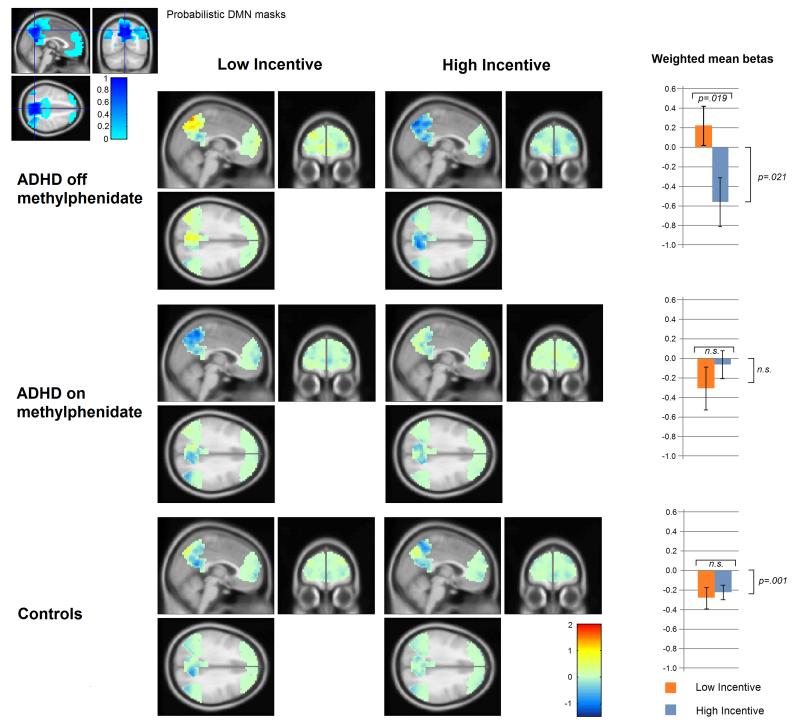
Three homologous pairs (left and right hemisphere) of ROIs (frontal; medial posterior; lateral posterior) were defined within the DMN image. The frontal ROIs were defined as the left and right frontal regions of the DMN image. For the medial and lateral posterior ROIs, binary masks for left and right precuneus plus posterior cingulate gyrus, and left and right angular gyrus plus middle temporal gyrus, respectively, were generated in SPM using an automated anatomical labeling utility (Tzourio-Mazoyer et al., 2002). Each of these was then multiplied by the DMN image to give six ROI masks weighted voxel-wise by the probability of being within their respective regions of the DMN. The extent of the masks is shown in the inset in Figure 2, and coordinates of peak values within each of the ROIs are given in Supplementary Table 1.
Figure 2.
Three Trial-types (Hits, Failed Inhibitions, and Correct Inhibitions) were selected as events-of-interest. Mean beta images (across scanning runs) for the regressors corresponding to each of these Trial-types in each motivational condition were computed for each subject, for each day. The weighted mean voxel value for each mean beta image for each ROI was then calculated. These weighted means were then analysed in a four-way omnibus ANOVA with 2 levels of Diagnosis (ADHD versus Control); 2 levels of Motivational condition (Low versus High Incentive); 2 levels of Medication Day (ADHD participants On or Off-methylphenidate); and 3 levels of Trial Type (Hits; Successful Inhibitions; Failed Inhibitions), data being collapsed initially across ROIs (hemisphere and region). Any significant Diagnosis-by-Motivation or Diagnosis-by-Medication Day interactions were investigated by means of follow-up ANOVAs conducted on Days, Diagnostic groups, and Motivational conditions separately. To establish whether significant net task-related DMN deactivation had occurred, where appropriate, the intercepts of the ANOVA models were tested for significant deviation from zero. This test is equivalent to a one-sample t-test on the combined data. Finally, any effects of Motivational condition or diagnosis were tested for interactions with ROI region.
For the two participants for whom data were missing for the “On Medication” day (one ADHD, one Control), the missing data were replaced by the mean. Diagnostics were examined for variables exerting undue leverage on the results (Cook’s Distance ≥1). One subject’s data for one Trial Type (Control participant; “On medication” day; False Alarms) were found to do so, and were replaced by the variable means. To ensure that missing data were not influencing the results, the analyses were re-run with pairwise deletion of participants with missing data.
Results
Psychometric scores
Although there were no group differences in age and SES, the ADHD group IQ (mean=91.7, SD=11.1) was significantly depressed relative to both the population norm, t(17)=3.159, p=.006 and the Control group mean (FSIQ mean=103.2, SD=15.1), t(17)=2.682, p=.016. IQ in the control group did not differ significantly from population norms.
Behavioural Data
Behaviourally, across all participants, increased incentive raised scores on three measures of inhibitory control: overall inhibition rate; d’ (indexing the degree to which restraint and speed had been co-maximised); and “bias-to-inhibit” (the degree to which the balance between the two had shifted in favour of restraint). Participants with ADHD, off-methylphenidate, had significantly lower d’ scores and higher miss rates compared with typically developing controls, and with their own scores when medicated. These results, with statistical tests, are shown in Table 1. No significant difference was found on any behavioural measure when the ADHD children on-methylphenidate were compared to their typically developing controls on the equivalent day.
Table 1.
Behavioural results and statistical comparisons between Control and ADHD participants when off medication (upper rows) and between ADHD off and on medication (lower rows). There was no significant difference between Control and ADHD children on any behavioural measure for the day on which the children were on medication.
| Control, N=18 | ADHD, N=18 | Main effect of Incentive (df=1,17) |
Main effect of diagnostic group (df=1,17) |
Incentive × Group (df=1,17) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incentive: | Low | High | Low | High | ||||||||||||||
| Mean z | SD | rate | Mean z | SD | rate | Mean z | SD | rate | Mean z | SD | rate | F | p | F | p | F | p | |
| Inhibition | −0.34 | 0.45 | 37% | −0.09 | 0.35 | 47% | −0.47 | 0.58 | 32% | −0.30 | 0.50 | 38% | 13.886 | 0.002 | 1.03 | 0.324 | <1 | |
| Misses | −1.88 | 0.52 | 3% | −1.85 | 0.61 | 3% | −1.54 | 0.59 | 6% | −1.48 | 0.51 | 7% | 2.05 | 0.169 | 4.561 | 0.047 | <1 | |
| D prime | 1.54 | 0.56 | 94% | 1.77 | 0.74 | 96% | 1.07 | 0.72 | 86% | 1.18 | 0.52 | 88% | 4.689 | 0.044 | 7.926 | 0.011 | <1 | |
| Bias-to-inhibit | −2.22 | 0.79 | 1% | −1.94 | 0.66 | 3% | −2.01 | 0.92 | 2% | −1.77 | 0.87 | 4% | 19.096 | <0.001 | 0.523 | 0.479 | <1 | |
| ADHD, N=17, off methylphenidate |
ADHD, N=17, on methylphenidate |
Main effect of Incentive (df=1,16) |
Main effect of Methylphenidate (df=1,16) |
Incentive × Methylphenidate (df=1,16) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibition | −0.49 | 0.59 | 31% | −0.31 | 0.51 | 38% | −0.38 | 0.63 | 35% | −0.17 | 0.41 | 43% | 7.63 | 0.014 | 1.172 | 0.295 | <1 | |
| Misses | −1.54 | 0.6 | 6% | −1.48 | 0.53 | 7% | −1.91 | 0.73 | 3% | −1.94 | 0.67 | 3% | 0.083 | 0.777 | 6.077 | 0.025 | 1.284 | 0.274 |
| D prime | 1.04 | 0.73 | 85% | 1.17 | 0.53 | 88% | 1.52 | 1.08 | 94% | 1.76 | 0.91 | 96% | 5.745 | 0.029 | 6.912 | 0.018 | <1 | |
| Bias-to-inhibit | −2.03 | 0.94 | 2% | −1.79 | 0.89 | 4% | −2.29 | 0.83 | 1% | −2.11 | 0.65 | 2% | 5.038 | 0.039 | 2.094 | 0.167 | <1 | |
As intended, the time-window available for responses generated by the tracking algorithm did not differ significantly between groups, nor, in the case of the ADHD participants, medication status. However, as anticipated, the median time window was significantly greater for all participants in the Low Incentive condition than in the High Incentive (Low: mean=354 ms, SD=20ms; High: mean=288ms, SD=19ms), F(1, 16)=14.889, p=.001.
DMN deactivation: Group differences
Figure 2 shows the degree of event-related deactivation in each voxel weighted by its value in the DMN mask. The weighted means of each ROI were analysed by the following ANOVAs.
The four-way ANOVA (2 levels of Medication Day; 2 levels of diagnostic Group; 2 levels of Motivational condition, 3 levels of Trial Type) returned a statistically significant Medication - by-Diagnosis-by-Motivation interaction, F(1,17)=8.904, p=.008. There were no significant effects of Trial Type, and no other main effects or interactions. The model intercept was significantly below zero, F(1,17)=7.291, p=.015, indicating significant net event-related DMN deactivation across diagnostic groups, medication days and motivational conditions.
In order to interpret the three-way Medication-by-Diagnosis-by-Motivation interaction, separate follow-up ANOVAs were conducted with the data from each medication day. For the on-methylphenidate day data, a three-way ANOVA (2 levels of Diagnosis; 2 levels of Motivation; 3 levels of Trial Type) returned no significant interactions nor main effects, indicating that when the ADHD participants were taking their usual dose of methylphenidate, event-related DMN deactivation did not differ significantly between diagnostic groups in either magnitude or degree of motivational modulation, for any trial type. However, for the off-methylphenidate day, this three-way ANOVA showed a significant Diagnosis-by-Motivation interaction, F(1, 17)=6.904, p=.018, the within-subjects effect of motivational condition being significantly greater in the ADHD group. There were no significant main effects or other interactions. A two-way ANOVA (2 levels of Motivation, 3 levels of Trial-type) conducted on the ADHD data for this off-methylphenidate day returned a significant main effect of Motivation, F(1,17)=6.713, p=.019, in which mean DMN deactivation was significantly attenuated in the Low Incentive relative to the High Incentive condition. When the intercepts were tested separately in each condition for deviation from zero, there was no significant net DMN deactivation in the Low Incentive condition, whereas in the High Incentive condition, the intercept was significantly below zero, F(1,17)=6.507, p=.021, indicating significant net DMN deactivation across Trial Types in the High Incentive condition. To test whether DMN deactivation in ADHD differed from that of control children in the same motivational condition, follow-up ANOVAs conducted on each motivational condition separately (2 levels of Diagnosis, 3 levels of Trial Type) indicated that DMN deactivation was significantly attenuated in children with ADHD relative to controls in the Low Incentive condition, F(1,17)=4.608, p=.047, but not in the High Incentive condition.
To test whether modulation of DMN deactivation by motivational condition was significantly greater when the ADHD participants were off-methylphenidate as compared to on-methylphenidate, a three-way ANOVA (Medication ; Motivation; Trial Type) was conducted. This showed a significant 3-way interaction, F(1,17)=8.484, p=.010, with no other significant interactions or main effects. A two-way ANOVA (2 levels of Motivation, 3 levels of Trial-type) conducted with data from ADHD participants’ on-methylphenidate day, returned no significant effects, indicating no significant modulation of DMN deactivation by motivational incentive when the children were on-methylphenidate.
To test for significant motivational modulation of DMN deactivation in the typically developing Control participants, a two-way ANOVA (2 levels of Motivation, 3 levels of Trial Type) was conducted on their data, collapsed over both days (as they were never medicated). This returned no significant main effects or interactions, and the intercept for the model was significantly below zero, F(1,17)=16.899, p=.001, indicating significant net DMN deactivation across motivational conditions.
There were no significant interactions between ROI region and any other factor, and all findings remained robust at p <.05 when repeated with the 15 pairs of participant for whom full datasets were available.
Supplementary Figures 1 and 2 show the results of voxel-based analyses of the control participants’ data, confirming that task-related activations and deactivations had occurred in the expected regions. Supplementary Figure 3 shows the results of a pair-wise voxel-analysis of the Diagnosis-by-Motivation finding from the ADHD participants when off-methylphenidate.
To summarise the fMRI results: Control children showed significant net event-related DMN deactivation. When off-methylphenidate, children with ADHD showed significantly greater modulation of DMN deactivation by incentive than control children. In the Low Incentive condition, DMN deactivation in children with ADHD was significantly attenuated relative to controls, whereas in the High Incentive condition, there was no significant difference between diagnostic groups. When taking methylphenidate as usual, there were no significant differences between ADHD children and controls, either in degree of motivational modulation, or in magnitude of DMN deactivation, and net-event-related DMN deactivation was significant across the two groups.
DMN deactivation: Effects of age and IQ
To investigate possible developmental effects, the within-pair ANOVAs were repeated with age entered as a covariate. There were no significant main effects of age, nor any significant interactions between age and other factors. To investigate effects of IQ, within-group (Control and ADHD off-methylphenidate) ANOVAs were repeated with FSIQ as a covariate. In neither group were there significant main effects of FSIQ, nor significant interactions between FSIQ and Trial Type or Motivational condition. To check that the effects of diagnosis were not accounted for by the FSIQ differences between the groups (mean FSIQ difference=11.4, SD=18.1), all the ANOVAs were repeated with FSIQ between-pair differences as a covariate. All the findings remained robust, and there were no significant main effects of FSIQ difference nor significant interactions with diagnosis, indicating that diagnosis was accounting for more of the variance in DMN deactivation than FSIQ difference.
Discussion
The typically developing children in this study showed significant net event-related deactivation in the DMN during an inhibitory control task. DMN deactivation was not significantly modulated in this group by motivational incentive to balance speed against restraint, nor was it significantly modulated by incentive in the ADHD children when taking their usual methylphenidate dose. However, when participants with ADHD were withdrawn from methylphenidate, motivational incentive to balance speed against restraint had a marked effect on DMN deactivation. In the Low Incentive condition, children with ADHD showed significantly attenuated DMN deactivation compared with typically developing controls. With a high incentive however, children with ADHD showed significantly increased net event-related DMN deactivation compared to the deactivation they exhibited under low incentives, abolishing the group difference in this motivational condition. This suggests that the deactivation response of the DMN to task-relevant stimuli is not in itself impaired in ADHD, but that the motivational threshold at which task-relevant stimuli become sufficiently salient to trigger DMN deactivation is raised. When on their usual methylphenidate dose, neither mean DMN deactivation nor motivational modulation of DMN deactivation in children with ADHD were significantly different to that of their typically developing peers.
Our fMRI findings are consistent with previous findings of attenuated task-related DMN deactivation in ADHD (Fassbender, et al., 2009; Peterson, et al., 2009), but for the first time we report its modulation by motivation, and the normalisation of this motivational modulation by methylphenidate. Our findings are thus consistent with the Sonuga-Barke and Castellanos (2007) hypothesis that DMN dysfunction may account for impaired task-performance in ADHD under sub-optimal conditions. However, they suggest an extension to this hypothesis, namely that ADHD is characterised by a raised (relative to typically developing children) motivational threshold at which event-related DMN deactivation occurs. In other words, children with ADHD may require a higher incentive than typically developing children to produce a comparable degree of task-related DMN deactivation. Our findings thus bring together attentional with motivational accounts of ADHD deficits (Sagvolden, et al., 1998), and, moreover, do so within the context of an inhibitory control task.
While our non-blinded study design precludes the inference that the observed medication effects reflect direct pharmacological action, our findings are consistent with Volkow et al’s (2008) finding that methylphenidate resulted in reduced metabolic increases in the DMN during a cognitive task in healthy adults. In Volkow’s study, these reduced metabolic increases in the DMN were associated with improved performance in subjects who activated these regions under placebo. This raises the possibility that in our ADHD participants, methylphenidate may have lowered the motivational threshold for task-related deactivation of the DMN, accounting for the observed normalisation of inhibitory performance.
Our study design does not lend itself to inferences about relationships between performance and DMN deactivation. However, our finding that phasic task-related DMN deactivation is modulated by motivation in ADHD raises a number of possibilities for further investigation in future studies. The hypothesis that attenuated DMN deactivation interferes with performance by increasing the likelihood of attentional lapses, is supported by Fassbender et al’s (2009) finding of between-subjects correlations between attenuated task-related DMN deactivation and a measure of distractibility. Alternatively, or additionally, a raised threshold for motivational salience may impair the calibration of motor restraint in ADHD (Liddle, et al., 2009) due to deficits in phasic dopamine release circuits implicated in Hebbian learning: “To learn, you must pay attention” (Caron, et al., 2009). In the absence of a supra-threshold incentive, children with ADHD may fail to learn optimal response timing. To distinguish between these mechanisms, future studies powered to allow trial-by-trial analysis could determine whether DMN deactivation on a given trial predicts success on that trial, as predicted by a lapse-of-attention hypothesis, or whether, alternatively, attenuated or unreliable phasic task-related deactivation interferes with the learning of optimally timed motor responses, thus affecting overall performance across trials. The fact that we found no significant difference in the degree of DMN deactivation between trial types (including correct and incorrect trials) raises the possibility that the latter interpretation may be correct.
Finally, in our study, socio-economic matching was chosen over IQ matching as our inclusion criterion ensured that our ADHD sample was drawn from a homogeneously severe population of children with combined subtype ADHD, corresponding to ICD-10 hyperkinetic disorder. This subtype is likely to exhibit a broad range of executive and other neuropsychological deficits that may depress IQ (Rhodes, Coghill, & Matthews, 2005), and, indeed, our ADHD group had a mean IQ significantly below the population mean. However, as our diagnostic and medication findings concerned a within-subject manipulation (motivational incentive) the reported effects would seem unlikely to be due to global cognitive delay. Future studies with a more heterogeneous sample of children with ADHD may shed light on how specific our findings are to the ADHD combined subtype.
Conclusion
In children with ADHD, attenuated DMN deactivation during an inhibitory control task can be normalised either by task-related motivational incentives or by methylphenidate (an indirect dopamine agonist), rendering their pattern of task-related DMN deactivation indistinguishable from that of typically developing children. This motivational modulation was not observed in their typically developing peers, who showed significant phasic task-related DMN deactivation across motivational conditions. We suggest that, relative to controls, children with ADHD (combined subtype) exhibit a raised motivational threshold at which task-relevant stimuli in an inhibitory control task acquire the salience necessary for the degree of phasic task-related DMN deactivation observed in typically developing children, and that methylphenidate normalises this threshold. Our findings suggest that a raised motivational/task-salience threshold in ADHD may contribute to impaired inhibitory control performance by disrupting phasic task-related DMN deactivation.
Supplementary Material
Key points.
Children with ADHD show deficits in attention and inhibitory control, yet perform better when motivated.
These deficits may reflect attenuated task-related deactivation of the Default Mode Network (DMN).
Using fMRI, we show that children with ADHD, when withdrawn from methylphenidate, show reduced task-related DMN deactivation relative to controls when the incentive to inhibit a response is low, but that DMN deactivation is normalised when incentives are increased.
We also show that methylphenidate eliminates this motivational modulation, and normalises DMN deactivation patterns.
Our findings suggest a raised motivational threshold for task-related DMN deactivation in ADHD that is normalised by methylphenidate.
Acknowledgements
This study was supported by a grant from the Wellcome Trust (grant number: 076448/Z/05/Z).
We are grateful to Drs Carolyn Nahman and Rachel Duffy for the clinical assessment of patients, to Carolyn Costigan for assistance with scanning, and to all the families who took part.
Footnotes
Philips Medical systems, Best, The Netherlands
Wellcome Trust Centre for Neuroimaging. Statistical Parametric Mapping. SPM5 ed 2005
References
- Band GPH, Ridderinkhof KR, van der Molen MW. Speed-accuracy modulation in case of conflict: the roles of activation and inhibition. Psychological Research-Psychologische Forschung. 2003;67(4):266–279. doi: 10.1007/s00426-002-0127-0. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Borger N, van der Meere J. Motor control and state regulation in children with ADHD: a cardiac response study. Biological Psychology. 2000;51(2-3):247–267. doi: 10.1016/s0301-0511(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Caron MG, Wightman RM. To learn, you must pay attention. Molecular insights into teachers' wisdom. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7267–7268. doi: 10.1073/pnas.0903306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C. Conners Rating Scales - Revised (SRS-R) Psychological Assessment Resources, Inc.; London: 1996. [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional Brain Networks Develop from a “Local to Distributed” Organization. Plos Computational Biology. 2009;5(5) doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Research. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AR, Pritchard A, Calhoun VD, Mayer AR. Interrater and intermethod reliability of default mode network selection. Human Brain Mapping. 2009;30(7):2293–2303. doi: 10.1002/hbm.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gao W, Zhu HT, Giovanello KS, Smith JK, Shen DG, Gilmore JH, Lin WL. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. Psychometric Properties of the Strengths and Difficulties Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(11):1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-being Assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41(5):645–655. [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. Journal of Cognitive Neuroscience. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen EB, Aase H, Meyer A, Sagvolden T. Attention-deficit/hyperactivity disorder (ADHD) behaviour explained by dysfunctioning reinforcement and extinction processes. Behavioural Brain Research. 2002;130(1-2):37–45. doi: 10.1016/s0166-4328(01)00434-x. [DOI] [PubMed] [Google Scholar]
- Li CSR, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle EB, Scerif G, Hollis CP, Batty MJ, Groom MJ, Liotti M, Liddle PF. Looking before you leap: A theory of motivated control of action. Cognition. 2009;112(1):141–158. doi: 10.1016/j.cognition.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clinical Psychology Review. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, Reiss A. Artifact Repair for fMRI Data from High Motion Clinical Subjects. Paper presented at the Human Brain Mapping.2007. [Google Scholar]
- McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29(4):1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An fMRI Study of the Effects of Psychostimulants on Default-Mode Processing During Stroop Task Performance in Youths With ADHD. Am J Psychiatry. 2009;166(11):1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SM, Coghill DR, Matthews K. Neuropsychological functioning in stimulant-naive boys with hyperkinetic disorder. Psychological Medicine. 2005;35(8):1109–1120. doi: 10.1017/s0033291705004599. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Berument SK, Le Couter A, Lord C, Pickles A. Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder - from brain dysfunctions to behaviour. Behavioural Brain Research. 1998;94(1):1–10. [PubMed] [Google Scholar]
- Sergeant J. The cognitive-energetic model: an empirical approach to Attention-Deficit Hyperactivity Disorder. Neuroscience and Biobehavioral Reviews. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41(1):100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Slusarek M, Velling S, Bunk D, Eggers C. Motivational effects on inhibitory control in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(3):355–363. doi: 10.1097/00004583-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neuroscience and Biobehavioral Reviews. 2007;31(7):977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of Neuroscience Methods. 2008;169(1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One. 2008;3(4):e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, Gatley SJ, Pappas N, Wong C, Vaska P, Zhu W, Swanson JM. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. American Journal of Psychiatry. 2004;161(7):1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed Psychological Corporation, Harcourt Brace and Co.; London: 1992. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation: Harcourt Brace; San Antonio: 1999. [Google Scholar]
- Wilkison PC, Kircher JC, McMahon WM, Sloane HN. Effects of methylphendate on reward strength in boys with Attention-Deficit Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(7):897–901. doi: 10.1097/00004583-199507000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.