Abstract
Functional maturation of afferent synaptic connections to inner hair cells (IHCs) involves pruning of excess synapses formed during development, as well as the strengthening and survival of the retained synapses. These events take place during the thyroid hormone (TH)-critical period of cochlear development, which is in the perinatal period for mice and in the third trimester for humans. Here, we used the hypothyroid Snell dwarf mouse (Pit1dw) as a model to study the role of TH in afferent type I synaptic refinement and functional maturation. We observed defects in afferent synaptic pruning and delays in calcium channel clustering in the IHCs of Pit1dw mice. Nevertheless, calcium currents and capacitance reached near normal levels in Pit1dw IHCs by the age of onset of hearing, despite the excess number of retained synapses. We restored normal synaptic pruning in Pit1dw IHCs by supplementing with TH from postnatal day (P)3 to P8, establishing this window as being critical for TH action on this process. Afferent terminals of older Pit1dw IHCs showed evidence of excitotoxic damage accompanied by a concomitant reduction in the levels of the glial glutamate transporter, GLAST. Our results indicate that a lack of TH during a critical period of inner ear development causes defects in pruning and long-term homeostatic maintenance of afferent synapses.
Keywords: auditory system, calcium current, capacitance measurements, glutamate transporter
Introduction
Early neuronal development is marked by overabundant outgrowth of axons and dendrites that innervate multiple targets. Inappropriate or excess neuronal connections are subsequently pruned back, resulting in a mature neuronal circuit. Defective or abnormal neuronal pruning can result in various neurological and behavioural disorders (Paolicelli et al., 2011; Zhan et al., 2014). Cochlear afferent innervation also undergoes pruning as the organ of Corti matures into a fully functioning organ (Sobkowicz et al., 1982; Huang et al., 2012). However, the mechanisms regulating the postnatal events that lead to a mature pattern of afferent innervation in the cochlea are not well understood. It is well known that thyroid hormone (TH) is required for normal development of both the central and peripheral nervous systems in a temporally sensitive manner (Bernal, 2005). Previous studies have shown that hypothyroidism alters neuronal connectivity to inner hair cells (IHCs) and outer hair cells, impairs their function, disrupts synaptic pruning, and alters vesicle release (Uziel et al., 1983; Rusch et al., 2001; Rueda et al., 2003; Brandt et al., 2007; Sendin et al., 2007), indicating an important role of TH action on these processes. These studies also raised several interesting questions regarding the mechanisms that control the retraction of excess neurites and the strengthening and survival of the retained connections such as: (i) what are the quantitative effects of hypothyroidism on presynaptic and postsynaptic pruning; (ii) are the excess synapses in hypothyroid IHCs functionally mature; (iii) what is the developmental time window for TH action on synaptic pruning; and (iv) does disrupted synaptic pruning resulting from hypothyroidism have long-term effects on synaptic maintenance? Addressing these questions will allow us to gain insights into the mechanisms regulating the pruning and survival of afferent IHC synapses in the cochlea, and reveal the functional consequences of these mechanisms being disrupted.
In this study, we used the Snell dwarf (Pit1dw, officially Pou1f1dw) hypothyroid mouse (Karolyi et al., 2007; Mustapha et al., 2009) model to address the questions outlined above focusing on IHC afferent synapses. We quantified the number of synapses under hypothyroid conditions at different time points during the postnatal period of the TH-critical window for cochlear development, which extends from embryonic day 18 to postnatal day (P)12 (Deol, 1973; Uziel, 1986; Knipper et al., 1999). We determined the functional status of the afferent synapses in the Pit1dw IHCs by analysing the recruitment of calcium channels to the synapses and by measuring calcium current and membrane capacitance. To determine the effect of hypothyroidism on the long-term maintenance of afferent IHC synapses, we examined the synaptic structure in older (P42) mice. We also determined the critical window for TH action on synaptic pruning by carrying out TH replacement for finite periods in the Pit1dw mice. Our results provide several novel insights into the structure and function of afferent synapses in hypothyroid conditions, the effect of TH on long-term synaptic maintenance, and the critical window required for TH action on synaptic pruning.
Materials and methods
Mice
DW/J Mlphln Pitdw/J mice were obtained from the Jackson Laboratory in 1990, and are maintained at Stanford University. Established procedures for animal care and genotyping were used, including feeding the mice a higher-fat chow designed for breeding (PMI5020), delayed weaning of mutants at P35, and housing mutants with normal littermates to provide warmth (Karolyi et al., 2007). P0 is the day of birth. All experiments were approved by the University Committee on the Use and Care of Animals, and conducted in accord with the principles and procedures outlined in the National Institutes of Health Guidelines for the Care and Use of Experimental Animals.
Immunofluorescence staining and confocal microscopy
RIBEYE and SHANK1 were used as presynaptic and postsynaptic markers, respectively. Another marker, CaV1.3, identified this calcium channel at the synapse. Inner ears were dissected into cold phosphate-buffered saline (PBS), and, after opening of the oval and round windows and the bone at the cochlear apex, cochleas were perfused with 4% paraformaldehyde in PBS and left in fixative solution for an additional 10 min. Samples were washed in PBS for 10 min, and then blocked in PBS containing 0.5% Triton X-100 and 5% bovine serum albumin for 30 min at room temperature. The same blocking buffer was used for diluting antibodies. Primary antibodies were incubated at 4 °C for 36 h, and this was followed by three washes in PBS with 0.1% Tween. Secondary antibodies were added for 1 h at room temperature, and this was followed by three washes in PBS with 0.1% Tween. The following polyclonal primary antibodies were used: goat anti-CtBP2 (1 : 200; Santa Cruz Biotechnology) for RIBEYE, rabbit anti-SHANK1 (1 : 200; Neuromics), rabbit anti-CaV1.3 (1 : 500; Alomone), goat anti-GLAST (1 : 200; Cell Signaling), and rabbit anti-myosin VIIa (1 : 300; Proteus Biosciences). The secondary antibodies used were Alexa Fluor 488-conjugated anti-goat and Alexa Fluor 546-conjugated anti-rabbit (1 : 500; Invitrogen). Subsequently, the cochleas were decalcified in 10% EDTA for 1 h at room temperature, and further fixed by immersion in 4% paraformaldehyde in PBS for 15 min. Cochleas were washed in PBS and mounted on slides in ProLong (Invitrogen) anti-fading medium. Confocal images of cochlear whole mount preparations were reconstructed in z-stacks with volocity 3d image analysis software to count the numbers of synaptic puncta. The data shown are from the mid-turn of the cochlea.
Quantification and statistical analysis
Quantification of RIBEYE, SHANK1, CaV1.3 and co-localized RIBEYE and SHANK1 (representing a ‘synapse’) or CaV1.3 puncta was performed as previously described (Mendus et al., 2014). A minimum of 10 IHCs per mouse and four to seven mice per genotype or treatment group were used for each marker. Marker counts are shown as box and whisker plots that represent every data point in the set. The whiskers represent the minimum and maximum values in the specific group examined. Outliers in the data set are also shown. Statistical analyses were performed with anova [spss statistics premium grad pack (IBM), or matlab (Mathworks, Natick, MA, USA)]. Two-way anova of the immunoh istochemistry data was performed with synaptic marker counts as the dependent variable, and with genotype (wild type or Pit1dw) and treatment or age as independent factors for these tests. On the basis of significant main effects and interactions from this statistical test, planned comparisons were performed by the use of Student’s t-test or a one-way anova with marker count as the dependent variable, followed by Scheffe’s post hoc test to identify differences between genotypes, or across ages or treatment groups (spss and microsoft excel). In all statistical tests, a P-value of <0.05 was considered to be statistically significant.
Cochlear RNA extraction and quantitative RT-PCR
Cochleas were dissected, and RNA extraction and cDNA preparation were performed as previously described (Mendus et al., 2014). To quantify mRNA expression levels, the cDNA was amplified in Taqman PCR assays (Applied Biosystems, Foster City, CA, USA). We used proprietary probes and primers from Applied Biosystems for CaV1.3 (assay ID Mm01209919_m1), GLAST (assay ID Mm00600697_m1), and the glyceraldehyde-3-phosphate dehydrogenase gene (assay ID Mm99999915_g1). All Taqman quantitative PCR assays were performed on a Bio-Rad CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA) with accompanying software for data analysis. Expression of the gene of interest was normalized with respect to the internal standard, the glyceraldehyde-3-phosphate dehydrogenase gene. RNA for each genotype was prepared by pooling RNA from three or four mice. At least four such independent experiments with all samples in triplicate were performed. Statistical analyses were performed with Student’s t-test on microsoft excel (Microsoft, Redmond, WA, USA).
Calcium current and capacitance measurements
Mice of different ages were decapitated, and cochleas were removed as previously described (Peng et al., 2013). Organs of Corti were dissected in a solution containing 140 mM NaCl, 2 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, 6 mM glucose, 2 mM pyruvate, and 2 mM creatine (pH 7.4 and 300-310 mOsm). The tectorial membrane was removed, and the midturn of the organ of Corti was mounted in a recording chamber with single strands of dental floss. The bath was perfused at rates of 0.4-0.5 mL/min and maintained at 20-23 °C. Whole cell patch-clamp was achieved on IHCs from the middle to apical cochlear turns with an Axon 200B amplifier (Molecular Devices) and thick-walled borosilicate patch pipettes (<4 MΩ). Pipettes were filled with an intracellular solution containing 125 mM CsCl, 3.5 mM MgCl2, 5mM ATP, 5 mM creatine phosphate, 10 mM HEPES, 1 mM caesium BAPTA, and 2 mM ascorbate (pH 7.2, 280–290 mOsm). Data were collected by use of an IOTech A/D (DAQ3000) with jclamp software (SciSoft) at a rate of 30 kHz, and filtered at 10 kHz. Capacitance was tracked with the dual sinusoidal approach (Schnee et al., 2011). This is a fast Fourier transfer-based method (Santos-Sacchi, 2004; Santos-Sacchi et al., 1998), and is based on a component solution of a simple model for a patched cell. Two voltage frequencies, f1 and f2, were summed, and the real and imaginary outputs of the current were used to determine the model components. We used an f1 frequency of 3125 Hz. Data were quantified and figures were generated with microsoft excel, microcal origin, and adobe illustrator.
TH injection
Pit1dw mice and control wild-type (WT) mice were subcutaneously injected daily from P3 to P8 with either 20 ng 3,3,5-triiodo-L-thyronine sodium salt (T3) solution/g body weight or PBS as a negative control. T3 solution (20 ng/μL) was prepared by dissolving 1 mg of T3 (Sigma Aldrich, St Louis, MO, USA) in 1 mL of 1 M NaOH and 49 mL of PBS. Radioimmunoassay was used to confirm that this dose of T3 was sufficient to induce normal TH levels in Pit1dw mice. Other groups have shown that replacement with T3 rather than T4 (thyroxine) is more effective, becauseit bypasses the necessary conversion of T4 to the more active T3 in vivo (Weiss et al., 1998; Sui et al., 2006). Mice were killed at P14.
Transmission electron microscopy
Transmission electron microscopy (TEM) analyses were performed as previously described (Mustapha et al., 2009). Briefly, mice were anaesthetized and fixed with an intracardiac perfusion of 2.5% glutaraldehyde in 0.15 m cacodylate buffer (pH 7.2) with 0.1% tannic acid. Inner ears were removed and further fixed in the same solution for 2 h. The ears were then decalcified with 3% EDTA with 0.25% glutaraldehyde for 1 week at 4 °C. They were then postfixed in 1% osmium tetraoxide, dehydrated with increasing ethanol concentrations, and embedded in Embed 812 epoxy resin. Embedded ears were sectioned, stained with uranyl acetate and lead citrate, and examined on a Phillips CM-100 transmission electron microscope. A minimum of four mice per group were examined.
Results
Elimination of excess presynaptic and postsynaptic structures formed during development is disrupted in hypothyroid IHCs
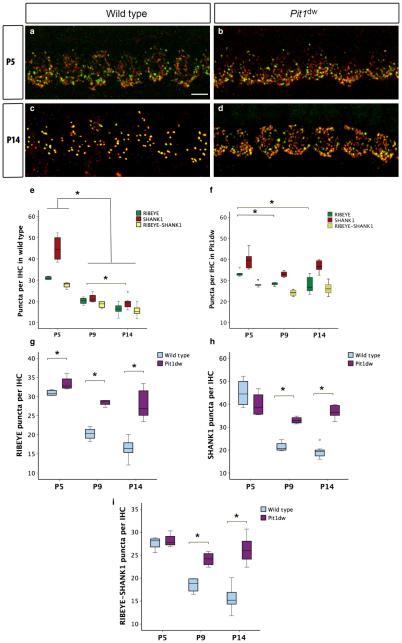
In mice, the development of IHC afferent innervation normally starts as early as embryonic day 16, and continues through birth until the onset of hearing at P12 (Ehret, 1976). The pruning of excessive type I afferent neurite branches and synapses formed during development at IHCs is an important aspect of this maturation process (Huang et al., 2007, 2012). We examined the IHC synapses in control WT and Pit1dw mice by immunohistochemistry with the presynaptic ribbon marker RIBEYE and the postsynaptic markers SHANK1 and GLUTR2/3 at P5, P9, and P14. The results of quantification of both SHANK1 and GLUT2/3 puncta were similar. However, we found that the anti-SHANK1 antibody gave us more consistent results. Therefore, we used SHANK1 data for all subsequent analyses. We analysed both the apical turn and mid-turn of the cochlea. As the results from both cochlear regions were similar, images and quantitative analyses from the mid-turn are shown henceforth. Representative images of immunohistochemistry for RIBEYE and SHANK1 are shown in Fig. 1a–d for P5 and P14 in both WT and Pit1dwmice. We quantified puncta that were RIBEYE-positive only, puncta that were SHANK1-positive only, and puncta in which both markers were co-localized (henceforth referred to as RIBEYE–SHANK1). For statistical analysis, two-way ANOVAs were performed with RIBEYE, SHANK1 or RIBEYE– SHANK1 count as the dependent variable, and with genotype (WT or Pit1dw) and age (P5, P9, and P14) as independent factors. This analysis showed a significant main effect of genotype × age interaction for all three markers (F2,24 = 35.640, P = 0.014 for RIBEYE; F2,24 = 314.714, P = 2.2E-5 for SHANK1; F2,24 = 55.326, P = 0.002 for RIBEYE–SHANK1). Planned comparisons were performed with a one-way anova, with a specific marker count (RIBEYE, SHANK1, or RIBEYE–SHANK1) as the dependent variable and either age or genotype as the independent factor, as indicated below. This analysis was followed by Scheffe’s post hoc test to identify differences between genotypes or across the ages tested.
Fig. 1.
Synaptic pruning is disrupted in Pit1dw IHCs. (a and b) Projection of confocal sections obtained from the mid-turn of cochlear whole mounts stained with afferent presynaptic (RIBEYE; green) and postsynaptic (SHANK1; red) markers at P5 in WT mice (a) and Pit1dw mice (b). (c and d) The same markers at P14 in WT mice (c) and Pit1dw mice (d). (e–i) Box plots of the quantification of RIBEYE, SHANK1 and RIBEYE–SHANK1 puncta from the mid-turn of the cochlea in WT and Pit1dw mice. (e and f) RIBEYE, SHANK1 and RIBEYE–SHANK1 counts at P5, P9 and P14 in WT mice (e) and Pit1dw mice (f). (g–i) Comparison of RIBEYE (g), SHANK1 (h) and RIBEYE–SHANK1 (i) counts between WT and Pit1dw mice. significant comparisons (P < 0.05) are indicated with asterisks. The dots indicate outliers in the data.
WT IHCs showed a normal pattern of synaptic pruning (Sobkowicz et al., 1982; Huang et al., 2012) (Fig. 1a, c and e). Actual counts for marker puncta per IHC are shown in Fig. 1e–i. A one-way anova with RIBEYE count as the variable and age as the independent factor was significant (F2,12 = 65.544; P = 3E-6). Scheffe’s post hoc test showed that the number of RIBEYE puncta was significantly lower at P9 (34%) and at P14 (47%) than at P5 (P = 3.2E-5 for P9 vs. P5; P = 3.7E-7 for P14 vs. P5) (Fig. 1e and g). Similar one-way ANOVAs for SHANK1 and RIBEYE–SHANK1 counts were also significant (F2,12 = 62.291 and P = 0.001 for SHANK1; F2,12 = 38.430 and P = 3E-5 for RIBEYE–SHANK1). SHANK1 counts were markedly reduced at P9 (52%) and at P14 (57%) as compared with P5 (P = 7E-6 for P9 vs. P5; P = 7.3E-7 for P14 vs. P5; Scheffe’s post hoc test) (Fig. 1e and h). RIBEYE–SHANK1 puncta counts were also significantly lower at P9 (33%), and at P14 (43%) than at P5 in WT mice (P = 0.0003 for P9 vs. P5; P = 7E-6 for P14 vs. P5; Scheffe’s post hoc test) (Fig. 1e and i). These results are consistent with a normal pattern of both presynaptic and postsynaptic pruning and elimination of excess synapses in these mice during development. In comparison, age-matched Pit1dw IHCs showed reduced pruning in the mid-turn (Fig. 1b, d and f). A one-way anova for RIBEYE counts in these mice was performed (F2,12 = 12.616, P = 0.001). Pitdw IHCs showed a smaller reduction in RIBEYE counts at P9 (13%, P = 0.032; Scheffe’s post hoc test) and P14 (21%, P = 0.001; Scheffe’s post hoc test) than at P5. In contrast, there were no significant changes in SHANK1 and RIBEYE–SHANK1 puncta in Pit1dw mice across the ages tested by one-way anova (P = 0.048 for SHANK1; P = 0.121 for RIBEYE–SHANK1). This result implied that, although a normal rate of synapse elimination was not observed in the Pit1dw IHCs, pruning had occurred to some extent by P14 only on the presynaptic side.
We found that, at all ages examined, Pit1dw IHCs showed a distinctly different synaptic staining pattern from those in WT mice of the same age. At P5, RIBEYE counts were slightly (P = 0.028; Scheffe’s post hoc test following a one-way anova) lower in WT mice than in Pit1dw mice, but SHANK1 and RIBEYE–SHANK1 puncta counts were similar in both genotypes (Fig. 1a, b, g, h and i), suggesting that presynaptic pruning was already underway in WT mice by P5. Moving further along the refinement window at P9 and P14, we found that all three markers were significantly lower in WT than in Pitdw mice (RIBEYE, P = 0.025 at P5, P = 0.0001 at P9, and P = 0.0002 at P14; SHANK1, P = 0.0001 at P9 and P = 2E-6 at P14; RIBEYE–SHANK1, P = 0.002 at P9 and P = 0.001 at P14; Scheffe’s post hoc test following a one-way anova) (Fig. 1g–i). Taken together, these data indicated that pruning of excess afferent synapses failed in the hypothyroid cochlea.
We also found an abnormal pattern of afferent synapses in adult Pit1dw IHCs by ultrastructural analysis with TEM. Pit1dw IHCs showed a large immature synaptic zone with multiple ribbons, as compared with a single ribbon in WT IHCs, as previously described (Sendin et al., 2007) (data not shown). To determine whether the defective pruning of synapses in the hypothyroid IHCs correlated with functional alterations, we examined the composition and function of the IHC afferent synapses in these mice.
Composition and function of IHC afferent presynaptic proteins in Pit1dw mice
Calcium channel expression and current measurements in Pitdw and WT IHCs
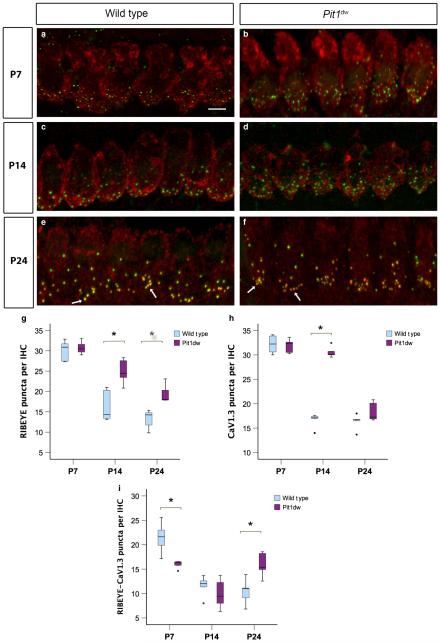
In order to determine the percentage of RIBEYE–SHANK1 or co-localized puncta that were functional, we examined the expression and distribution of the calcium channel, CaV1.3. CaV1.3 is the primary calcium channel of both mature and immature IHCs (Engel et al., 2002; Brandt et al., 2003, 2005; Michna et al., 2003). We examined whether there was any alteration in the level of gene expression and distribution of this protein in Pit1dw mice vs. control mice. The mRNA expression levels of CaV1.3 in the organ of Corti at both P4 and P7 were not significantly different between Pit1dw and WT cochlea as determined by quantitative PCR (data not shown). We investigated the expression pattern and presynaptic distribution of the CaV1.3 protein by using immunohistochemistry with anti-CaV1.3 and anti-RIBEYE antibodies at P7, P14, and P24, followed by confocal imaging and counting of labelled puncta (Fig. 2a–f). We then performed a two-way anova with marker count as the dependent variable and age and genotype as independent factors. This analysis showed a significant genotype × age interaction (F2,28 = 8.157, P = 0.002 for RIBEYE; F2,28 = 75.039,P = 5.6E-12forCaV1.3;andF2,28 = 5.440, P = 3.1E5 for co-localized RIBEYE and CaV1.3). This analysis was followed by one-way anova for planned comparisons between genotypes or across ages, followed by Scheffe’s post hoc test. The number of co-localized CaV1.3–RIBEYE puncta was significantly lower at P7 in Pit1dw IHCs than in WT IHCs (P = 0.004), but not different at P14 (Fig. 2a–d and i). These data suggested that clustering of CaV1.3 at ribbon synapses was delayed at P7 in Pit1dw IHCs but reached normal levels by P14. The number of CaV1.3 puncta at P14 was, however, significantly higher in Pit1dw IHCs than in WT IHCs (P = 2.2E-9) (Fig. 2c, d and h). We inferred from these data that the excess ribbon synapses seen at P14 in Pit1dw mice were not coupled with CaV1.3, and therefore may be functionally inactive. At P24, however, most of the RIBEYE puncta were juxtaposed with CaV1.3 puncta in Pit1dw IHCs, and, interestingly, there were significantly higher (P = 0.01) numbers of these functional synaptic puncta in Pit1dw IHCs than in control IHCs at this age (Fig. 2e, f and i). We also examined the changes in expression for these markers across the three ages tested (P7, P14, and P24) within a given genotype (WT or Pit1dw mice) to determine the developmental time course for the formation of mature ribbon synapses that contain CaV1.3. WT IHCs had counts for CaV1.3 and co-localized RIBEYE–CaV1.3 puncta that were comparable at P14 and P24 (P = 0.15) but significantly lower than the respective values at P7 (P = 3.3E-10 for P14 vs. P7; P = 4.6E-10 for P24 vs. P7), indicating that functional synapses had undergone refinement in WT mice by P14 (Fig. 2h and i). In Pit1dw mice, however, CaV1.3 counts at P7 and P14 were comparable, whereas those at P24 were significantly lower than at either of these ages (P = 1.5E-9 and P = 6.5E-9 for P24 vs. P7 and P24 vs. P14, respectively) (Fig. 2h). Interestingly, the numbers of colocalized RIBEYE–CaV1.3 puncta at P14 in Pit1dw mice were significantly lower than those at both P7 and P24 (P = 0.001 for both comparisons) (Fig. 2i).
Fig. 2.
Abnormal CaV1.3 puncta clustering at the synapses of Pit1dw IHCs. (a and b) Projection of confocal sections obtained from the mid-turn of cochlear whole mounts stained with the afferent presynaptic marker RIBEYE (green) and the calcium channel CaV1.3 (red) at P7 in WT mice (a) and Pit1dw mice (b). (c–f) The same markers at P14 for WT (c) and Pit1dw mice (d), and at P24 for WT (e) and Pit1dw mice (f). (g–i) Box plots of the quantification of RIBEYE (g), CaV1.3 (h) and RIBEYE–CaV1.3 (i) puncta from the mid-turn of the cochlea in WT and Pit1dw mice. significant comparisons (P < 0.05) between genotypes are indicated with asterisks. To maintain clarity of the figure, P-values for significant comparisons across different ages within the same genotype are not indicated on the graph, but are mentioned in the relevant Results section. The dots indicate outliers in the data.
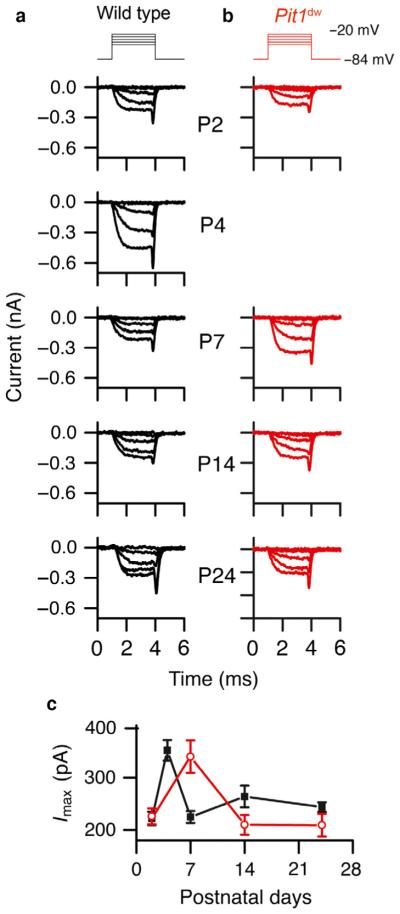
To examine whether the presence of excess synapses or the immature synaptic properties of Pit1dw IHCs affected their function, we measured calcium currents and vesicle fusion changes in Pit1dw and WT IHCs at various time points during development (between P2 and P24). In these experiments, calcium currents from both Pit1dw and WT IHCs matured at different ages during the first postnatal week (Beutner & Moser, 2001; Johnson et al., 2005). The calcium current was high initially in WT IHCs, but plateaued from P7 onwards (one-way anova with current measurement as the dependent variable and age as the independent factor followed by Scheffe’s post-hoc tests showed that calcium currents at P7, P14 and P24 were comparable to each other, but were all significantly lower than the calcium current at P4: P = 5.2E-5 for P4 vs. P7; P = 0.002 for P4 vs. P14; P = 2.3E-7 for P4 vs. P24) (Marcotti et al., 2003). In Pit1dw IHCs, however, the calcium current was still high at P7, and maturation was delayed (Fig. 3c). It eventually matured by P14 in Pit1dw IHCs (a similar one-way anova for calcium current data from Pit1dw IHCs, followed by Scheffe’s post hoc tests, showed that calcium currents at P14 and P21 were not significantly different from each other, but were both lower than the calcium current at P7: P = 0.017 for P7 vs. P14; P = 0.013 for P7 vs. P24) (Fig. 3a–c). The calcium current at P14 in Pit1dw IHCs was comparable to the calcium current in WT IHCs. No significant differences were observed in the voltage of half-activation during development for either the WT or the Pit1dw mice (data not shown). Taken together, these data suggest that TH is required for the normal clustering and/ or pruning of CaV1.3 during development, similarly to what we observed with RIBEYE (Fig. 1a and b). Our electrophysiology measurements showed that, after an initial delay in maturation, there were normal calcium currents at P14 and at P24 in Pit1dw IHCs. The excess number of CaV1.3 puncta and functional synapses at these ages did not correlate with the measured calcium current amplitude, suggesting that the number of channels per puncta was reduced or perhaps that single-channel conductance was altered during normal hair cell maturation, as previously described (Zampini et al., 2010).
Fig. 3.
(a and b) Calcium current measurements from WT (a) and Pit1dw (b) IHCs. The stimulus is shown at the top. Measurements were made at the ages indicated on the right. Hair cells were held at −84 mV and depolarized in 10-mV steps from −120 mV to +20 mV. The data shown include 20-mV steps from −80 mV to −60 mV. (c) Plots of the calcium current amplitudes at different postnatal days for WT (black) and Pit1dw (red) IHCs. The number of WT and Pit1dw IHCs recorded were, respectively, P2 (12 and 5), P4 (4 and 0), P7 (13 and 4), P14 (11 and 20), and P24 (9 and 12).
Two sine capacitance measurements in Pit1 and WT IHCs
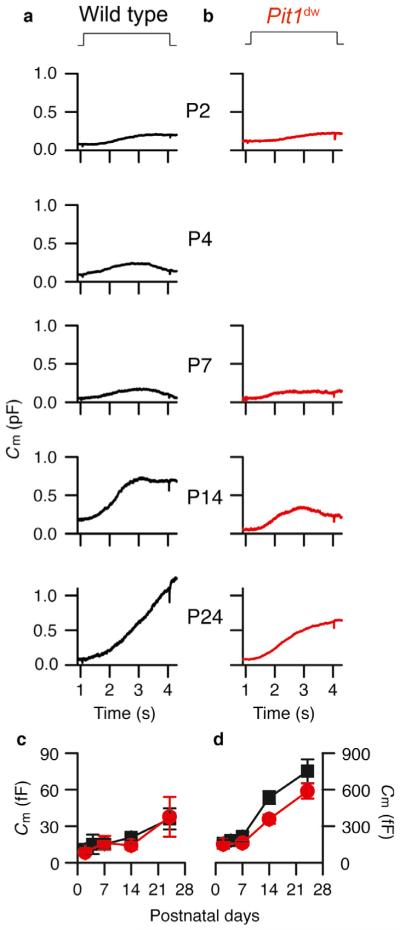
Both vesicle trafficking and vesicle fusion are triggered by calcium influx through CaV1.3 calcium channels (Beutner & Moser, 2001; Brandt et al., 2003, 2005; Fuchs et al., 2003; Moser et al., 2006). A change in the calcium channel distribution is predicted to alter synaptic release properties. Therefore, we investigated whether the initial delay in CaV1.3 current maturation in Pit1dw IHCs mice altered neurotransmitter release during development. We used two sine capacitance measurements that allowed us to monitor capacitance continually during a depolarization (Schnee et al., 2011). Previous work identified two components of release, i.e. an initial fast component that saturated quickly and correlated with vesicles immediately near to the synapse, and a second, larger component of release, termed superlinear, that required recruitment of additional vesicles. Using this method, we found that the initial delay in calcium current maturation during the early postnatal period (P2 and P7) did not affect total vesicle release in Pit1dw IHCs as compared with WT IHCs (Fig. 4a, b and d). At P14, however, despite similar calcium current measurements in both Pit1dw and WT IHCs, capacitance measurements showed normal first-component release (release at 200 ms) (Fig. 4c) but lower total vesicle release (Fig. 4d), suggesting reduced vesicle replenishment or second-component release in Pit1dw IHCs as compared with WT IHCs. At P24, total vesicle release reached nearly normal levels in Pit1dw IHCs (Fig. 4d), indicating that the reduced vesicle replenishment phenotype seen at P14 in these mice was likely to be the result of a developmental delay.
Fig. 4.
Two sine capacitance measurements from WT (a) and Pit1dw (b) IHCs at different postnatal ages, as indicated. Capacitance was measured in response to a 3-s depolarization to −34 mV from a holding potential of −84 mV. The first component was measured at 200 ms into the step depolarization, and is summarized in (c), and full release is summarized in (d). The number of measurements were as follows for WT and Pit1dw IHCs, respectively: P2 (6 and 4), P4 (2 and 0), P7 (4 and 3), P14 (6 and 5), and P24 (5 and 5).
Our study revealed that the absence of TH during the early postnatal period of cochlear neuronal development caused a delay in the pruning and clustering of presynaptic elements important for the function of afferent IHC synapses. Despite the excess number of synapses in these hypothyroid IHCs, the number of functional synapses reached normal levels by the age of onset of hearing (P14). This subset of structurally and functionally mature synapses was sufficient to drive a normal calcium current and first-component vesicle release at P14 and P24. The higher number of functional synapses at P24 in Pit1dw IHCs indicates that calcium current and vesicle release per synapse were perhaps reduced as compared with WT synapses, resulting in comparable calcium current and capacitance. We next examined whether TH replacement would restore normal synaptic pruning in the afferent synapses of Pit1dw IHCs.
TH replacement rescues normal synaptic pruning in Pit1dw IHCs
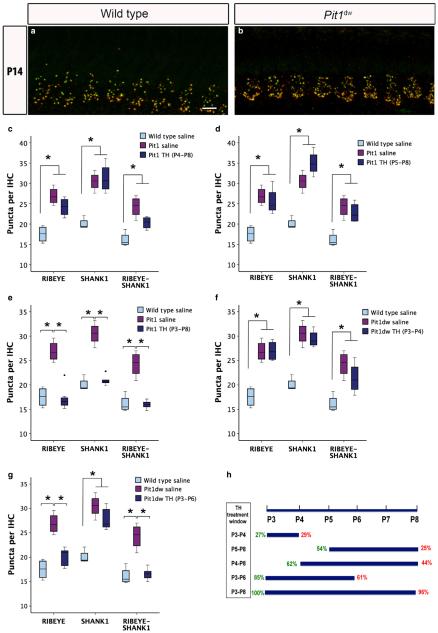
We injected Pit1dw mice with TH for specific time windows during the early postnatal period (Fig. 5h). These time windows were initially chosen on the basis of reports of higher deiodinase 2 activity and TH transporter levels in the cochlea from P5 to P8 suggesting that a greater amount of the active T3 form of TH was available at this period (Campos-Barros et al., 2000; Sharlin et al., 2011). We hypothesized that, if TH were required for pruning in this time window, supplementing with TH during this period would rescue the abnormal synaptic pruning phenotype that we saw in the Pit1dw mice (Fig. 1). We examined the synapses in the IHCs of TH-treated Pit1dw mice by immunohistochemistry. Our initial studies with TH replacement in two distinct time windows (from P5 to P8, and from P4 to P8) did not show significant differences between the treated and untreated groups at P14, with respect to number of puncta labelled with RIBEYE and SHANK1 (Fig. 5c, d and h). We then extended the duration of TH replacement from P3 to P8. Confocal images of cochlear whole mounts stained with antibodies against RIBEYE and SHANK1 are shown from the mid-turn for salineinjected WT and TH-treated Pit1dw mice at P14 in Fig. 5a and b. We quantified the marker (RIBEYE, SHANK1 and RIBEYE–SHANK1) puncta, and then performed one-way anova with marker count as the dependent variable and treatment group as the independent factor (F2,12 = 28.429, P = 0.001 for RIBEYE; F2,12 = 65.263, P = 0.001for SHANK1; F2,12 = 35.686, P = 0.008 for RIBEYE–SHANK1). RIBEYE, SHANK1 and RIBEYE–SHANK1 counts in IHCs of TH-treated Pit1dw mice (Fig. 5b and e) were similar to those in saline-treated WT mice at P14 (Fig. 5a and e). Also, as expected, RIBEYE, SHANK1 and RIBEYE–SHANK1 marker counts were significantly lower in IHCs of TH-treated Pit1dw mice than in those of saline-treated Pit1dw mice (P = 5E-5 for RIBEYE; P = 1E-6 for SHANK1; P = 1.7E-5 for RIBEYE–SHANK1; Scheffe’s post hoc test). Treatment with TH from P3 to P8 restored a normal synaptic pattern and a normal number in Pit1dw mice, as indicated by the results described above. We then investigated whether TH-mediated pruning can be completed before P8 by supplementing with TH from P3 to P4 (Fig. 5f) and from P3 to P6 (Fig. 5g). For the P3 to P4 window, RIBEYE, SHANK1 and RIBEYE–SHANK1 marker counts in Pit1dw mice treated with TH were similar to those in saline-treated Pit1dw mice (Fig. 5f). This result indicated that TH treatment from P3 to P4 was insufficient to restore normal pruning in these mice. Treatment of Pit1dw mice with TH from of P3 to P6 resulted in a significant reduction in the numbers of both RIBEYE (P = 0.002) and RIBEYE–SHANK1 (P = 0.006) puncta, but not of SHANK1 puncta, as compared with saline-treated Pit1dw mice (Fig. 5g). Likewise, counts for RIBEYE and RIBEYE-SHANK1 puncta were comparable to those in WT mice, but that for SHANK1 was still significantly higher than in WT mice in the P3 to P6 treatment group (P = 0.001). Therefore, we inferred that the window from P6 to P8 might be required to complete postsynaptic pruning. The different TH treatment windows and percentages of presynaptic and postsynaptic pruning with respect to pruning in WT mice are summarized in Fig. 5h. Additionally, we considered two more TH treatment windows, i.e. from P0 to P5 and from P5 to P10, to rule out the possibility that any 5-day period of TH supplementation during the early postnatal days would produce the same benefits as the P3 to P8 treatment. Quantification of RIBEYE and SHANK1 puncta from the cochleas of Pit1dw mice treated from P0 to P5 or from P5 to P10 did not show normal synaptic pruning as seen with the P3 to P8 treatment (data not shown). In addition to RIBEYE and SHANK1, we also investigated whether TH supplementation would restore the normal CaV1.3 distribution in Pit1dw mice, but found no changes in this regard with TH treatment from P3 to P8 (data not shown). Perhaps more prolonged or even permanent TH supplementation may be necessary to establish normal CaV1.3 clustering, such as that performed by Sendin et al. (2007). Taken together, these results showed that the critical critical time window for TH action on afferent synaptic pruning in the cochlea was from P3 to P8. These findings constitute the first direct demonstration of TH rescuing a synaptic pruning defect in TH-deficient animals. We next investigated whether TH is important for the long-term maintenance of afferent synapses.
Fig. 5.
TH treatment from P3 to P8 restores normal synaptic counts in Pit1dw IHCs. (a and b) Projection of confocal sections obtained from the mid-turn of cochlear whole mounts stained with RIBEYE (green) and SHANK1 (red) markers at P14 in saline-treated WT mice (a) and Pit1dw mice (b) treated with TH from P3 to P8. Scale bar: 10 lm. (c–g) Box plots of the quantification of RIBEYE, SHANK1 and RIBEYE–SHANK1 puncta from the mid-turn of the cochlea in saline-treated WT and Pit1dw mice, and in TH-treated Pit1dw mice, for the following treatment windows: P4 to P8 (c), P5 to P8 (d), P3 to P8 (e), P3 to P4 (f), and P3 to P6 (g). significant comparisons (P < 0.05) are indicated with asterisks. The dots indicate outliers in the data. (h) Percentage of presynaptic (green) and postsynaptic (red) pruning for the different TH treatment groups with respect to WT controls. The duration of each treatment period is indicated with respect to the entire period examined from P3 to P8.
Homeostatic maintenance of afferent synapses is disrupted in young adult Pitdwmice
Reduced afferent postsynaptic puncta in hypothyroid IHCs with age
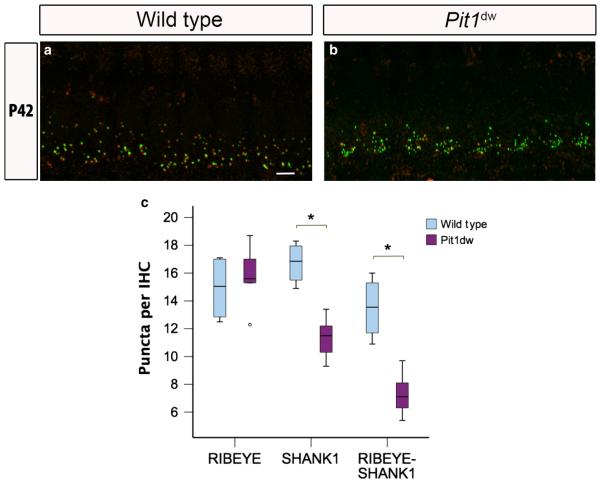
Spiral ganglion neurons are target cells of TH in the developing and adult cochlea. The observation that the TH receptor α-subunit continues to be expressed in adult spiral ganglion neurons suggests a more sustained role for TH in the ear (Lautermann & ten Cate, 1997). We next examined whether, in addition to its role in synaptic development and pruning, TH was also involved in the homeostatic maintenance of healthy synapses over a long-term period, such as during aging. We examined WT and Pit1dw IHCs by using immuno-histochemistry with RIBEYE and SHANK1 on whole mount preparations of the organ of Corti from mice at P42 (Fig. 6a–c; only midturn shown). At P42, the total numbers of RIBEYE puncta per IHC dw those in saline-treated WT mice at P14 (Fig. 5a and e). Also, as were comparable in Pit1dw and WT IHCs (P = 0.61, Student’s t-test) (Fig. 6c). However, the number of postsynaptic SHANK1 and RIBEYE–SHANK1 puncta at P42 were significantly reduced in Pit1dw IHCs as compared with WT IHCs (SHANK1, P = 0.001; synapses, P = 0.002; Student’s t-test). Taken together, these obser-vations suggested degeneration of afferent terminals at the base of Pit1dw IHCs with age.
Fig. 6.
Reduced number of afferent postsynaptic counts in young adult Pit1dw IHCs. (a and b) Projection of confocal sections obtained from the mid-turn of cochlear whole mounts stained with RIBEYE (green) and SHANK1 (red) markers in WT mice (a) and Pit1dw mice (b) at P42. Scale bar: 10 lm. (c) Box plot of the quantification of RIBEYE, SHANK1 and RIBEYE–SHANK1 puncta from the mid-turn of WT and Pit1dw cochlea at P42. significant comparisons (P < 0.05) are indicated with asterisks. The dots indicate outliers in the data.
Degeneration of afferent terminals at the base of the IHCs in Pitdw mice
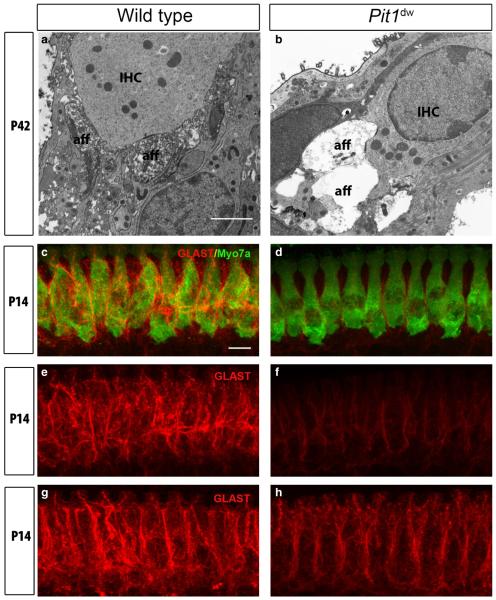
We used TEM to further examine the potential degeneration of afferent terminals at the base of Pit1dw IHCs and compared them with WT IHCs. We observed swollen afferent dendrites filled with vacuoles at P21 and P42 in Pit1dw IHCs but not in WT IHCs (Fig. 7a and b; only P42 data shown). This novel feature suggested synaptic damage in the form of excitotoxicity to the afferent dendritic region of Pit1dw IHCs, which has, so far, never been reported in any hypothyroid animal model. A similar pattern of dendritic damage was also seen in guinea pigs treated with the glutamate receptor agonist AMPA, and in mice lacking the glutamate transporter GLAST (Puel et al., 1991; Hakuba et al., 2000). Given that the glial high-affinity glutamate transporters GLT-1 and GLAST are encoded by TH-regulated genes (Mendes-de-Aguiar et al., 2008; Zamoner et al., 2008), we hypothesized that lower GLAST levels in the supporting cells surrounding the IHCs in hypothyroid animals might lead to less glutamate being cleared from the synaptic cleft. This buildup of glutamate, over a period of time, could result in excitotoxic damage to the afferent dendrites connected to IHCs, as observed in the TEM images above (Fig. 7a and b).
Fig. 7.
Swollen afferent type I terminals and reduced GLAST expression in young adult Pit1dw mice. (a and b) Representative TEM images from the mid-turn of Pit1dw and WT cochleas at P42. IHC and afferent boutons (aff) are indicated. Scale bar: 2 lm. (c and d) Projections of confocal sections obtained from the mid-turn of cochlear whole mounts co-stained with anti-GLAST (red) and anti-myosin VIIa (green) antibodies in WT and Pit1dw mice at P14. (e and f) GLAST expression (red) alone in WT and Pit1dw mice at P14 before TH treatment. (g and h) GLAST expression (red) at P14 in saline-treated WT mice and in TH-treated Pit1dw mice from P3 to P8. Scale bar: 10 lm.
To examine whether the buildup of glutamate at the synaptic cleft could cause damage to the afferent terminals in Pit1dw mice, we checked the transcript and protein levels of GLAST by quantitative RT-PCR and immunofluorescence staining. We found no significant differences in the levels of GLAST mRNA in the organ of Corti of Pit1dw vs. WT mice at P9 and P12 (data not shown). We then examined the protein expression of GLAST by immunohistochemistry at P14 on whole mount cochlear tissue. The IHCs were marked with an anti-myosin VIIa antibody in WT and Pitdw mice (Fig. 7c and d). We saw a substantial reduction in GLAST staining along the plasma membrane of the inner phalangeal cells in Pitdw cochlea as compared with WT controls (Fig. 7e and f). We next investigated whether normal levels of GLAST protein expression could be restored in Pitdw mice treated with TH. TH treatment from P3 to P8 at least partially restored GLAST expression in the inner phalangeal cells of Pitdw mice at P14 as compared with saline-treated WT mice (Fig. 7g and h). Together, these data suggested an important role for TH in the homeostatic maintenance of healthy synapses by regulating GLAST expression post-transcriptionally.
Discussion
This study represents a comprehensive analysis concerning the effect of TH deficiency on the functional maturation and maintenance of IHC afferent synapses. We observed abnormal persistence of excess afferent synaptic connectivity in the absence of TH. We found that only a subset of excess synapses in Pit1dw mice were structurally and functionally mature, but this group was sufficient to drive normal calcium currents and vesicle release by adulthood. We restored synaptic pruning in Pit1dw IHCs by TH replacement from P3 to P8, establishing this window as being critical for the effect of TH on this process. Finally, we discovered a new role for TH in the homeostatic maintenance of IHC afferent terminals as they age, via regulation of GLAST protein levels.
Critical window of TH action on IHC afferent synaptic pruning
Defective cochlear afferent fibre retraction and abnormal persistence of multi-ribbon formation at the active zone in outer hair cells and IHCs, respectively, have been reported in different hypothyroid models (Uziel et al., 1983; Sendin et al., 2007). Our present study suggests that the last stage of afferent neurite development when excess connections undergo extensive pruning and retained synapses mature functionally was impaired in the absence of TH.
Synaptic pruning takes place after the peak of synapse formation that occurs at around P4 in rodents (Sobkowicz et al., 1982; Huang et al., 2012; Wong et al., 2014). We observed significantly higher numbers of synapses at P9 and P14 in Pit1dw IHCs than in WT IHCs, suggesting defective pruning in hypothyroid IHCs. It has been reported that the activity of the enzyme deiodinase 2, which converts TH from T4 to the more active T3 form, peaks from P5 to P8 in the rodent cochlea (Campos-Barros et al., 2000). This increase in deiodinase 2 activity also coincides with high expression levels of TH transporters in the cochlea (Sharlin et al., 2011; Ng et al., 2013). These observations suggest that TH is most active in the cochlea during this period. However, injecting Pit1dw mice with TH from P3 to P8, rather than from P3 to P4, from P4 to P8, or from P5 to P8, was most effective in rescuing the synapse-pruning defect in these mice, indicating that the earlier time point is critical for TH action on this process. P3 to P6 treatment was almost as effective as P3 to P8 treatment for presynaptic pruning, but postsynaptic pruning was not completed by P6. This result indicates that TH action was required up to P8 for completion of postsynaptic pruning. It also suggests that presynaptic pruning may precede postsynaptic disassembly.
Further evidence to support this idea comes from our observation of partial pruning of only the presynaptic ribbons in Pit1dw mice at later ages during the refinement period. This result indicates that presynaptic pruning occurs to at least some extent in these mice, even when there is no pruning postsynaptically. Partial presynaptic pruning in Pit1dw IHCs could be attributable to the involvement of a cofactor that acts synergistically with TH to mediate pruning, or could perhaps occur because TH modulates the rate of existing mechanisms and its absence merely delays these processes.
Afferent synaptic activity in hypothyroid IHCs
In both the developing central and peripheral nervous systems, synaptic activity-dependent and synaptic activity-independent mechanisms have been implicated in synapse formation and pruning (Verhage, 2000; Cohen-Cory, 2002; Molnar & Isaac, 2002; Goda & Davis, 2003). Mechanisms underlying these processes in the cochlea are poorly understood; however, it is well known that the initiation of synaptic activity precedes the completion of synapse formation and pruning (Beutner & Moser, 2001; Johnson et al., 2013). Interestingly, synapse formation and pruning do not seem to depend on the synaptic activity of the IHCs, as suggested by studies in mice deficient for CaV1.3 and otoferlin (Nemzou et al., 2006; Roux et al., 2006). In addition, mice lacking a functional bassoon protein also show generally intact calcium-dependent exocytosis activity, even though ribbon formation was disrupted in these mice (Jing et al., 2013). These studies suggest that synaptic activity of IHCs may be independent of synapse formation and pruning processes and vice versa. In Pit1dw mice, our electrophysiological data showed that, despite an initial delay, the normal developmental maturation/ reduction of the maximum calcium current amplitude had taken place in the absence of TH by the age of onset of hearing. Our results are, in some ways, similar to previous reports indicating a developmental delay in synaptic functional maturation in the absence of TH (Brandt et al., 2007; Sendin et al., 2007). The difference, however, is that whereas they reported that calcium currents remained abnormally elevated near the level of the immature maximum peak current in their hypothyroid models, we found recovery to normal levels in Pit1dw mice by the age of onset of hearing. These differences in observations can perhaps be attributed to the diversity in the genetic backgrounds of the hypothyroid models studied. With respect to capacitance measurements, we found that the first component of release was unaffected in Pit1dw IHCs throughout development and up to P24. However, total release data suggested a delay in vesicle replenishment at P14, despite the normal calcium current. When correlated with morphological data, our immunohistochemistry study revealed that the excess synaptic (RIBEYE–SHANK1) puncta in hypothyroid IHCs might not represent ‘functional’ synapses, owing to the absence of co-localized CaV1.3 puncta in them. The subset of functional synapses in Pit1dw IHCs, however, appear to be sufficient to generate normal calcium currents and a normal first component of release from the age of onset of hearing and also in the young adults. The increased number of functional synapses seen at P24 in Pit1dw IHCs may reflect an attempt to compensate for the deficit in vesicle replenishment seen at P14 in Pit1dw IHCs.
On the basis of these data, we propose that TH is required for elimination of the excess synapses formed during development and for triggering mechanisms involved in the development of the initial calcium current, but not for the later stages of functional maturation in these cells.
Homeostatic maintenance of afferent synapses in young adult Pit1dw mice
In addition to the immediate effects of TH on synapse pruning in the early postnatal period, the effects of TH and/or other factors on the long-term maintenance of mature synapses are also of interest to our group (Mendus et al., 2014). One group of genes that are TH-regulated and are crucial for synapse maintenance are those encoding the glial glutamate transporters GLAST and GLT-1 (Mendes-de-Aguiar et al., 2008; Zamoner et al., 2008). In this study, we have shown, for the first time, that the expression level of GLAST protein (but not mRNA) in the organ of Corti is TH-dependent. This result suggests that TH may regulate GLAST expression at the post-transcriptional stage, such as in the trafficking of the protein to cell membranes. GLAST is the only glutamate transporter detected in the organ of Corti, and is responsible for transmitter uptake at IHC afferent synapses (Li et al., 1994; Rebillard et al., 2003; Glowatzki et al., 2006). Reduction of GLAST expression in supporting cells could explain the glutamate excitotoxicity phenotype of the afferent nerve endings in Pit1dw mice at later ages. Similar afferent terminal damage was seen in noise-exposed GLAST- deficient mice (Hakuba et al., 2000). In addition, excitotoxicity resulting from extracellular glutamate buildup was shown in the brains of hypothyroid rats, and was linked to reduced GLAST levels, supporting the idea that similar mechanisms may operate in the hypothyroid cochlea (Cattani et al., 2013). A cartoon summarizing these observations with respect to synaptic pruning and maintenance in WT and hypothyroid IHCs is given in Fig. 8.
Fig. 8.
A cartoon representing normal and hypothyroid adult IHC synapses. (a) WT IHC. (b) Hypothyroid IHC. (c) Magnification of the box in b. A hypothyroid IHC is characterized by an excess of afferent synapses caused by the abnormal retention of afferent neurite branches and terminals, calcium channels being more widely distributed around the hair cell, and higher glutamate buildup at the synaptic cleft, owing to lower GLAST levels (b and c). In comparison, a normal IHC shows fewer but well-organized afferent terminals and a more clustered pattern of CaV1.3 calcium channel expression at the basal region of the hair cell. a, afferent fibre; e, efferent fibre; r, ribbon synapse.
The next set of experiments will be directed towards defining the cell types and the network of TH-regulated genes that drive afferent synaptic pruning, maturation, and maintenance. Recent studies from our laboratory and others have provided further support for the growing evidence in favour of a major role for the supporting cells and the spiral ganglion neurons in cochlear synapse formation and maturation (Tritsch et al., 2007; Wan et al., 2013; Mendus et al., 2014; Yu & Goodrich, 2014). Developing a mouse model with cellspecific TH deficiency can be a valuable tool to help identify candidate regulatory factors expressed in the different cochlear cell types. The relevant mouse models for these candidates can then be studied to answer unresolved questions in the field, such as whether the persistence of transient synapses would affect normal hearing during development.
Acknowledgements
This work was supported by research and core grants from the National Institute in Deafness and other Communicative Disorders: R01 DC09590 (M. Mustapha), R01 DC009913 (A. Ricci), and P30 DC010363 (S. Heller, Stanford University). We thank Lars Becker for help with computer-related issues and the schematic cartoon. We also acknowledge support from Dr Sally Camper and the March of Dimes Foundation during the initial phase of this work. The authors sincerely thank Dr Srikantan Nagarajan for advice on statistics, and Dr Sally Camper for helpful comments and critical discussions throughout the duration of this study. The authors declare no competing financial interests.
Abbreviations
- IHC
inner hair cell
- P
postnatal day
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcription-polymerase chain reaction
- T3
3,5,3′-triiodothyronine sodium salt
- T4
thyroxine
- TEM
transmission electron microscopy
- TH
thyroid hormone
- WT
wild-type
References
- Bernal J. Thyroid hormones and brain development. Vitam. Horm. 2005;71:95–122. doi: 10.1016/S0083-6729(05)71004-9. [DOI] [PubMed] [Google Scholar]
- Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J. Neurosci. 2001;21:4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J. Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J. Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Kuhn S, Münkner S, Braig C, Winter H, Blin N, Engel J. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. J. Neurosci. 2007;27:3174–3186. doi: 10.1523/JNEUROSCI.3965-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc. Natl. Acad. Sci. USA. 2000;97:1287–1292. doi: 10.1073/pnas.97.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattani D, Goulart PB, Cavalli VLDLO, Winkelmann-Duarte E, Dos Santos AQ, Pierozan P, Zamoner A. Congenital hypothyroidism alters the oxidative status, enzyme activities and morphological parameters in the hippocampus of developing rats. Mol. Cell. Endocrinol. 2013;375:14–26. doi: 10.1016/j.mce.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science (New York) 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- Deol MS. Congenital deafness and hypothyroidism. Lancet. 1973;2:105–106. doi: 10.1016/s0140-6736(73)93310-2. [DOI] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory threshold in the house mouse (Mus musculus) J. Am. Audiol. Soc. 1976;1:179–184. [PubMed] [Google Scholar]
- Engel J, Michna M, Platzer J, Striessnig J. Calcium channels in mouse hair cells: function, properties and pharmacology. Adv. Otorhinolaryng. 2002;59:35–41. doi: 10.1159/000059243. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr. Opin. Neurobiol. 2003;13:452–458. doi: 10.1016/s0959-4388(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Cheng N, Hiel H, Yi E, Tanaka K, Ellis-Davies GCR, Bergles DE. The glutamate–aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J. Neurosci. 2006;26:7659–7664. doi: 10.1523/JNEUROSCI.1545-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Hakuba N, Koga K, Gyo K, Usami SI, Tanaka K. Exacerbation of noise-induced hearing loss in mice lacking the glutamate transporter GLAST. J. Neurosci. 2000;20:8750–8753. doi: 10.1523/JNEUROSCI.20-23-08750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–2933. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- Huang L-C, Barclay M, Lee K, Peter S, Housley GD, Thorne PR, Montgomery JM. Synaptic profiles during neurite extension, refinement and retraction in the developing cochlea. Neural Dev. 2012;7:38. doi: 10.1186/1749-8104-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z, Rutherford MA, Takago H, Frank T, Fejtova A, Khimich D, Strenzke N. Disruption of the presynaptic cytomatrix protein bassoon degrades ribbon anchorage, multiquantal release, and sound encoding at the hair cell afferent synapse. J. Neurosci. 2013;33:4456–4467. doi: 10.1523/JNEUROSCI.3491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J. Physiol. 2005;563(Pt 1):177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kuhn S, Franz C, Ingham N, Furness DN, Knipper M, Marcotti W. Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity. Proc. Natl. Acad. Sci. USA. 2013;110:8720–8725. doi: 10.1073/pnas.1219578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolyi IJ, Dootz GA, Halsey K, Beyer L, Probst FJ, Johnson KR, Camper SA. Dietary thyroid hormone replacement ameliorates hearing deficits in hypothyroid mice. Mamm. Genome. 2007;18:596–608. doi: 10.1007/s00335-007-9038-0. [DOI] [PubMed] [Google Scholar]
- Knipper M, Gestwa L, Ten Cate WJ, Lautermann J, Brugger H, Maier H, Zenner HP. Distinct thyroid hormone-dependent expression of TrKB and p75NGFR in nonneuronal cells during the critical TH-dependent period of the cochlea. J. Neurobiol. 1999;38:338–356. [PubMed] [Google Scholar]
- Lautermann J, ten Cate WJ. Postnatal expression of the alphathyroid hormone receptor in the rat cochlea. Hear. Res. 1997;107:23–28. doi: 10.1016/s0378-5955(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Li H-S, Niedzielski AS, Beisel KW, Hiel H, Wenthold RJ, Morley BJ. Identification of a glutamate/aspartate transporter in the rat cochlea. Hear. Res. 1994;78:235–242. doi: 10.1016/0378-5955(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J. Physiol. 2003;548(Pt 2):383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-de-Aguiar CB, Alchini R, Decker H, Alvarez-Silva M, Tasca CI, Trentin AG. Thyroid hormone increases astrocytic glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J. Neurosci. Res. 2008;86:3117–3125. doi: 10.1002/jnr.21755. [DOI] [PubMed] [Google Scholar]
- Mendus D, Sundaresan S, Grillet N, Wangsawihardja F, Leu R, Müller U, Mustapha M. Thrombospondins 1 and 2 are important for afferent synapse formation and function in the inner ear. Eur. J. Neurosci. 2014;39:1256–1267. doi: 10.1111/ejn.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna M, Knirsch M, Hoda J-C, Muenkner S, Langer P, Platzer J, Engel J. Cav1.3 (alpha1D) Ca2+ currents in neonatal outer hair cells of mice. J. Physiol. 2003;553(Pt 3):747–758. doi: 10.1113/jphysiol.2003.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar E, Isaac JTR. Developmental and activity dependent regulation of ionotropic glutamate receptors at synapses. Scientific World J. 2002;2:27–47. doi: 10.1100/tsw.2002.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Brandt A, Lysakowski A. Hair cell ribbon synapses. Cell Tissue Res. 2006;326:347–359. doi: 10.1007/s00441-006-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha M, Fang Q, Gong TW, Dolan DF, Raphael Y, Camper SA, Duncan RK. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J. Neurosci. 2009;29:1212–1223. doi: 10.1523/JNEUROSCI.4957-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemzou NR, Bulankina AV, Khimich D, Giese A, Moser T. Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels. Neuroscience. 2006;141:1849–1860. doi: 10.1016/j.neuroscience.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Ng L, Kelley MW, Forrest D. Making sense with thyroid hormone – the role of T(3) in auditory development. Nat. Rev. Endocrinol. 2013;9:296–307. doi: 10.1038/nrendo.2013.58. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science (New York) 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Peng AW, Effertz T, Ricci AJ. Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron. 2013;80:960–972. doi: 10.1016/j.neuron.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel J-L, Pujol R, Ladrech S, Eybalin M. Alpha-amino-3-hydroxy-5-methyl-4-isoxazole proionic acid electrophysiological and neurotoxic effects in the guinea-pig cochlea. Neuroscience. 1991;45:63–72. doi: 10.1016/0306-4522(91)90103-u. [DOI] [PubMed] [Google Scholar]
- Rebillard G, Ruel J, Nouvian R, Saleh H, Pujol R, Dehnes Y, Devau G. Glutamate transporters in the guinea-pig cochlea: partial mRNA sequences, cellular expression and functional implications. Eur. J. Neurosci. 2003;17:83–92. doi: 10.1046/j.1460-9568.2003.02429.x. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler M-C, Bahloul A, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Rueda J, Prieto JJ, Cantos R, Sala ML, Merchan JA. Hypothyroidism prevents developmental neuronal loss during auditory organ development. Neurosci. Res. 2003;45:401–408. doi: 10.1016/s0168-0102(03)00009-9. [DOI] [PubMed] [Google Scholar]
- Rusch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Forrest D. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J. Neurosci. 2001;21:9792–9800. doi: 10.1523/JNEUROSCI.21-24-09792.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Determination of cell capacitance using the exact empirical solution of partial differential Y/partial differential Cm and its phase angle. Biophys J. 2004;87:714–727. doi: 10.1529/biophysj.103.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Kakehata S, Takahashi S. Effects of membrane potential on the voltage dependence of motility-related charge in outer hair cells of the guinea-pig. J. Physiol. 1998;510(Pt 1):225–235. doi: 10.1111/j.1469-7793.1998.225bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee ME, Kong J, Santos-sacchi J, Ricci AJ. Tracking vesicle fusion from hair cell ribbon synapses using a high frequency, dual sine wave stimulus paradigm. Commun. Integr. Biol. 2011;4:785–787. doi: 10.4161/cib.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin G, Bulankina AV, Riedel D, Moser T. Maturation of ribbon synapses in hair cells is driven by thyroid hormone. J. Neurosci. 2007;27:3163–3173. doi: 10.1523/JNEUROSCI.3974-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharlin DS, Visser TJ, Forrest D. Developmental and cell-specific expression of thyroid hormone transporters in the mouse cochlea. Endocrinology. 2011;152:5053–5064. doi: 10.1210/en.2011-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J. Neurosci. 1982;2:942–957. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, Wang F, Li BM. Adult-onset hypothyroidism impairs paired-pulse facilitation and long-term potentiation of the rat dorsal hippocampo-medial prefrontal cortex pathway in vivo. Brain Res. 2006;1096:53–60. doi: 10.1016/j.brainres.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Uziel A. Periods of sensitivity to thyroid hormone during the development of the organ of Corti. Acta Otolaryngol. Suppl. 1986;429:23–27. doi: 10.3109/00016488609122726. [DOI] [PubMed] [Google Scholar]
- Uziel A, Pujol R, Legrand C, Legrand J. Cochlear synaptogenesis in the hypothyroid rat. Brain Res. 1983;283:295–301. doi: 10.1016/0165-3806(83)90186-4. [DOI] [PubMed] [Google Scholar]
- Verhage M. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Wan G, Corfas G, Stone JS. Inner ear supporting cells: rethinking the silent majority. Semin. Cell Dev. Biol. 2013;24:448–459. doi: 10.1016/j.semcdb.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RE, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S. Thyroid hormone action on liver, heart, and energy expenditure in thyroid hormone receptor beta-deficient mice. Endocrinology. 1998;139:4945–4952. doi: 10.1210/endo.139.12.6412. [DOI] [PubMed] [Google Scholar]
- Wong AB, Rutherford MA, Gabrielaitis M, Pangrsic T, Göttfert F, Frank T, Moser T. Developmental refinement of hair cell synapses tightens the coupling of Ca2+ influx to exocytosis. EMBO J. 2014;33:247–264. doi: 10.1002/embj.201387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W-M, Goodrich LV. Morphological and physiological development of auditory synapses. Hear. Res. 2014;311:3–16. doi: 10.1016/j.heares.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoner A, Heimfarth L, Pessoa-Pureur R. Congenital hypothyroidism is associated with intermediate filament misregulation, glutamate transporters down-regulation and MAPK activation in developing rat brain. Neurotoxicology. 2008;29:1092–1099. doi: 10.1016/j.neuro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Zampini V, Johnson SL, Franz C, Lawrence ND, Münkner S, Engel J, Marcotti W. Elementary properties of CaV1.3 Ca(2+) channels expressed in mouse cochlear inner hair cells. J. Physiol. 2010;588(Pt 1):187–199. doi: 10.1113/jphysiol.2009.181917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Gross CT. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]