Abstract
Objective
Evaluate upper genital tract (UGT) presence of vaginal bacterial species using sensitive molecular methods capable of detecting fastidious bacterial vaginosis (BV)-associated bacteria.
Study Design
Vaginal swabs were collected prior to hysterectomy. The excised uterus was sterilely opened and swabs collected from endometrium and upper endocervix. DNA was tested in 11 quantitative PCR (qPCR) assays for 12 bacterial species: Lactobacillus iners, L. crispatus, L. jensenii, Gardnerella vaginalis, Atopobium vaginae, Megasphaera spp., Prevotella spp., Leptotrichia/Sneathia, BVAB1, BVAB2, BVAB3 and a broad-range16S rRNA gene assay. Endometrial fluid was tested with Luminex and ELISA for cytokines and defensins, and tissue for gene expression of defensins and cathelicidin.
Results
We enrolled 58 women: mean age 43 + 7 years, mostly white (n = 46; 79%) and BV-negative (n = 43; 74%). By species-specific qPCR, 55 (95%) had UGT colonization with at least one species (n = 52), or were positive by 16S PCR (n = 3). The most common species were L. iners (45% UGT, 61% vagina), Prevotella spp. (33% UGT, 76% vagina) and L. crispatus (33% UGT, 56% vagina). Median quantities of bacteria in the UGT were lower than vaginal levels by 2–4 log10 rRNA gene copies/swab. There were no differences in endometrial inflammatory markers between women with no bacteria, Lactobacillus only or any BV-associated species in the UGT.
Conclusion
Our data suggest that the endometrial cavity is not sterile in most women undergoing hysterectomy, and that the presence of low levels of bacteria in the uterus is not associated with significant inflammation.
Keywords: Intrauterine bacteria, endometritis, upper genital tract infection, reproductive tract microbiota, uterine cavity, endometrium, sterile
Introduction
Bacterial colonization of the uterus is associated with adverse reproductive health outcomes, including preterm delivery and chorioamnionitis,1 pelvic inflammatory disease and endometritis2,3 and miscarriage.4 Upper genital tract infection has been presumed to be due to pathologic ascent of vaginal bacteria in to the upper genital tract. The physical barrier of cervical mucous, its high concentrations of antimicrobial peptides and inflammatory cytokines,5–9 and possibly immunoglobulins10 or matrix degrading enzymes 11 in the mucous plug are thought to provide a defense against bacterial ascent and the uterine cavity of healthy women has long been considered sterile.
However, radioactively labeled albumin spheres placed in the vagina ascend into the uterus as early as 2 minutes after instillation,12 suggesting that fluid and particles move between the vagina and uterus relatively freely. Studies of ostensibly healthy women report a variable rate of uterine bacterial colonization by culture, ranging from 0–82%.13–22 This wide range is due in part to differences in sample collection: studies using hysterectomy or transfundal sampling had lower rates (0–24%) 13–16,22 compared to those using transcervical sampling (33–82%).17,18,21
Many studies using molecular characterization of the microbiota have demonstrated the ubiquitous presence of bacteria throughout the body, and their influence on health. 23,24 We hypothesized that bacterial colonization of the upper genital tract may be quite common and not pathologic in many cases. We undertook this study to assess the prevalence and concentrations of bacteria in the upper genital tract (UGT) using sensitive molecular methods in sterilely sampled hysterectomy specimens. Additionally, we measured the endometrial immune response to determine whether intrauterine bacterial colonization was associated with epithelial inflammation, which could suggest an adverse effect of the bacteria.
Materials & Methods
Study cohort and sample collection
Women undergoing hysterectomy for non-cancer indications were eligible. Exclusion criteria included presence of an IUD, use of antibiotics, endometrial biopsy, IUD removal or hysteroscopy in the past 30 days, or concern for cervical or endometrial neoplasia. Total laparoscopic or laparoscopically-assisted vaginal hysterectomy specimens were only collected if the surgeon was able to complete the procedure using a non-invasive vaginal fornix delineator (Colpo-Probe, Cooper Surgical, Trumbull, CT) or a vaginal sponge stick rather than an intracervical manipulator. The University of Washington Human Subjects Division approved the study. All subjects signed informed consent. All patients received standard pre-operative antibiotic prophylaxis at least 30 minutes prior to surgery.
Prior to vaginal exams or prep, flocked swabs (Copan Diagnostics Inc., Murrieta, CA) were inserted 3–4cm into the vagina for 5 seconds. One was smeared on a glass slide for Gram stain and Nugent scoring.25 The uterus was removed, wrapped in a sterile towel, taken to pathology without fixation and incised sagitally under sterile conditions, beginning at the fundus. Swabs were collected first from the endometrium and then from the upper endocervix by rolling the swab 2–3 times across the epithelium and frozen at −80ºC. In a subset of participants (n = 30, 52%) swabs were collected in the Port-A-Cul anaerobic system (Beckton, Dickinson and Company, Franklin Lakes, NJ), cultured in standard fashion, including selective broth to allow growth of mycoplasma species and isolates identified by routine biochemical methods. Tissue sections were collected from the endometrium contralateral to the swab collection, cut into 1 × 1 cm blocks, placed in RNALater at 4ºC for 24 hours, then placed at −80ºC.
Bacterial PCR assays
Frozen swabs were thawed and 400 uL of PBS added, mixed by vortex shaker for 1 minute, then the swab removed and the sample spun at 17,000 x g for ten minutes (all at 4 degrees). The pellet underwent DNA extraction with the MoBio Bacteremia DNA Isolation Kit (MoBio, Carlsbad, CA), while the supernatant was aliquoted and frozen for Luminex analysis. DNA underwent taxon-directed 16S rRNA gene TaqMan format qPCR assays for the following bacterial species: Lactobacillus crispatus, L. jensenii, L. iners, Gardnerella vaginalis, Atopobium vaginae, Megasphaera genus, Prevotella genus, Bacterial Vaginosis Associated Bacterium 1 (BVAB1), BVAB2, BVAB3 and an assay detecting two closely related bacteria (Leptotrichia and Sneathia).26,27 For the Prevotella genus assay, the forward primer 384F (5′ - GC CTG AAC CAG CCA AGT A – 3′), reverse primer 513R (5′ - GGA ATT AGC CGG TCC TTA TT - 3′) and a taxon-specific probe (6FAM - GTG CAG GAI GAC GGC C – MGBNFQ) were used. The thermocycler (ABI 7500 Thermocycler, Applied Biosystems, Foster City, CA) program was 2 minutes 50°C, 10 minutes 95°C, and then 45 cycles of 15 seconds 95°C, 39 seconds 59°C and 30 seconds 72°C. UGT swabs were also tested using a broad-range 16S rRNA gene assay to assess for the presence of any bacteria. Limits of detection for the assays were as follows: L. crispatus 75 gene copies/swab, L. jensenii 125 gene copies/swab, all other species-specific assays 150 gene copies/swab, and broad-range 16S 6,400 gene copies/swab. 28 Negative assays were assigned a value of half the lower limit of detection for that assay.
Measurement of cytokines, chemokines and antimicrobial peptides
Supernatant from endometrial swabs was submitted for Luminex (Luminex Corporation, Austin, TX) analysis. Seven of the 14 analytes (IL4, IL10, IL17, IFN-γ, IFNα, TNFα, MIP1α) were undetectable in over 95% of samples and were not included in the final analysis. ELISA for human beta defensin 2 (HBD2), HBD3 (Alpha Diagnostics International, San Antonio, TX) and human alpha defensins 1-3 (HNP 1-3; Hycult Biotech, Plymouth Meeting, PA) was performed. Homogenized endometrial tissue sections underwent RNA extraction using the RNEasy Fibrous Tissue Kit (Qiagen Inc., Valencia, CA). RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories, Waltham, MA) and amplified using primers and probes from Applied Biosystems (Foster City, California) for HBD2, HBD3, cathelicidin (CAMP) and IL1β, as well as the housekeeping gene β-actin.
Statistical analysis
All analysis was performed using Stata v.10. Prevalences were compared between groups using the chi square test. Quantities of bacteria and concentrations of cytokines were not normally distributed, so were compared across groups using Wilcoxon rank-sum or Kruskall Wallis tests.
Results
Cohort
We enrolled 58 women with mean age of 43 ± 7 years. Participants were primarily white (n = 46; 79%), with a small proportion of African American (n = 6; 10%) and Hispanic (n = 4; 7%) women (2 declined to answer the question). All underwent hysterectomy for benign disease: primarily bleeding (n = 20; 34%), fibroids (n = 15; 26%), pain (n = 17; 29%). Most had a normal Nugent score (n = 43; 74%), while 6 (10%) had bacterial vaginosis, 7 (12%) had an intermediate score and 2 (3%) could not be scored. Most (37; 64%) were on no hormonal medications, 5 (9%) were taking oral contraceptives, 13 (22%) were using Lupron, and 2 (3%) were using a different hormonal medication (testosterone, hormone replacement therapy). Eight women (14%) reported being menopausal. Only 37/50 (74%) pre-menopausal women provided information about last menstrual period (LMP). The median number of days since LMP was 28 (IQR 12, 64), and of the 24 women reporting < 40 days since their LMP only 8 (33%) were in the first 14 days of their cycle. Most women had never douched (n = 32; 55%) or douched more than 1 week prior to surgery (n = 10; 17%), with a minority who had douched within the past week (n = 2; 3%) and 14 (24%) who did not answer the question. Nineteen women (33%) reported sexual intercourse in the week prior to surgery.
UGT colonization
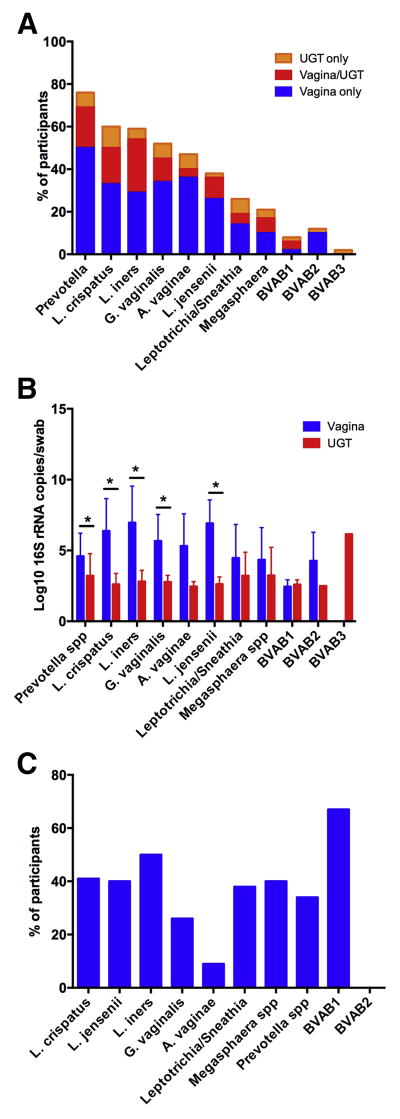
By species-specific qPCR, 55 (95%) of women had UGT colonization (i.e. in the endometrium or upper endocervix) with at least one of the assayed species (n = 52), or were positive by broad range 16S PCR (n = 3). The most commonly detected species in the vagina were Prevotella spp. (76%) L. iners (61%), and L. crispatus (56%). These were also the most commonly detected species in the UGT: L. iners (45%), Prevotella spp. (33%) and L. crispatus (33%)(Figure 1a). G. vaginalis, A. vaginae and L. jensenii, were detected in the vagina in over 40% of women, but were detected less frequently in the UGT (in 19%, 10% and 20%, of women). Mean quantities of bacteria detected in the UGT were lower than levels in the vagina by 2–4 log10 rRNA gene copies (Figure 1b). When detected in the vagina, A. vaginae was the least likely species to also be detected in the UGT, while BVAB1 and L. iners were the most likely (Figure 1c). The mean vaginal quantity of L. crispatus and G. vaginalis was significantly higher in women who had UGT colonization with those species: 7.7 ± 1 vs. 5.5 ± 2.5 gene copies/swab for L. crispatus (p = 0.006) and 7.8 ± 1.2 vs. 4.9 ± 1.5 gene copies/swab for G. vaginalis (p < 0.001). There were no significant differences in vaginal quantity between women with and without UGT colonization with other bacteria (data not shown). The median number of species detected in the UGT was 2 (IQR 1,3; range 0–8), while the median number of species detected in the vagina was 3 (IQR 2,5; range 0–9). There was no correlation between number of species detected in the vagina and the UGT (correlation coefficient 0.21, p = 0.12). Of note, in several cases an organism present in the UGT was not present in the vagina (Figure 2).
Figure 1. Distribution of upper genital tract bacterial colonization.
A. Proportion of participants with detection of bacteria by species-specific qPCR in the vagina alone, both vagina and upper genital tract (UGT) and UGT alone. B. Comparison of mean quantity of bacteria in the vagina and UGT of women with either vaginal or upper genital tract detection of that species. * denotes comparisons that are significantly different (p < 0.05) by t-test. C. Proportion of women with vaginal detection of a given species by qPCR who also had UGT detection of that species.
Figure 2. Comparison of vaginal and upper genital tract detection of bacteria.
Detection and quantity of each species in the vagina and UGT for each participant, organized by Nugent score. Each bacterium is represented by a row, and the quantity is represented by a gradient of color, with darker colors representing higher quantities. The color gradients represent grouping of 1–100, 101–10,000, 10,000 – 1,000,000 and > 1,000,000 16S rRNA gene copies/swab. A white space means that the bacterium was not detected in that sample.
As almost all women had at least one species detected in the UGT we were unable to evaluate risk factors for colonization in general. We divided women into those with no bacteria detected in the UGT (n = 3), Lactobacillus species only (n = 18; 31%), any non-Lactobacillus species (n = 34; 59%), 16S positive only (n = 3). The only demographic difference between these groups was race: UGT colonization with a non-Lactobacillus species was more common in African American women (5/6; 83%) and Hispanic women (3/4; 75%) than white women (25/46; 54%)(p = 0.01). Rates of BV were slightly different between these groups: 17% for African American, 0% for Hispanic and 11% for white women. There was a trend to increasing UGT colonization by non-Lactobacillus species with increasing Nugent score: with Nugent score 0–3 the rate was 51% (22/43), score of 4–6 71% (5/7) and score 7–10 83% (5/6) (p = 0.24). However, the six women with the highest levels of non-Lactobacillus species detected in the UGT all had a Nugent score < 7. Age, menopausal status, treatment with GnRH agonist, gravidity, parity, douching or sex in the past week were not significantly different between the groups. (data not shown)
Of the subset of 30 women who also had cultures performed of upper genital tract swabs, 28 (93%) had bacteria detected by qPCR, and 26/30 (87%) had bacteria detected by culture. Both women with negative qPCR results were also negative by culture. The most commonly cultured organisms were Diphtheroids (n = 15; 50%), followed by anaerobic gram-positive cocci (12; 40%), Proprionibacterium spp. (n = 9; 30%) and Lactobacillus species (n = 8 species from 5 women; 17%) (Supplementary Table 1).
Immune response
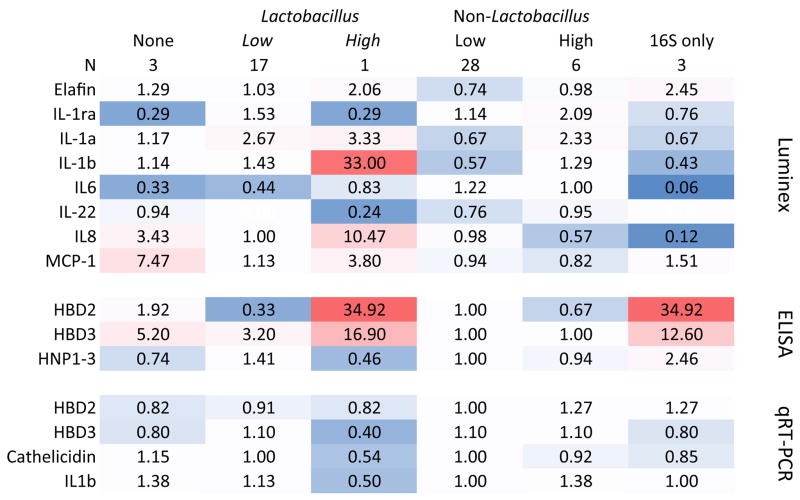
Soluble markers of inflammation were measured from endometrial swabs by Luminex, antimicrobial peptides by ELISA, and gene expression for defensins, cathelicidin and IL1β from tissue RNA and results compared between women with no bacteria, only Lactobacillus species or any non-Lactobacillus species detected in the upper genital tract. (Figure 3) There were no significant differences in the median values of these markers between groups. However, the lowest quantities of beta-defensin proteins seemed to be samples from women with non-Lactobacillus species in the UGT. The one woman with high (> 100,000 gene copies/swab) of L. iners in the UGT had relatively high levels of several inflammatory markers – but the lowest levels of gene expression for the beta defensins, cathelicidin and IL1β. When compared between women who had surgery for fibroids, bleeding, pain or other reasons, the only analyte that was significantly different between the groups was IL6: median 6 pg/mL (Interquartile Range (IQR) 1, 28) in women having surgery for fibroids, 21.9 pg/mL (IQR 9,154) in women having surgery for bleeding, 32 pg/mL (IQR 14, 323) in women having surgery for pain, and 1 (IQR 1, 7.8). There was no difference in the distribution of women with only Lactobacillus spp. in the UGT versus non-Lactobacillus species between the surgical indications. (data not shown)
Figure 3. Endometrial immune markers.
Comparison of markers of the immune response in the upper genital tract between women with no bacteria detected by PCR in the upper genital tract, only Lactobacillus species detected, or any non-Lactobacillus species detected. Numbers in boxes are multiple of the median, calculated by taking the median value for the whole cohort and dividing the individual group value by that number. There were no significant differences between these three groups. Values highlighted in red are higher than the group median, and those in blue are lower than the group median.
Comment
We detected UGT bacteria by PCR in 95% of women undergoing hysterectomy for benign gynecologic conditions. These results confirm the growing consenus that the endometrial cavity is not sterile. However, the quantity of bacteria present in the uterus and high endocervix was significantly lower than that in the vagina, suggesting that either the cervix serves as a partial filter to ascent, or that the endometrial immune response clears bacteria that do ascend, or a combination of both. We found a much higher prevalence of UGT colonization, but less correlation between vaginal and UGT samples than we anticipated. In women with vaginal colonization by a given species, rates of UGT colonization varied widely, suggesting differences in microbial ability to evade cervical immunity, or greater permissiveness to some species. Many groups have shown that the vaginal microbial community is dynamic.27,29 Our results suggest that microbes may remain in the UGT after they disappear from the vagina and/or have better growth in the UGT than the vagina.
Studies using a similar strategy of incising a hysterectomy specimen to collect samples, but using culture to identify bacterial colonization, report rates of intrauterine bacterial colonization ranging from 0% among 10 women from Finland22 to 31% in a cohort of 100 women from England.16 Studies using transcervical sampling report higher rates of intrauterine bacterial colonization, ranging from 33%18 to 60%,17 but the degree of cervical or vaginal contamination of the endometrial specimen is unknown.30 Our qPCR results from surgically obtained samples suggest an even higher rate of low-level bacterial presence in the upper genital tract than culture-based studies using transcervical sampling. Many of the bacteria identified by qPCR in this study, such as BVAB1-3 and Leptotrichia/Sneathia, are fastidious and difficult to culture, which may account for the differences between our data and previous reports. The bacteria we identified by culture from a subset of women include several taxa that have been identified in vaginal communities but were not targeted by our PCR assays: Corynebacteria (Diptheroids), Proprionibacteria, Ureaplasma, coagulase-negative Staphylococcus, as well as several anaerobic colonies that could represent any number of other common vaginal species. All women in this study received pre-operative antibiotics intravenously, which likely affected our culture results.
Surprisingly, we saw few differences in endometrial immune markers between women with and without upper genital tract colonization by BV-associated microbes. This could be due to trauma-induced cytokine release at the time of surgical removal of the tissue, but our median values are similar to reported median values from endometrial aspirates in women with intact uteri undergoing in-vitro fertilization procedures31 suggesting this is not the case. Alternatively, cytokines may be impacted by hormonal status, the underlying pathology leading to hysterectomy, or by viral or fungal pathogens not measured in our study. Our data suggest that a low quantity of upper genital tract bacterial colonization by common vaginal species does not induce a strong inflammatory stimulus in most cases.
This is an exploratory analysis with a small sample size, which limits our ability to detect small associations or to perform well-powered subgroup analyses to look at factors associated with UGT colonization for different species. However, it is the first study using molecular methods to assess upper genital tract colonization in non-pregnant women. Our analysis is cross-sectional, which limits our ability to make conclusions about causation or direction of associations. However, opportunities to sterilely collect endometrial samples with minimal risk of contamination from the lower genital tract are becoming scarce, and preclude longitudinal sample collection from the UGT. Changing patterns of surgery mean that fewer hysterectomies are being performed, and many are now performed use minimally invasive techniques where an intracervical manipulation device is used. Intracervical instrumentation could introduce an uncontrolled amount of endometrial contamination, so these cases were excluded. Another limitation of our study is the use of selective qPCRs, which do not capture the entire microbiota. However, we did not have sufficient concentrations of bacterial DNA to perform broad range bacterial PCR with pyrosequencing. We did obtain additional information using bacterial culture in some cases, but prophylactic antibiotics potentially impact the sensitivity of cultures, and culture is not able to identify fastidious bacterial species. Finally, since these samples were collected from surgical specimens, all participants had pathology and thus the study population may not reflect the conditions present in normal, healthy women.
Recent advances in our understanding of the human microbiome reveals the important role that microbes play in many facets of human health.32 The microbiome plays an important role in the immunologic homeostasis of the gut, encouraging proper development of mucosal immunity and preventing excessive inflammation (reviewed in 33). T-regulatory cells in the gut mucosa maintain a tolerogenic environment and appear to be selected by interactions with commensal gut microbiota.34 In the uterus, T-regulatory cells are important for implantation of the embryo and early placental development.35,36 In one study, the presence of hydrogen peroxide producing Lactobacillus species on the tip of the embryo transfer catheter for in-vitro fertilization increased the chance of live birth compared to women who did not have these bacteria detected by culture.37 This finding raises the possibility that the presence of intrauterine commensal bacteria may have a similar role in the selection of uterine T-regulatory cells as commensal gut microbiota do for colonic T-regulatory cells. While many other factors contribute to a successful pregnancy, it is worth noting that germ-free mice raised in sterile conditions have lower rates of reproductive success after embryo transfer than conventional animals,38 suggesting a potential role for intrauterine bacteria in successful pregnancy. This potentially critical issue is largely unexplored.
It is clear that intrauterine bacterial colonization is not always benign, nor positive. In patients undergoing in vitro fertilization cycles, the presence of Streptococcus viridans on the embryo transfer catheter tip was associated with decreased chance of live birth compared to women without S. viridans detected.37 Women with preterm birth are more likely to have intra-uterine placental infection than women who deliver at term. (reviewed in 39) Women with significant inflammatory sequelae in the upper genital tract with pelvic inflammatory disease often have anaerobic Gram-negative rods and mixed communities of bacteria in the uterus and fallopian tubes.3,40 Pathologic effects of intrauterine bacteria may occur only with particularly virulent strains or species, only with high concentrations of bacteria, or only in the presence of a mixed bacterial community at the endometrial surface.
In summary, these data indicate that the endometrial cavity is not sterile in most women undergoing hysterectomy for benign indications. Additionally, detection of bacteria in the upper genital tract is not associated with a significant inflammatory immune response. While bacterial concentrations in the endometrium are much lower than that in the vagina, a low-level bacterial presence in the uterus appears common and not pathologic.
Supplementary Material
Acknowledgments
Funding: This work was supported by a K08 from NIAID (1K08AI087969 – 01; CM) and by a grant from the University of Washington Royalty Research Fund (CM). Neither funding source had any role in collection, analysis, presentation or decision to publish the data.
The authors would like to acknowledge Xuezhou Hou, who developed the Prevotella genus assay in Dr. Fredricks’ laboratory at the Fred Hutchinson Cancer Research Institute, as well as the surgeons in the Gynecology Division at the University of Washington who facilitated collection of specimens.
Footnotes
This study was conducted in Seattle, WA.
The authors report no conflicts of interest.
These findings were presented in part at the Annual Meeting of the Infectious Diseases Society of Obstetrics & Gynecology in Whistler, British Columbia, August 9–11 2012
Reprints will not be available
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. The New England journal of medicine. 1995 Dec 28;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 2.Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sexually transmitted diseases. 2013 Feb;40(2):117–122. doi: 10.1097/OLQ.0b013e31827c5a5b. [DOI] [PubMed] [Google Scholar]
- 3.Hillier SL, Kiviat NB, Hawes SE, et al. Role of bacterial vaginosis-associated microorganisms in endometritis. American journal of obstetrics and gynecology. 1996 Aug;175(2):435–441. doi: 10.1016/s0002-9378(96)70158-8. [DOI] [PubMed] [Google Scholar]
- 4.Nelson DB, Bellamy S, Nachamkin I, Ness RB, Macones GA, Allen-Taylor L. First trimester bacterial vaginosis, individual microorganism levels, and risk of second trimester pregnancy loss among urban women. Fertility and sterility. 2007 Nov;88(5):1396–1403. doi: 10.1016/j.fertnstert.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulcova-Gallova Z. Immunological and physicochemical properties of cervical ovulatory mucus. Journal of reproductive immunology. 2010 Nov;86(2):115–121. doi: 10.1016/j.jri.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JA, Moscicki AB, Sumerel JL, Ma Y, Scott ME. Determination of cytokine protein levels in cervical mucus samples from young women by a multiplex immunoassay method and assessment of correlates. Clin Vaccine Immunol. 2008 Jan;15(1):49–54. doi: 10.1128/CVI.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ming L, Xiaoling P, Yan L, et al. Purification of antimicrobial factors from human cervical mucus. Human reproduction (Oxford, England) 2007 Jul;22(7):1810–1815. doi: 10.1093/humrep/dem128. [DOI] [PubMed] [Google Scholar]
- 8.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. American journal of obstetrics and gynecology. 2001 Sep;185(3):586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 9.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. American journal of obstetrics and gynecology. 2002 Jul;187(1):137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 10.Hein M, Petersen AC, Helmig RB, Uldbjerg N, Reinholdt J. Immunoglobulin levels and phagocytes in the cervical mucus plug at term of pregnancy. Acta obstetricia et gynecologica Scandinavica. 2005 Aug;84(8):734–742. doi: 10.1111/j.0001-6349.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 11.Becher N, Hein M, Danielsen CC, Uldbjerg N. Matrix metalloproteinases in the cervical mucus plug in relation to gestational age, plug compartment, and preterm labor. Reprod Biol Endocrinol. 2010;8:113. doi: 10.1186/1477-7827-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zervomanolakis I, Ott HW, Hadziomerovic D, et al. Physiology of upward transport in the human female genital tract. Annals of the New York Academy of Sciences. 2007 Apr;1101:1–20. doi: 10.1196/annals.1389.032. [DOI] [PubMed] [Google Scholar]
- 13.Ansbacher R, Boyson WA, Morris JA. Sterility of the uterine cavity. American journal of obstetrics and gynecology. 1967 Oct 1;99(3):394–396. doi: 10.1016/s0002-9378(16)34549-5. [DOI] [PubMed] [Google Scholar]
- 14.Spore WW, Moskal PA, Nakamura RM, Mishell DR., Jr Bacteriology of postpartum oviducts and endometrium. American journal of obstetrics and gynecology. 1970 Jun 15;107(4):572–577. doi: 10.1016/s0002-9378(16)33944-8. [DOI] [PubMed] [Google Scholar]
- 15.Moller BR, Kristiansen FV, Thorsen P, Frost L, Mogensen SC. Sterility of the uterine cavity. Acta obstetricia et gynecologica Scandinavica. 1995 Mar;74(3):216–219. doi: 10.3109/00016349509008942. [DOI] [PubMed] [Google Scholar]
- 16.Cowling P, McCoy DR, Marshall RJ, Padfield CJ, Reeves DS. Bacterial colonization of the non-pregnant uterus: a study of pre-menopausal abdominal hysterectomy specimens. Eur J Clin Microbiol Infect Dis. 1992 Feb;11(2):204–205. doi: 10.1007/BF01967084. [DOI] [PubMed] [Google Scholar]
- 17.Bollinger CC. Bacterial Flora of the Nonpregnant Uterus: A New Culture Technic. Obstetrics and gynecology. 1964 Feb;23:251–255. [PubMed] [Google Scholar]
- 18.Hemsell DL, Obregon VL, Heard MC, Nobles BJ. Endometrial bacteria in asymptomatic, nonpregnant women. The Journal of reproductive medicine. 1989 Nov;34(11):872–874. [PubMed] [Google Scholar]
- 19.Mishell DR, Jr, Bell JH, Good RG, Moyer DL. The intrauterine device: a bacteriologic study of the endometrial cavity. American journal of obstetrics and gynecology. 1966 Sep 1;96(1):119–126. doi: 10.1016/s0002-9378(16)34650-6. [DOI] [PubMed] [Google Scholar]
- 20.Sparks RA, Purrier BG, Watt PJ, Elstein M. The bacteriology of the cervix and uterus. Br J Obstet Gynaecol. 1977 Sep;84(9):701–704. doi: 10.1111/j.1471-0528.1977.tb12684.x. [DOI] [PubMed] [Google Scholar]
- 21.Andrews WW, Hauth JC, Cliver SP, Conner MG, Goldenberg RL, Goepfert AR. Association of asymptomatic bacterial vaginosis with endometrial microbial colonization and plasma cell endometritis in nonpregnant women. American journal of obstetrics and gynecology. 2006 Dec;195(6):1611–1616. doi: 10.1016/j.ajog.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Teisala K. Endometrial microbial flora of hysterectomy specimens. European journal of obstetrics, gynecology, and reproductive biology. 1987 Oct;26(2):151–155. doi: 10.1016/0028-2243(87)90050-5. [DOI] [PubMed] [Google Scholar]
- 23.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012 Sep 13;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 24.Consortium THMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012 Jun 14;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of clinical microbiology. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in Vaginal Bacterial Concentrations with Intravaginal Metronidazole Therapy for Bacterial Vaginosis as Assessed by Quantitative PCR. Journal of clinical microbiology. 2009 Jan 14; doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012 May 2;4(132):132ra152. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eschenbach DA, Rosene K, Tompkins LS, Watkins H, Gravett MG. Endometrial cultures obtained by a triple-lumen method from afebrile and febrile postpartum women. The Journal of infectious diseases. 1986 Jun;153(6):1038–1045. doi: 10.1093/infdis/153.6.1038. [DOI] [PubMed] [Google Scholar]
- 31.Boomsma CM, Kavelaars A, Eijkemans MJ, et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Human reproduction (Oxford, England) 2009 Jun;24(6):1427–1435. doi: 10.1093/humrep/dep011. [DOI] [PubMed] [Google Scholar]
- 32.Relman DA. Microbiology: Learning about who we are. Nature. 2012 Jun 14;486(7402):194–195. doi: 10.1038/486194a. [DOI] [PubMed] [Google Scholar]
- 33.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science (New York, NY) 2012 Jun 8;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011 Oct 13;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shima T, Sasaki Y, Itoh M, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. Journal of reproductive immunology. 2010 Jun;85(2):121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Wang Z, Zhao X, Wang J, Sun H, Hu Y. An increase of Treg cells in the peripheral blood is associated with a better in vitro fertilization treatment outcome. Am J Reprod Immunol. 2012 Aug;68(2):100–106. doi: 10.1111/j.1600-0897.2012.01153.x. [DOI] [PubMed] [Google Scholar]
- 37.Moore DE, Soules MR, Klein NA, Fujimoto VY, Agnew KJ, Eschenbach DA. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertility and sterility. 2000 Dec;74(6):1118–1124. doi: 10.1016/s0015-0282(00)01624-1. [DOI] [PubMed] [Google Scholar]
- 38.Inzunza J, Midtvedt T, Fartoo M, et al. Germfree status of mice obtained by embryo transfer in an isolator environment. Lab Anim. 2005 Oct;39(4):421–427. doi: 10.1258/002367705774286439. [DOI] [PubMed] [Google Scholar]
- 39.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. The New England journal of medicine. 2000 May 18;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 40.Kiviat NB, Wolner-Hanssen P, Eschenbach DA, et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol. 1990 Feb;14(2):167–175. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.