Abstract
Importance
The translation of evidence of comparative effectiveness of antidepressants into practice is suboptimal. This deficit directly affects outcomes and quality of care for patients with depression. To overcome this problem, we developed the Depression Medication Choice (DMC) encounter decision aid, designed to help patients and clinicians consider the available antidepressants and the extent to which they improved depression and other patient important issues.
Objective
Estimate the effect of DMC on quality of the decision-making process and depression outcomes.
Design
We conducted a cluster randomized trial by which primary care practices were randomly allocated to treatment of depression with or without use of the DMC decision aid.
Setting
Ten rural, suburban, and urban primary care practices across Minnesota and Wisconsin, U.S.
Participants
Adults with moderate to severe depression considering treatment with an antidepressant and their primary care clinicians.
Intervention
DMC, a series of cards, each highlighting the effect of the available options on an issue of importance to patients for use during face-to-face consultations.
Main outcomes and measures
Decision-making quality as judged by patient knowledge and involvement in decision making, patient and clinician decisional comfort (Decisional Conflict Scale) and satisfaction, and encounter duration, medication adherence and depression symptoms (PHQ-9).
Results
We enrolled 117 clinicians and 301 patients [67% women; mean age=44 (SD=15); mean PHQ-9=15 (SD=4)] into the trial. Compared to usual care (UC), use of DMC significantly improved patients’ decisional comfort (DMC=80% vs. UC=75%, p=.02), knowledge (DMC=65% vs.UC=56%, p=.03), satisfaction (RR=1.25, p=.81 to RR= 2.4, p=.002), and involvement (DMC=47% vs. UC=33%, p=<.001). It also improved clinicians’ decisional comfort (DMC=80% vs. UC=68%, p<.0001) and satisfaction (RR=1.64, p=.02). There were no differences in encounter duration, medication adherence, or improvement of depression control between arms.
Conclusion and Relevance
The DMC decision aid helped primary care clinicians and patients with moderate to severe depression select antidepressants together, improving the decision-making process without extending the visit. On the other hand, DMC had no discernible effect on medication adherence and depression outcomes. By translating comparative effectiveness into patient-centered care, use of DMC improved the quality of primary care for patients with depression.
BACKGROUND
Depression is a common, debilitating, and costly chronic mental illness affecting 17% of Americans.1–4 Depression burdens patients, their families, and the healthcare system.1–4 Fortunately, treatment can effectively mitigate this burden.5,6 Of the available treatments, pharmacotherapy has become the main modality, particularly for patients with moderate to severe depression.7,8 Unfortunately, the efficacy of antidepressants is reduced by low patient adherence rates (13–60%) and premature discontinuation (33–42%) that contributes to avoidable disability, increased risk of relapse, and higher healthcare utilization.9–11 Patients stop taking antidepressants because of unrealistic expectations, lack of treatment efficacy, or unacceptable side effects.12,13 It would seem critical, therefore, to improve the process by which patients and clinicians select and implement antidepressants.
Choosing the “right” antidepressant is difficult because of limitations in the evidence of their comparative effectiveness.14,15 More certain are their effects on other important outcomes (i.e., weight gain) that matter to patients. Clinicians however struggle to present this information to patients in meaningful ways and have a difficult time parting from their preferred antidepressants.16,17 Clinicians and patients have more to gain by identifying which agent is better given the patient’s circumstances and preferences, rather than selecting what might be deemed the “most effective” choice. This shared decision making (SDM) approach forms informed preferences that lead to patient-centered choices. To arrive at informed preferences, patients and clinicians must access and make sense of the best research evidence, which unfortunately has not effectively reached the point of care.18,19 This suboptimal translation of evidence into practice directly affects outcomes and quality of care for patients with depression.18–20
Decision aids are evidence-based interventions designed to engage patients and clinicians in a SDM process and translate research evidence into patient-centered care.21–24 A body of evidence (115 randomized trials) demonstrates that decision aids increase patients’ knowledge, and engagement in and comfort with the decision-making process.24 Most of this evidence reflects the use of educational tools distributed to patients before making a decision, with the goal to empower patients to participate in decision-making through information.24 Yet, only a minority of these trials sought to determine if SDM actually took place. An evolving body of evidence, on the other hand, is finding that decision aids can effectively promote SDM when used during the clinical encounter.25
In collaboration with patients, clinicians, and stakeholders, we developed an encounter decision aid, Depression Medication Choice, to support pharmacotherapy choices when this approach is being considered in the primary care of patients with moderate to severe depression.26 We hypothesized that its use would improve patient engagement, the quality of decision-making as perceived by patients and clinicians, and depression outcomes.
METHODS
Study Design
We conducted a cluster randomized trial in which we randomly allocated primary care practices to treat depression with (intervention) or without (control) Depression Medication Choice. Patients and clinicians were surveyed regarding the quality and outcome of the decision-making process, and patient adherence and clinical outcomes were followed for six months after the encounter. Mayo Clinic Institutional Review Board, IRB of record for participating practices, and Hennepin County Medical Center Human Subjects Research Committee approved the study procedures. The trial is registered at Clinicaltrials.gov (NCT01502891) and details of the study procedures are found elsewhere.26
Study Setting and Participants
This study took place between December 2011 and November 2013 in 10 rural, suburban, and urban primary care practices across Minnesota and Wisconsin, USA. Primary care practices were eligible for the study if they had at least two clinicians interested in participating, a clinical champion (i.e. outstanding clinician who has earned the respect of their peers and is willing to build support for and actively promote the current project), eligible patients, and the willingness to have an on-site study coordinator for the duration of the trial.26 Clinicians were invited to participate if they cared for patients with depression. Eligible adult patients (>18 years) had moderate to severe depression and a Patient Health Questionnaire (PHQ-9)27 score ≥10, no bipolar disorder, an appointment with a member of their primary care team, and no major barriers to providing informed consent. Eligibility criteria (i.e. severity of depression, PHQ-9) were set so that identified patients, whether on a medication or not, were most likely to engage in antidepressants conversations (i.e. start, increase, or switch).
Stakeholder Engagement
We developed Depression Medication Choice in close collaboration with two patient advisory groups and with an External Advisory Council comprised of 24 stakeholders (i.e. patients, clinicians, policy makers) from 12 different organizations. We further engaged these groups throughout the set-up and conduct of the trial, seeking insights primarily on eligibility criteria, choice of outcomes, and recruitment strategies (i.e. identifying respectful ways to recruit patients with depression).
Recruitment
We invited practices that were part of the External Advisory Council to participate in the trial, and then extended our reach to Mayo Clinic and Mayo Clinic Health Systems practices. Clinicians were approached for participation during an initial on-site meeting or, afterward, by the study coordinator.26 On-site study coordinators identified potentially eligible patients from the appointment schedules of clinicians and approached them at the time of appointment. Clinicians and patients both provided written informed consent and neither received financial incentives to participate in the trial.
Allocation Procedure
We initially had set to stratify practices according to their number of clinicians as an indicator of their potential for enrolment but later replaced it with their history of accrual (low vs. high) in past studies, an indicator we deemed more effective in addressing recruitment rate per arm. The lead study statistician therefore stratified practices by their history of accrual and the presence of the DIAMOND program (a practice redesign initiative to improve depression care present in numerous MN practices at the time of the study),28 and centrally randomized practices within these strata to either care with or without Depression Medication Choice. Study team members, practices, and clinicians were aware of the assigned arms. Patients were kept unaware of the study hypothesis and nature of the intervention.
Intervention and Control
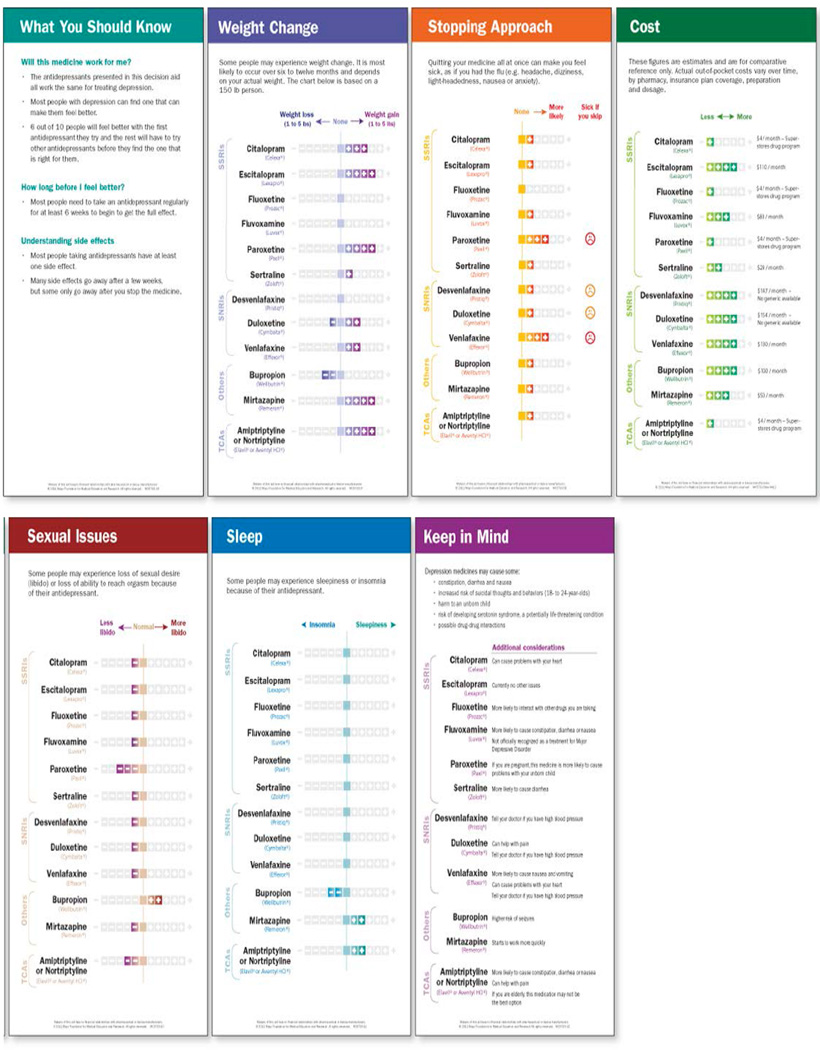
We described the development of Depression Medication Choice elsewhere.26 The decision aid, laminated 4”x10” cards, presents general considerations about antidepressant efficacy and then side effects in terms that matter to patients: weight change, sleep, libido, discontinuation, and cost (Figure 1). We briefly (<10 min) demonstrated to clinicians how to use the decision aid prior to enrollment of their first patient. A videoclip and storyboard demonstrating the basic use of the decision aid remained available as well as a leaflet for patients to take home (eFigures 1–2).
Figure 1.
This decision aid presents itself in form of 4”x10” laminated cards
The Depression Medication Choice Encounter Decision Aid
Clinicians in the intervention group were to use the decision aid during the consultation with their patients, while clinicians in the control arm did not have access to the decision aid (usual care).
Outcomes and Data Collection
The evaluation of the study was guided by the RE-AIM framework (Reach, Effectiveness, Adoption, Implementation, Maintenance). This paper focuses on the effectiveness of Depression Medication Choice to improve decision-making and clinical outcomes.
Patient Level Outcomes
Decision-Making Quality Outcomes
Decisional conflict, defined as personal uncertainty about which course of action to take when choice among competing options involves risk or challenge to personal life values, was our primary outcome. Patients completed the Decisional Conflict Scale (DCS) immediately after the clinical encounter.29 We report results as level of comfort with the decision (0=conflict, 100=comfort). Other measures of decision-making quality were obtained from patients’ post encounter: knowledge and acceptability of information sharing (i.e. satisfaction).30–32
Clinical Outcomes
Patients self-completed the PHQ-9, a measure of depression symptoms, at entry in the study, 3 months, and 6 months.27 We extracted PHQ-9 recorded in the medical records of patients during the trial period, to be used as proxy for unreturned ones, if completed with +/− 2 weeks of the 3 and 6 month period.
Adherence
Patients reported on medication usage at time of appointment and after the clinical encounter. We collected pharmacy records and reviewed medical records for the trial period. For patients with pharmacy data, we calculated primary medication adherence as proportion of patients who filled their prescription within 30 days, and secondary adherence as the proportion of patients with a percentage of days covered (PDC) >80%.33 PDC was defined as the number of days a patient had a supply of each medication divided by the number of days of eligibility for that medication, for each antidepressant prescribed.33
Clinician Level and Encounter Level Outcomes
Decision-Making Quality Outcomes
Clinicians completed the DCS (clinician version) and an acceptability of information sharing (i.e., satisfaction) scale following each encounter with a participating patient.31,32,34 We video-recorded encounters in which both patient and clinician gave us consent to record. From these recordings, we assessed the extent to which clinicians sought to engage patients in the decision-making process using the OPTION scale.35 We also assessed the extent to which they used the decision aid as intended using a fidelity checklist.26,36 Study coordinators captured the number of minutes patients remained in the consultation room as a proxy for the impact of using the decision aid on encounter duration and for disruption of clinic flow.
Sample Size
We used a formula for a clustered-adjusted t-test to estimate that the recruitment of 300 patients (30 per practice) would give the study a power of 90% to detect a difference of 9.8 point or greater on the DCS with a 2 sided alpha level of 0.05.37 Assumptions were based on results from trials of similar design and outcomes: DCS variances are as reported in the Statin Choice Trial (16.9, 14.1), there is a modest correlation of outcomes across practices [intracluster correlation coefficient (ICC) of 0.05], and a 10% average attrition rate.31,32,38 Assuming a similar ICC and attrition rate for other outcomes, this sample size would have 99% power to detect a 1.0 standard deviation difference in any continuous measure, and 80% power to detect a 30% difference in 6-month adherence rates assuming a control adherence rate of 50%.
Statistical Analysis
All outcomes were analyzed according to the intention-to-treat principle.37 Because clinicians and patients were randomized in clusters (practices), we used cluster-adjusted t-tests, χ2 tests, and hierarchical generalized linear models (HGLMs) to compare variables between groups.39 In particular, HGLMs allowed us to account for the correlation of patient outcomes within clinicians and practices explicitly, by modelling the intercept as a 3-level effect, with random effects at clinician and practice level.39
We summarized patient and clinician characteristics by arm, testing for differences using cluster adjusted tests or HGLMs with random effects for practice for patient characteristics and χ2-tests for clinician characteristics. We summarized outcomes by arm, and then, to assess the effect of the intervention on outcomes, we estimated a series of HGLMs with logit or linear response, with random effects for clinician and practice, and including randomization group as a binary independent variable. We then tested whether there was an intervention effect by testing whether the coefficient of the group indicator was significantly different from null and reported the P-value for this test for all outcomes. Patient prescription, filled status, and adherence outcomes were compared only among those with pharmacy data. Analysis and data management were conducted using SAS 9.2, Stata 13.1, and REDCap Management system.
RESULTS
Participants Flow and Characteristics
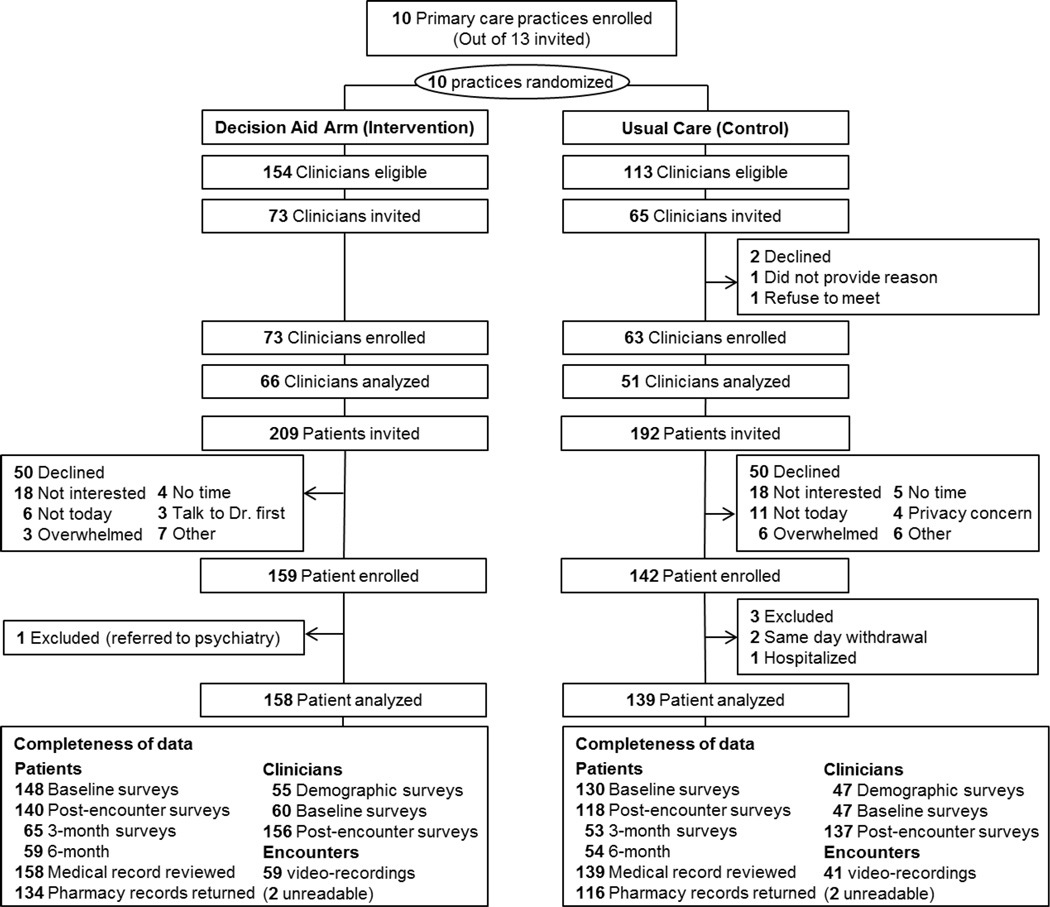
Figure 2 describes the flow of participants and completeness of data. A total of 117 clinicians [median(range): 7(4–30) per practice] and 297 patients [median(range): 34(15–40) per practice, 2(1–14) per clinician], from 10 practices (1 rural, 1 suburban, 8 urban) were included in the analysis. There was no difference in the attrition of participants or completeness of the data across arms (Figure 2). Characteristics of participants are summarized in Table 1. Although gender and ethnicity differed moderately, there were no significant differences in participant characteristics across arms.
Figure 2.
Flow of Participants
Table 1.
Sociodemographic Characteristics of Study Participants
| Characteristics | Usual Care | Decision Aid |
|---|---|---|
| Patients | N=139 | N=158 |
| Female, n (%) | 86 (61.9) | 114 (72.2) |
| Age, mean (SD), y | 43.9 (15.1) | 43.2 (15.6) |
| > 40 y, n (%) | 80 (57.6) | 87 (55.1) |
| Ethnicity, Caucasian, n (%) | 92 (66.2) | 118 (74.7) |
| Missing | 22 (15.8) | 18 (11.4) |
| Marital status, n (%) | ||
| Married/ Living with someone | 50 (36.0) | 61 (38.6) |
| Separated/ divorced/ widowed | 24 (20.4) | 36 (22.8) |
| Never married | 40 (28.8) | 38 (24.1) |
| Missing | 25 (18.0) | 23 (14.6) |
| Education, n (%) | ||
| High school degree or less | 36 (25.9) | 44 (27.9) |
| College (with or without degree) | 68 (58.3) | 78 (49.3) |
| Graduate or professional | 6 (4.3) | 10 (6.3) |
| Other | 4 (2.9) | 3 (1.9) |
| Missing | 25 (18.0) | 23 (14.6) |
| Income, n (%) | ||
| Less than $30,000 | 59 (32.5) | 51 (32.3) |
| $30,000 to $59,999 | 17 (12.2) | 26 (16.5) |
| $60,000 to $99,999 | 22 (15.8) | 39 (24.7) |
| $100,000 or more | 11 (7.9) | 15 (9.5) |
| Missing/ did not want to disclose | 25 (18.0) | 26 (16.5) |
| Preference in decision-making style, n (%) | ||
| Make decision alone | 3 ( 2.2) | 4 (2.5) |
| Make decision after considering clinician | 20 (14.4) | 29 (18.4) |
| Share the decision | 68 (48.9) | 76 (48.1) |
| Clinician makes decision after considering me | 21 (15.1) | 23 (14.6) |
| Leave decision to clinician | 4 (2.9) | 5 (3.2) |
| Missing | 23 (16.5) | 21 (13.3) |
| Literacy and numeracy, n (%) | ||
| Less than adequate literacy | 48 (34.5) | 38 (24.1) |
| Missing | 9 (6.5) | 11 (7.0) |
| Less than adequate numeracy | 66 (47.5) | 67 (42.4) |
| Missing | 14 (10.1) | 12 (7.6) |
| PHQ-9, mean (SD) | 15 (4) | 15 (4) |
| Clinicians | N=51 | N=66 |
| Female, n (%) | 30 (58.8) | 37 (56.1) |
| Age, mean (SD), y | 41 (12) | 40 (12) |
| Resident, n (%) | 19 (37.3) | 22 (33.3) |
| Years in practice, >10y, n (%) | 15 (29.4) | 16 (24.2) |
| Missing | 4 (7.8) | 11 (16.7) |
| Direct patient care, >50% of time, n (%) | 26 (60.0) | 47 (71.2) |
| Missing | 22 (43.1) | 15 (22.7) |
| Preference in decision-making style | ||
| Make decision alone | 1 (2.0) | 1 (1.5) |
| Make decision after considering patient | 0 (0.0) | 3 (4.5) |
| Share the decisions | 42 (84.0) | 48 (71.6) |
| Patient makes decision after considering me | 4 (8.0) | 8 (11.9) |
| Leave decision to patient | 0 (0.0) | 0 (0.0) |
| Missing | 3 (6.0) | 7 (10.4) |
Patients Decision Making Quality and Clinical Outcomes
After the encounters with their clinicians, patients in the decision aid arm reported significantly higher comfort with the decision [mean difference(95%CI): 5.3 out of 100(1.1, 9.5), p=0.01], and were more knowledgeable [OR(95%CI): 9.5(0.8, 18.2), p=.03] and satisfied [RR: 1.25(p=.81) to 2.40(p=.002)] compared to patients in the control arm (Table 2). There was no observed difference across arms in control of depression symptoms (mean PHQ-9), remission rate (PHQ-9 <5) or responsiveness (>50% PHQ-9 improvement) at 3 and 6 months, or in medication use or adherence (Table 2).
Table 2.
Patient Decision-Making Quality & Clinical Outcomes
| Usual Care (N=139) |
Decision Aid (N=158) |
Mean Diff (95%CI)a |
P-Value | |
|---|---|---|---|---|
| Decision-Making Quality Outcomes | ||||
| Continuous outcomes, mean (95%CI)a | ||||
| Decisional Conflict(0=conflict, 100=comfort)b |
||||
| Informed subscale | 72.1 (68.5, 75.7) | 79.6 (76.4, 82.7) | 7.8 (1.9, 13.6) | 0.009 |
| Clarity subscale | 73.3 (69.6, 76.9) | 81.3 (78.2, 84.4) | 8.4 (3.3, 13.5) | 0.001 |
| Support subscale | 79.3 (75.6, 83.0) | 83.4 (80.3, 86.4) | 4.5 (−1.4, 10.4) | 0.13 |
| Uncertainty subscale | 71.7 (67.5, 75.9) | 74.6 (71.0, 78.1) | 2.9 (−2.5, 8.3) | 0.30 |
| Effectiveness subscale | 75.8 (72.3, 79.3) | 79.1 (76.3, 81.9) | 3.3 (−1.1, 7.7) | 0.14 |
| Overall | 74.5 (71.4, 77.6) | 79.7 (77.0, 82.5) | 5.3 (1.1, 9.5) | 0.01 |
| Missing,c n (%) | 25 (22.7) | 20 (14.9) | ||
| Knowledge(0=no correct, 100= all correct) |
ORd (95%CI)a | |||
| Tailored to information in the decision aid | 46.6 (42.6, 50.5) | 58.1 (53.6, 62.6) | 13.2 (6.4, 19.9) | 0.0001 |
| Generic (i.e. depression in general) | 72.4 (67.3, 77.5) | 72.5 (68.0, 77.0) | 2.8 (−9.3, 14.8) | 0.65 |
| Overall (i.e. both tailored and generic) | 56.3 (52.9, 59.6) | 63.5 (59.9, 67.1) | 9.5 (0.8, 18.2) | 0.03 |
| Missing, n (%) | 23 (20.9) | 21 (15.7) | ||
| Categorical Outcomes, N (%) | ORd(95%CI)a | |||
| Satisfactione | ||||
| Right amount of Information given | 102 (91.9) | 124 (92.5) | 1.25 (0.21, 7.52) | 0.81 |
| Information given was extremely clear | 64 (58.7) | 92 (68.7) | 1.17 | 0.09 |
| Information given was extremely helpful | 57 (52.8) | 92 (69.2) | 1.77 (0.92, 3.38) | 0.01 |
| Strongly desire to receive Information this way for other treatment decisions |
55 (50.5) | 90 (68.2) | 2.01 (1.18, 3.40) | 0.005 |
| Strongly recommend the way information was shared to others |
65 (59.1) | 104 (77.6) | 2.40 (1.38, 4.19) | 0.002 |
| Missingc | 30 (27.5) | 26 (19.7) | ||
| Clinical Outcomes | ||||
| Continuous outcomes, mean (95%CI)a | Mean Diff (95%CI) | |||
| Depression symptoms | ||||
| PHQ-9, 3 monthf | 9.0 (7.7, 10.2) | 9.2 (8.0, 10.3) | 0.4 (−2.5, 3.4) | 0.78 |
| Missing | 38 (34.5) | 44 (32.8) | ||
| PHQ-9, 6 monthg | 9.3 (8.2, 10.5) | 8.9 (7.8, 10.0) | − 0.2 (−2.9, 2.6) | 0.91 |
| Missing | 38 (34.5) | 49 (36.6) | ||
| Categorical outcomes, n (%) | ||||
| Depression symptoms | ||||
| Remission, 3 month | 26 (18.7) | 31 (19.6) | - | 0.85 |
| Response, 3 month | 43 (30.9) | 53 (33.5) | - | 0.77 |
| Missing | 38 (34.5) | 44(32.8) | ||
| Remission, 6 month | 20 (14.4) | 34 (21.5) | - | 0.18 |
| Response, 6 month | 38 (27.3) | 55 (34.8) | - | 0.15 |
| Missing | 38 (34.5) | 49 (36.6) | ||
| Adherence to medication | OR (95%CI) | |||
| On medication at time of encounter | 90 (64.7) | 93 (58.9) | - | 0.93 |
| On medication after the encounter | 110 (79.1) | 142 (89.9) | - | 0.15 |
| Pharmacy record available | 93 (66.9) | 113 (71.5) | ||
| Primary adherence (filled prescription)h | 82 (93.2) | 94 (86.2) | - | 0.19 |
| Missing | 5 (5.7) | 4(3.7) | ||
| Secondary adherencei | ||||
| %PDC >80% (of filled prescription)j | 85 (97.7) | 96 (98.0) | - | 0.25 |
| %PDC >80% (of all patients) | 91 (97.8) | 107 (94.7) | 0.67 | |
95% confidence intervals;
Reported variance σ2(site)=5.7; σ2(clinician)=0.00; σ2(patient)=254.1;
Reported as the highest proportion of missing across items;
Odd Ratios;
Relative Risk;
7 point-likert scale, reporting proportion of agree/strongly agree;
Reported variance σ2(site)=0.0; σ2(clinician)=3.5; σ2(patient)=35.2;
Reported variance σ2(site)=0.0; σ2(site)=3.0 ; σ2(patient)=31.7;
Calculated out of available pharmacy records;
PDC: Proportion of days covered
Clinicians Decision Making Quality and Encounter Outcomes
Table 3 shows that clinicians were more comfortable with the decisions made [mean difference(95%CI): 11.4 out of 100(17.1, 5.7), p<0.0001] and more satisfied with the process when they used the decision aid [RR: 1.64 (p=.02)]. In available video-recorded encounters (n=96), clinicians assigned to the use of the decision aid involved patients significantly more in the decision-making process [mean difference(95%CI): 15.8 out of 100(6.5, 25.0), p=0.001] (Table 3). Clinicians in the intervention arm reported actual use of the decision aid in 81% of the encounters, and of those with video-recordings (n=57) reached, on average, 54% of the targeted fidelity items (i.e. used the decision aid as intended). There was no difference in the duration of clinical encounters [mean(SD), 48(27) vs. 44(22) minutes, p=0.47, for control and intervention arm, respectively].
Table 3.
Clinicians Decision-Making Quality Outcomes
| Usual Care (N=139)a |
Decision Aid (N=158)a |
Mean Difference (95%CI)b |
P-Value | |
|---|---|---|---|---|
| Continuous outcomes, mean (95%CI)b | ||||
| Decisional Conflict(0=conflict, 100=comfort) | ||||
| Informed subscale | 65.3 (61.5, 69.1) | 79.1 (76.8, 81.5) | 14.4 (7.1, 21.6) | <0.0001 |
| Clarity subscale | 66.8 (62.2,69.4) | 85.3 (83.0, 87.6) | 19.9 (11.9, 27.9) | <0.0001 |
| Support subscale | 72.1 (69.1, 75.1) | 79.2 (76.7, 81.7) | 6.6 (1.8, 11.4)) | 0.007 |
| Uncertainty subscale | 64.4 (60.6, 68.3) | 74.1 (71.2, 77.1) | 9.2 (3.4, 15.0) | 0.002 |
| Effectiveness subscale | 71.8 (68.9, 74.8) | 81.2 (78.9, 83.5) | 9.1 (3.9, 14.2) | 0.001 |
| Overall | 68.3 (65.4, 71.2) | 79.7 (77.6, 81.8) | 11.4 (5.7, 17.1) | <0.0001 |
| Missing,c n (%) | 18 (13.1) | 17 (10.9) | ||
| Involvement of patients in the decision-making processd | 32.5 (28.5, 36.6) | 46.6 (42.3, 50.8) | 15.8 (6.5, 25.9) | 0.001 |
| Missing, n (%) | 100 (71.9) | 101 (63.9) | ||
| Categorical Outcomes, n (%) | RR (95%CI)e | |||
| Satisfaction,f satisfied extremely satisfied | 74 (54.0) | 119 (76.3) | 1.64 (1.25, 2.16) | 0.02 |
| Missing | 22 (16.1) | 19 (12.2) | ||
N=number of encounters (i.e. multiple entries per clinician);
95% confidence intervals;
Reported as the highest proportion of missing across items;
OPTION scores (12 items, 0–100 scale, 0= no involvement), inter-rater agreement calculated as Shrout & Fleiss’ ICC=0.96;
Relative Risk;
1 item, 5-point likert scale, reporting proportion of satisfied /extremely satisfied.
DISCUSSION
Main Findings
In this randomized trial, the use of Depression Medication Choice by primary care clinicians and patients with moderate to severe depression during clinical encounters was feasible, and effectively improved patient knowledge and engagement in the decision-making process, as well as patient and clinician satisfaction with that process. Use of the decision aid, on the other hand, had no discernible effect on encounter duration, depression control, and medication use and adherence.
Limitations
Our study is at risk of bias. Lack of blinding of participants may have affected questionnaire responses, and lack of blinding of analysts, particularly those reviewing videos, may have biased video-based outcomes. There was substantial loss to follow up (∼20%) for our primary endpoint, mainly due to logistical issues at the beginning of the study, where study coordinators were still adapting to the recruitment and follow up process. While these issues may increase the risk of bias in favor of the intervention, other limitations may bias it toward no difference. Because most clinicians used the decision aid with only two patients, it is possible that our trial underestimates the efficacy of the decision aid when used repeatedly and expertly.36 We did not access the content of patient-clinician interactions outside the index encounter yet, patients had on average 3 or more appointments (range 1–10) during the trial period (data not shown). We did not capture use of or adherence to co-interventions (i.e. psychotherapy).
This trial also yielded imprecise clinical outcome estimates, which limited our ability to detect meaningful differences in these secondary endpoints across arms. This was due to greater than anticipated ICC and loss to follow-up (30%) affecting both PHQ-9 measures and medication adherence data, and higher adherence rates in the trial (>80%) than the initial estimated rate (50%). Hence, estimates of the effect of the decision aid on clinical outcomes and adherence are favorable to the decision aid arm but too imprecise to draw definitive conclusions about their impact.
Comparison with other studies
This trial shares the strengths of our other practical real-world decision aid trials.31,32,38,40 We used a rigorous trial design with optimal allocation concealment, and high recruitment rates for clinicians and patients. It was conducted in rural, suburban, and urban, academic and non-academic, small and large practices caring for patients of various ethnic and socio-demographic backgrounds.
This trial is the first to assess the effectiveness of an encounter decision aid for antidepressants and one of a few assessing the impact of SDM in mental health.41 The magnitude of our findings, including impact on clinical outcomes, is consistent with the results of other trials of encounter decision aids in other contexts.31,32,40 Moreover, this trial is one of a few that can directly link the use of decision aids to improvements in observed decision-making quality.25 We also assessed the fidelity with which clinicians and patients used the decision aid as intended during clinical encounters, a rare feature in the SDM literature.24,42 This is also the first study to assess clinicians’ comfort with the decision-making process in the context of mental health. Importantly, clinicians were more satisfied with encounters which used the decision aid than those without, and the use of the decision aid did not add to the duration of the encounters, key findings for promoting implementation.
Implications for practice, policy, and research
There is substantial policy support for SDM. Although there are significant investments in generating comparative evidence of treatment for various conditions,43 including depression, there is limited information about methods for incorporating this evidence meaningfully in routine clinical practice.18–20 Depression Medication Choice offers one effective patient-centered method. Our results, however, do not support the notion that SDM will improve the efficacy or efficiency of care, an assumption policymakers often make when they advocate for patient involvement.44,45
Several practice guidelines call for SDM in the management of depression under the premise that for treatment to be effective, patients need to actively participate and adhere to these treatments despite their side effects, cost, and burden.10,11 This remains one of their least translated recommendations.46,47 Depression Medication Choice with its impact on patient decision-making process, efficient design (i.e. user friendly, easy to update evidence), straightforward implementation (minimal training and support), and clinician buy-in could provide a means to meet this recommendation.
Involving patients in fateful healthcare decisions is an integral component of patient-centered care, a necessary feature of high quality healthcare.48 When confronted with our findings –which are consistent with the systematic review of 115 randomized trials and with our own previous trials – policymakers will have to decide whether the value of decision aids as promoters of patient-centered care and informed patient engagement, as demonstrated in this trial, argues on its own merits, for its priority.
Further work in this area is necessary. The ideal decision support should probably include non-pharmacological options. A larger and longer trial to study the effect of the decision aid on adherence to therapy in patients selected because of non-adherence may be more informative.49 Larger studies are needed to identify subgroups (i.e., socioeconomic status)50 that may benefit more from using the decision aid. Identifying the amount and type of support needed to effectively embed the use of this decision aid in the routines of primary care practices to support its longitudinal use also remains to be determined.
CONCLUSION
Depression Medication Choice is a novel and efficient SDM tool. It effectively helps patients with moderate to severe depression and their primary care clinicians engage in collaborative deliberation by using evidence about the comparative effectiveness of antidepressants. Depression Medication Choice can help patients and their clinicians identify and implement treatment that best fits the patient’s values, preferences, and goals, in a timely way; a path to higher quality healthcare.
Acknowledgments
AL participated to the conception and design of the study, contributed to the analysis and interpretation of the data, supervised the conduct of the trial, and wrote the first draft of the manuscript. VMM conceived the study, applied for funding, contributed to the interpretation of the data, and provided critical revision to the manuscript. NDS, MDW, and KJY participated to the conception and design of the study, contributed to the interpretation of the data, and provided critical revision of the manuscript. JH participated to the conception and design of the study, led the statistical analysis and interpretation of the data, and provided critical revision of the manuscript. MEB and JWI contributed to the analysis and interpretation of the data, and provided critical revision of the manuscript. SRD and EMH contributed to the acquisition of the data, technical support (i.e., protection of participants’ confidentiality), and provided critical revision of the manuscript. ML, DHB, KMDW, and MRM contributed to the acquisition of the data, provided technical support (i.e. clinical champions) and critical revision of the manuscript. KKS led the design of the intervention and provided critical revision of the manuscript. All authors approved the final version of this manuscript. AL, VMM, and JH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
AL, VMM, NDS, MDW, KJY, MEB, JWI, SRD, EMH, ML, DHB, KMDW, MRM, and KKS report no potential conflict of interest.
The Agency for Healthcare and Quality Research funded this study under the American Recovery and Reinvestment Act of 2009 (iADAPT-1 R18 HS019214) and had no role in the design, execution, analyses, interpretation of the data or decision to submit results of this study. There was no commercial involvement in the trial.
We certify that this manuscript represent valid work and that neither this manuscript nor one with substantially similar content under our authorship has been publish or is being considered for publication elsewhere.
Footnotes
Trial Registration. Clinicaltrials.gov (NCT01502891) at https://clinicaltrials.gov/ct2/show/NCT01502891
REFERENCES
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 3.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27(8):959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merikangas KR, Ames M, Cui L, et al. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Arch Gen Psychiatry. 2007;64(10):1180–1188. doi: 10.1001/archpsyc.64.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon GE. Long-term prognosis of depression in primary care. Bull World Health Organ. 2000;78(4):439–445. [PMC free article] [PubMed] [Google Scholar]
- 6.Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(4 Suppl):1–45. [PubMed] [Google Scholar]
- 7.Coulehan JL, Schulberg HC, Block MR, Madonia MJ, Rodriguez E. Treating depressed primary care patients improves their physical, mental, and social functioning. Arch Intern Med. 1997;157(10):1113–1120. [PubMed] [Google Scholar]
- 8.Katzelnick D, Simon G, Pearson S, Manning W, Kobak K. Depression management programs. Arch Fam Med. 2000;9(8):689–670. [PubMed] [Google Scholar]
- 9.Akincigil A, Bowblis JR, Levin C, Walkup JT, Jan S, Crystal S. Adherence to antidepressant treatment among privately insured patients diagnosed with depression. Med Care. 2007;45(4):363–369. doi: 10.1097/01.mlr.0000254574.23418.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheehan DV, Keene MS, Eaddy M, Krulewicz S, Kraus JE, Carpenter DJ. Differences in medication adherence and healthcare resource utilization patterns: older versus newer antidepressant agents in patients with depression and/or anxiety disorders. CNS Drugs. 2008;22(11):963–973. doi: 10.2165/00023210-200822110-00005. [DOI] [PubMed] [Google Scholar]
- 11.Demyttenaere K, Enzlin P, Dewe W, et al. Compliance with antidepressants in a primary care setting, 1: Beyond lack of efficacy and adverse events. J Clin Psychiatry. 2001;62(Suppl 22):30–33. [PubMed] [Google Scholar]
- 12.Wierzbicki M, Pekarik G. A meta-analysis of psychotherapy dropout. Prof Psychol Res Pr. 1993;24(2) [Google Scholar]
- 13.Van Grieken RA, Beune EJ, Kirkenier AC, Koeter MW, van Zwieten MC, Schene AH. Patients perspectives on how treatment can impede their recovery from depression. J Affect Disord. 2014;167:153–159. doi: 10.1016/j.jad.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 14.Gartlehner G, Hansen R, Morgan L, et al. Prepared by the RTI International–University of North Carolina Evidence-based Practice Center, Contract No. 290-2007-10056-I. AHRQ Publication No. 12-EHC012-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. www.effectivehealthcare.ahrq.gov/reports/final.cfm) [PubMed] [Google Scholar]
- 15.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 16.Gaissmaier W, Anderson BL, Schulkin J. How do physicians provide statistical information about antidepressants to hypothetical patients? Med Decis Making. 2014;34(2):206–215. doi: 10.1177/0272989X13501720. [DOI] [PubMed] [Google Scholar]
- 17.Davies NM, Gunnell D, Thomas KH, Metcalfe C, Windmeijer F, Martin RM. Physicians’ prescribing preferences were a potential instrument for patients’ actual prescriptions of antidepressants. J Clin Epidemiol. 2013;66(12):1386–1396. doi: 10.1016/j.jclinepi.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sox HC. Comparative effectiveness research: a progress report. Ann Intern Med. 2010;153(7):469–472. doi: 10.7326/0003-4819-153-7-201010050-00269. [DOI] [PubMed] [Google Scholar]
- 19.Timbie JW, Fox DS, Van Busum K, Schneider EC. Five Reasons That Many Comparative Effectiveness Studies Fail To Change Patient Care And Clinical Practice. Health Affairs. 2012;31(10):2168–2175. doi: 10.1377/hlthaff.2012.0150. 2012. [DOI] [PubMed] [Google Scholar]
- 20.Morrato EH, Concannon TW, Meissner P, Shah ND, Turner BJ. Dissemination and implementation of comparative effectiveness evidence: key informant interviews with Clinical and Translational Science Award institutions. J Comp Eff Res. 2013;2(2):185–194. doi: 10.2217/cer.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. doi: 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 22.Shah N, Mullan R, Breslin MA, Yawn BP, Ting H, Montori VM. Translating comparative effectiveness into practice. The case of diabetes medications. Med Care. 2010;48(6 suppl):S153–S158. doi: 10.1097/MLR.0b013e3181d5956c. [DOI] [PubMed] [Google Scholar]
- 23.Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219–226. doi: 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 24.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Agoritsas T, Heen AF, Brandt L, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ. 2015;350:g7624. doi: 10.1136/bmj.g7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeBlanc A, Bodde AE, Branda ME, et al. Translating comparative effectiveness of depression medications into practice by comparing the depression medication choice ision aid to usual care: study protocol for a randomized controlled trial. Trials. 2013;14:127. doi: 10.1186/1745-6215-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A new direction in depression treatment in Minnesota: DIAMOND program, Institute for Clinical Systems Improvement, Bloomington, Minnesota. Psychiatr Serv. 2010;61(10):1042–1044. doi: 10.1176/ps.2010.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor A. User Manual _ Knowledge [Document on the Internet] 2000 http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Knowledge.pdf.
- 31.Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301. doi: 10.1186/1472-6963-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 33.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7-8):1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 34.LeBlanc A, Kenny DA, O’Connor AM, Légaré F. Decisional Conflict in Patients and Their Physicians: A Dyadic Approach to Shared Decision Making. Med Decis Making. 2009;29(1):61–68. doi: 10.1177/0272989X08327067. [DOI] [PubMed] [Google Scholar]
- 35.Elwyn G, Hutchings H, Edwards A, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect. 2005;8(1):34–42. doi: 10.1111/j.1369-7625.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyatt KD, Branda ME, Anderson RT, et al. Peering into the black box: a meta-analysis of how clinicians use decision aids during clinical encounters. Implement Sci. 2014;9:26. doi: 10.1186/1748-5908-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donner A, Klar NS. Design and Analysis of Cluster Randomisation Trials in Health Research. London: Hodder Arnold; 2000. [Google Scholar]
- 38.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007;167(10):1076–1082. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 39.Snijders T, Bosker Rj. Multileve analysis: An introduction to basic and advanced multilevel modeling. London: Sage; 2004. [Google Scholar]
- 40.Montori VM, Shah ND, Pencille LJ, et al. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am J Med. 2011;124(6):549–556. doi: 10.1016/j.amjmed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Duncan E, Best C, Hagen S. Shared decision making interventions for people with mental health conditions. Cochrane Database Syst Rev. 2010;(1):CD007297. doi: 10.1002/14651858.CD007297.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couet N, Desroches S, Robitaille H, et al. Assessments of the extent to which healthcare providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2013 doi: 10.1111/hex.12054. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKinney M. ‘Time is of the essence’. PCORI moves to implement comparative effectiveness research, funding. Modern healthcare. 2012;42(5):12–13. [PubMed] [Google Scholar]
- 44.Katz SJ, Hawley S. The value of sharing treatment decision making with patients: expecting too much? JAMA. 2013;310(15):1559–1560. doi: 10.1001/jama.2013.278944. [DOI] [PubMed] [Google Scholar]
- 45.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. NEJM. 2013;368(1):6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 46.Gilbody SM, Whitty PM, Grimshaw JM, Thomas RE. Improving the detection and management of depression in primary care. Qual Saf Health Care. 2003;12(2):149–155. doi: 10.1136/qhc.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harter M, Bermejo I, Ollenschlager G, et al. Improving quality of care for depression: the German Action Programme for the implementation of evidence-based guidelines. Int J Qual Health Care. 2006;18(2):113–119. doi: 10.1093/intqhc/mzi089. [DOI] [PubMed] [Google Scholar]
- 48.Institute of Medicine. Crossing the Quality Chasm. A new health system for the 21st century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 49.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coylewright M, Branda M, Inselman JW, et al. Impact of sociodemographic patient characteristics on the efficacy of decision AIDS: a patient-level meta-analysis of 7 randomized trials. Circ Cardiovasc Qual Outcomes. 2014;7(3):360–367. doi: 10.1161/HCQ.0000000000000006. [DOI] [PubMed] [Google Scholar]