Abstract
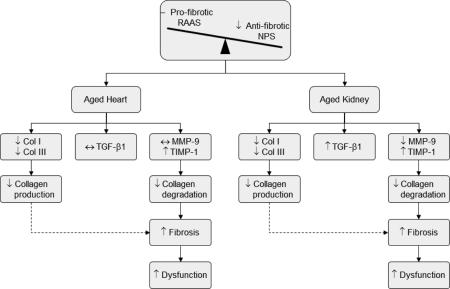
Cardiorenal fibrosis is a biological process that increases with age and contributes to dysfunction of the heart and kidney. While numerous circulating and tissue hormones, cytokines and enzymes have been identified in the development of cardiorenal fibrosis, several reports have suggested that the anti-fibrotic natriuretic peptide system (NPS), pro-fibrotic renin-angiotensin-aldosterone system (RAAS), transforming growth factor-beta 1 (TGF-β1), matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) are fundamental regulators and mediators of this process. However, the simultaneous assessment of these components in the development of age-mediated cardiorenal fibrotic remodeling is not completely understood. Thus, we assessed cardiorenal structure and function, the circulating NPS and RAAS and the cardiorenal tissue gene expression of collagen (Col) I, Col III, TGF-β1, MMP-9 and TIMP-1 in 2 and 20 month old Fischer rats. Our studies determined that aging was characterized by an increase in cardiorenal fibrosis that was accompanied with cardiorenal dysfunction. These alterations were associated with lower circulating atrial and C-type natriuretic peptides and higher angiotensin II and aldosterone levels in the aged rats. Moreover, we observed a decrease in Col I and III, an increase in TIMP-1 and no change in MMP-9 mRNA expressions in the aged heart and kidney, while TGF-β1 expression increased only in the aged kidney. We conclude that the age-mediated alterations in these fibrotic regulator and mediator profiles favors collagen accumulation due to an imbalance between the NPS and RAAS as well as a decline in the degradative pathway, thus suggesting a therapeutic opportunity to target these components.
Keywords: fibrosis, cardiorenal, natriuretic peptides, renin-angiotensin-aldosterone system, gene expression
Graphical Abstract
1. INTRODUCTION
The heart and kidney are hormonally linked via the natriuretic peptide system (NPS), where the cardiac and reno-endothelium derived atrial natriuretic peptide (ANP) and c-type natriuretic peptide (CNP) respectively, mediates the inhibition of cardiorenal fibrosis and adverse remodeling through the particulate guanylyl cyclase receptors [1, 2]. The NPS is counter-regulated by the renin-angiotensin-aldosterone system (RAAS) of which angiotensin II (ANG II) via the angiotensin receptor 1 (AT1) and aldosterone through the mineralocorticoid receptor contribute to adverse cardiac and renal remodeling and fibrosis [3]. Notably, combined fibrosis of the heart and kidney are hallmarks of aging [4, 5] and indeed in the extreme, contribute to both heart failure and chronic kidney disease. The interstitium of the heart and kidneys are dynamic structures that are reflective of a continuous process of synthesis and degradation of extracellular matrix (ECM) proteins including collagen and of which is influenced by circulating neurohumoral factors [6]. Indeed, an emerging view is that fibrosis of the heart and kidney involves simultaneous remodeling of both organs leading to chronic cardiorenal disease. Furthermore, the important relationship between the NPS and RAAS in the setting of age-related cardiorenal fibrosis is not clearly understood.
To date, ANP and CNP have been shown to inhibit DNA synthesis and fibroblast proliferation [7-9]. Whereas activated RAAS as well as transforming growth factor-beta 1(TGF-β1) markedly stimulate collagen deposition and the formation of cardiac and renal fibrosis [10, 11]. Moreover, the fibroblast is a key regulator collagen turnover by a balancing of collagen synthesis and degradation [12]. This regulatory process involves the interaction between the matrix metalloproteinases (MMPs) and the tissue inhibitors of metalloproteinases (TIMPs) [13-15], of which MMP-9 and TIMP-1 in particular, is altered in models of cardiac and renal disease [16-22]. While fibrosis remains the hallmark of cardiorenal aging and contributes to cardiorenal impairment, the mechanisms for accentuated collagen deposition in the aged heart and kidney remain poorly defined, in part, due to the lack of simultaneous assessment of myocardial and renal fibrosis in the same setting.
Here we wanted to confirm and extend investigations on age-mediated changes in the key regulators and mediators of fibrosis and dysfunction in both the heart and kidney. We utilized an experimental rat model of aging to assess the balance between the anti-fibrotic NPS and pro-fibrotic systems RAAS and TGF-β1 and its relationship with left ventricular (LV), renal cortical and renal medullary fibrosis as well as cardiorenal structure and function. We also defined the gene expressions of collagen (Col) I, Col III, MMP-9 and TIMP-1 in the heart and kidney. We hypothesized that aging would be associated with: 1) a relative deficiency of plasma ANP and CNP as well as an activation of circulating ANG II and aldosterone; 2) parallel increases in LV, cortical and medullary fibrosis coupled with impaired cardiorenal function and, 3) changes in the collagen degrading pathway due to an imbalance in the expression of MMP-9 and TIMP-1. Our study supports the concept that an imbalance between key regulators and mediators of fibrosis, such as the NPS, RAAS and TGF-β1 pathway, characterizes cardiorenal aging and represents a therapeutic opportunity.
2. METHODS
2.1 Animals
Studies were performed in 2- and 20-month old male Fischer rats (Harlan Laboratories; n=10 per age group). The Fischer (F344) rat is a widely utilized animal model for aging studies and was developed by the National Institute of Aging[23]. This inbred rat strain closely mimics many characteristics of CV and renal aging seen in humans and also exhibits a lower tumor rate than non-inbred rat strains[24-30]. This experimental study was performed with approval of the Mayo Clinic Institutional Animal Care and Use Committee and in accordance with the Animal Welfare Act.
2.2. Echocardiography
As routinely carried out in our laboratory, standard 2-dimensional echocardiography was performed on lightly anesthetized (1.5% isoflurane in oxygen) rats using the Vivid 7 ultrasound system (GE medical Systems) and 10S transducer with ECG monitoring. M-mode images and 2-dimensional parasternal short axis images were recorded for off-line analyses of left ventricular structure and function using EchoPAC software (EchoPAC PC BTO 9.0.0, GE Healthcare) are previously described in detail [29]. Diastolic wall strain (DWS), as an index of diastolic stiffness based on the linear elastic theory, was calculated with the following equation: (systolic posterior wall thickness – diastolic posterior wall thickness) / systolic posterior wall thickness [31, 32].
2.3. Renal Function
Rats were place in metabolic cages with free access to food and water and allowed to acclimatize for 24 hours prior to collection. A 24-hour urine collection was performed on ice after acclimatization for urinary protein excretion assessment as previously described [28]. Glomerular filtration rate (GFR) was also assessed by inulin clearance on anesthetized (2.0-2.5% isoflurane in oxygen) rats as described previously [28].
2.4. Blood Pressure and Plasma Collection
Blood pressure (BP) and plasma collection was performed as previously described [29].
2.5. LV and Renal Tissue Collection
The hearts and kidneys were removed from anesthetized (2.0-2.5% isoflurane in oxygen) rats and the LV, renal cortex and medulla were carefully dissected. A cross-section of the LV and kidney containing both cortex and medulla were preserved in 10% formalin for histological analysis of fibrosis. The remaining LV, renal cortex and medulla were quickly snap frozen in liquid nitrogen.
2.6. Neurohumoral Analysis
Plasma ANP [33], CNP [29], ANG II [34] and aldosterone [35] were determined by commercially available radioimmunoassays as described in prior studies.
2.7. Histological Analysis for Cardiorenal Fibrosis
Cross-sections of paraffin-embedded LV and renal tissue (4 μm) were stained with picrosirius red and quantified as previously described [28, 29].
2.8. Quantitative mRNA Expression
As previously described [36], real-time polymerase chain reaction (RT-PCR) was used to quantify Col I, Col III, TGF-β1, MMP-9, TIMP-1 and 18S gene expression levels. Briefly, total RNA was extracted from ~30 mg cardiac or renal cortical or medullary tissues using Qiagen RNeasy kit (Qiagen, Hilden, Germany). Then the cDNA was reverse transcribed and triplicate cDNA aliquots were amplified using sequence-specific primers (Geneworks, Adelaide, SA, Australia) with TagMan fluorogenic probe for TGF-β1 and 18S (Applied Biosystems) or SYBR Green detection for Col I, Col III, MMP-9 and TIMP-1 (Applied Biosystems) using an ABI prism 7900HT sequence Detection System (Applied Biosystems). The primer pairs were designed using Primer Express 2.0 software (Applied Biosystems) based on published sequences (http://www.ncbi.nlm.nih.gov). 18S rRNA was used as an endogenous control in all experiments and used to standardized quantitation for the expression of each gene.
2.9. Statistical Analyses
Our results are expressed as mean ± SE. Student unpaired t-tests were employed for single comparisons between age groups. All statistical analyses were performed using Graphpad Prism 6 software. Statistical significance was accepted as P<0.05.
3. RESULTS
3.1. Age and Cardiorenal Structure and Function
Cardiorenal structure and function as well as BP are reported in Table 1. As expected, there were significant increases in body, left ventricular, total kidney weights with aging. The aged rats exhibited significantly larger LV cavity dimension, elevated urinary protein excretion as well as significantly lower ejection fraction, diastolic wall strain and GFR. Analysis of anesthetized rats in vivo revealed a significantly higher systolic and diastolic BP, with no change in heart rate (data not shown) in the aged rats.
Table 1.
Cardiorenal structure and function and blood pressure between 2 and 20 months old Fischer rats
| Parameter | 2 months | 20 months |
|---|---|---|
| Body Weight (g) | 210 ± 3 | 449 ± 6† |
| Cardiac Structure and Function | ||
| LV Weight (mg) | 470 ± 7 | 812±22† |
| LVEDD (mm) | 6.74 ± 0.10 | 7.41 ± 0.08† |
| LVESD (mm) | 3.28 ± 0.10 | 4.35 ± 0.09† |
| EF (%) | 88 ± 1 | 79 ± 1† |
| Diastolic Wall Strain | 0.39 ± 0.01 | 0.26 ± 0.03† |
| Renal Structure and Function | ||
| Total Kidney Weights (mg) | 1623 ± 35 | 2729 ± 56† |
| GFR (ml/min/kg) | 3.95 ± 0.23 | 2.52 ± 0.33** |
| Protein Excretion Rate (μg/min) | 5.6± 0.4 | 15.6 ± 3.6† |
| Blood Pressure | ||
| Systolic BP (mmHg) | 101 ± 2 | 116 ± 3** |
| Diastolic BP (mmHg) | 91 ± 2 | 102 ± 4* |
Values are mean ± SE.
P<0.05
P<0.01 or
P<0.001 vs. 2 months. LV = left ventricular; LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; EF = ejective fraction; GFR = glomerular filtration rate; BP = blood pressure
3.2. Age on Circulating NPS and RAAS
Table 2 reports circulating ANP, CNP, angiotensin II and aldosterone with aging. Specifically, the aged rats had significantly lower plasma levels of ANP and CNP, whereas plasma levels of ANG II and aldosterone were significantly higher compared to the young rats.
Table 2.
Plasma natriuretic peptides, angiotensin II and aldosterone between 2 and 20 month old Fischer rats
| Parameter | 2 months | 20 months |
|---|---|---|
| ANP (pg/ml) | 27 ± 3 | 14 ± 1** |
| CNP (pg/ml) | 34 ± 4 | 10 ± 1** |
| ANG II (pg/ml) | 11 ± 3 | 20 ± 3* |
| Aldosterone (ng/dl) | 18 ± 3 | 40 ± 6** |
Values are mean ± SE.
P<0.05 or
P<0.01 vs. 2 months. ANP = atrial natriuretic peptide; CNP = c-type natriuretic peptide; ANG II = angiotensin II
3.3. Age and Cardiorenal Fibrosis and Fibrosis-Related Gene Expression
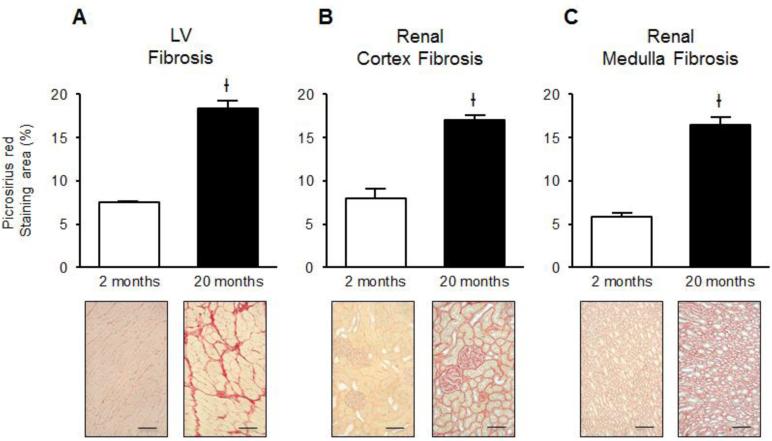
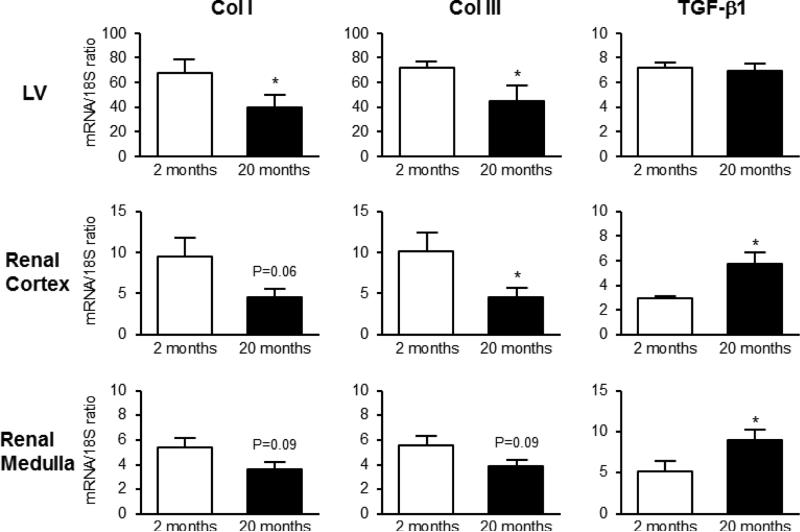
The magnitude of fibrosis in the LV, renal cortex and medulla (Figure 1A, B and C, respectively) was significantly increased in the aged rats compared to the young rats. As type I and III collagens are the major fibrillar collagens responsible for fibrosis in normal and diseased heart and kidneys, we examined the effect of age on collagen synthesis regulated at the transcriptional level by RT-PCR. As illustrated in Figure 2, the expression of mRNA for LV Col I and III significantly decreased with age. While in the kidney there was a strong trend for a reduction in Col I mRNA expression in the renal cortex and medulla, whereas there was significant reduction in Col III mRNA in the renal cortex and trended to decrease in the renal medulla. Aging did not change levels of TGF-β1 mRNA expression in the LV, although there was a significant increase in TGF-β1 transcript levels in the renal cortex and medulla.
Figure 1.
Effects of aging on LV (A), renal cortical (B) and medullary (C) fibrosis as determined by picrosirius red staining in 2 and 20 month old Fischer rats at 20x objective magnification. Ɨ P<0.001 vs. 2 months. Black scale bar = 100 μm.
Figure 2.
mRNA expression of pro-fibrotic genes Col I, Col III and TGF-β1 (first, second and third column, respectively) in the LV (upper row), renal cortex (middle row) and medulla (lower row) between 2 and 20 month old Fischer rats. * P<0.05 vs. 2 months.
3.4. Age and Cardiorenal Gene Expression of Regulators of Collagen Turnover
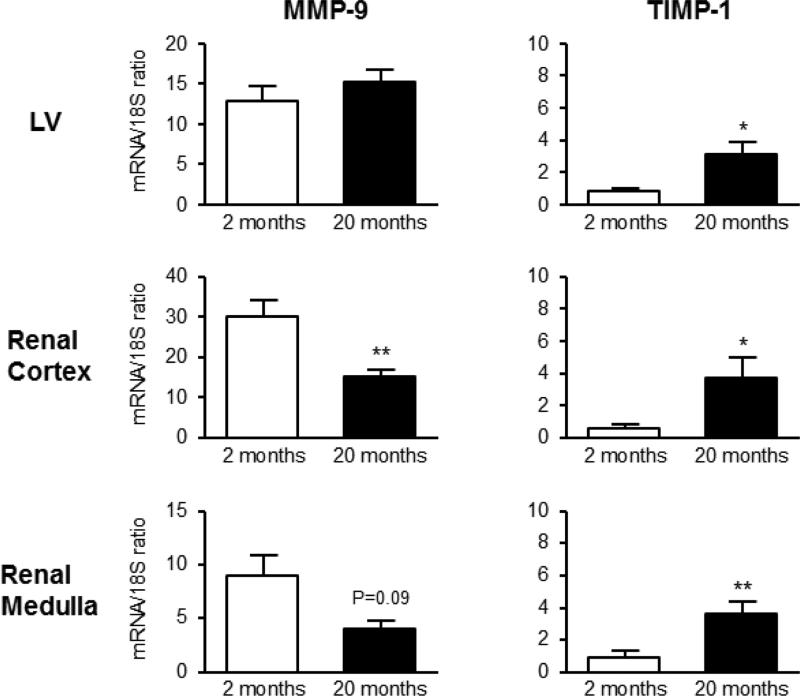
As illustrated in the Figure 3, mRNA expression of MMP-9 were not altered in the LV, however was significantly lower in the renal cortex and trended to be lower in the renal medulla in the aged rats. TIMP-1 gene expression in the LV, renal cortex and medulla significantly increased with aging. Moreover, an essential component to ECM degradation is reflected by the MMP-9/TIMP-1 ratio and this important ratio is reported in Table 3. The data presented here suggest that net cardiorenal MMP activity, based on the MMP-9/TIMP-1 ratio, was significantly lower in the LV, renal cortex and medulla, thus suggesting a decrease in collagen degradation.
Figure 3.
mRNA expression of regulators of collagen turnover genes MMP-9 and TIMP-1 (first and second column, respectively) in the LV (upper row), renal cortex (middle row) and medulla (lower row) between 2 and 20 month old Fischer rats. * P<0.05 or ** P<0.01 vs. 2 months.
Table 3.
MMP-9 to TIMP-1 ratio in the left ventricle, renal cortex and medulla between 2 and 20 month old Fischer rats
| Parameter | 2 months | 20 months |
|---|---|---|
| LV MMP-9/TIMP-1 | 17.01 ± 3.92 | 7.10 ± 1.14* |
| Renal Cortex MMP-9/TIMP-1 | 58.69 ± 8.13 | 6.07 ± 0.98** |
| Renal Medulla MMP-9/TIMP-1 | 21.68 ± 9.85 | 1.18± 0.10* |
Values are mean ± SE.
P<0.05 or
P<0.01 vs. 2 months. MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase
4. DISCUSSION
Our study defines the parallel increase in fibrosis and associated cardiorenal dysfunction in the heart and kidney, together with key circulating and tissue modulators of fibrogenesis in an experimental rat model of aging. Specifically, we found an important shift in the balance between the anti-fibrotic NPS, specifically ANP and CNP and the pro-fibrotic RAAS and TGF-β1 pathway that favors fibrogenesis. We also report that age mediated cardiorenal fibrosis is correlated with an overall decrease in collagen degradation, rather than an increase in collagen production, as the gene expression of TIMP-1 increased, while Col I, Col III and MMP-9/TIMP-1 ratio decreased. Together, these data suggest that altered collagen turnover at the gene level is modulated by a number of pathways that are associated with age-mediated cardiorenal fibrosis and dysfunction.
Fibrosis of the heart and kidney is a hallmark of aging [4, 5], however few investigations have simultaneously investigated key systems that contribute to this phenotype in both the heart and kidney. Here we confirm previous reports by our group and others [28, 29, 37, 38] that age-mediated cardiorenal fibrosis increases with aging in the rat. Interestingly, Col I and Col III mRNA transcripts decrease in the aging LV, renal cortex and medulla, thus suggesting the increase in fibrosis in the aged heart and kidney is not likely due to increase in collagen production. Indeed, the observation that BP is increased with age could support an important load dependent mechanism in the formation of fibrosis in the LV.
The RAAS, TGF-β1 and NPS are fundamental pathways in implicated in cardiorenal fibrogenesis due to disease and aging, with the RAAS and TGF-β1 promoting organ fibrosis through fibroblast proliferation, collagen synthesis and accumulation and the NPS counteracting these fibrotic processes [3, 39]. In the present investigation, we found a significant increase in the circulating ANG II and aldosterone in the aged rat. In vitro studies have demonstrated that ANG II or aldosterone increase collagen gene expression [40], stimulate TGF-β1 production in fibroblasts [41, 42] and induced fibroblast proliferation [43]. Indeed, as studies has suggested that TGF-β1 is key factor that contributes to the formation of cardiac and renal fibrosis in various disease states [44, 45], we also quantified the gene expression of TGF-β1 in the aging heart and kidney. We found that TGF-β1 mRNA remained unchanged between the young and aged LV, however was significantly up regulated in both the aged renal cortex and medulla. Our data differs, in part from previous studies, as elevated plasma ANG II and aldosterone seen the aged rats was not associated with increased Col I or III gene expression in the LV or kidney. However, the elevation in ANG II and aldosterone may have contributed to the increase in TGF-β1 gene expression seen the kidney and is consistent with previous studies demonstrating the RAAS effects on TGF-β1 production and renal fibrosis [11]. Notably, recent studies have reported that TGF-β1 can suppress collagen production through the induction of a negative regulator of collagen transcription such as CUX1 [46]. Thus, additional studies are needed to define the unique regulatory role and interaction of TGF-β1 and the RAAS on cardiorenal fibrogenesis in aging.
The relationship between the RAAS and NPS continues to evolve and in setting of aging, remains to fully be understood. Here, we confirmed our previous study [29] that the aged rat is characterized by a significant decrease of plasma CNP. Interestingly, we also observed a significant decline in circulating ANP in the aged rats. This decrease in ANP could be, in part, responsible for elevated ANG II and aldosterone as well as cardiorenal fibrosis. Specifically, ANP has been demonstrated to directly inhibit aldosterone production [47], aldosterone-stimulated nuclear translocation of the mineralocorticoid receptor [48] and renin release [49], as well as counteract the effects of ANG II [50] and suppress organ fibrosis through various mechanisms [7, 8, 51, 52]. While our work and by others [53], in part, supports the idea that ANP may be decreased with older age, review of the human and experimental literature, albeit limited, is generally in contrast this finding and support an elevation of ANP with aging [54, 55]. It is tempting to speculate that differences in rat strain, age groups or concomitant disease seen in older humans such as hypertension, chronic kidney disease or atrial fibrillation could account for this discrepancy. Although the overall deficiency of ANP and CNP may have a multifactorial role in the development of cardiorenal fibrosis seen in this experimental aging model, further studies in humans from children to the elderly, especially without concomitant disease, is warranted.
Our findings underscore the importance of collagen degradation in the setting of cardiorenal aging. Importantly organ fibrosis is regulated, in part, by a group of enzymes called MMPs, which degrade ECM proteins, and their endogenous inhibitors, TIMPs [13-15]. Given the decline in both Col I and III mRNA levels in the aging heart and kidney seen here, it is plausible that a reduction collagen turnover at the transcription level during cardiorenal aging may be a function of a decline in collagen degradation. Here, we found no change in MMP-9 gene expression in the LV, however there was decrease in MMP-9 mRNA expression the renal cortex and medulla, suggesting that there is an impairment in the regulation of collagen degradation. Our postulation is further supported by the fact that the gene expression of cardiac and renal TIMP-1, which has high specificity for MMP-9 [56], was significantly increased. As there was no increase in MMP-9 mRNA levels coupled with elevated levels of TIMP-1, this balance shift is suggestive of an impaired proteolytic process to degrade collagen in the aged heart and kidney. Our data are consistent with this notion as the cardiorenal MMP-9/TIMP-1 ratios were significantly lower in the aged rats compared to the young rats. This is consistent, in part, with the elegant study by Bonnema et al. [57], who reported changes in plasma MMPs, TIMPs and its ratio with human aging are in favor of decreased ECM degradative capacity. Thus the aged heart and kidney may be characterized as an environment with the inability to breakdown collagen resulting in fibrosis, which may be in contrast to processes that are initiated by disease and result in an increase in fibrosis.
There are several limitations to the current study and we should be cautious in interpreting the current data. Additional investigations are needed to evaluate the cardiorenal tissue expression of the NPS (i.e. atrial and ventricular levels) and RAAS including their receptors, as well as a detailed assessment of the MMP and TIMP protein and activity levels including and beyond MMP-9 and TIMP-1. Moreover, comprehensive studies are needed in higher species as well as multiple age groups in order to have a more complete assessment of these mediators and regulators of collagen turnover and fibrosis that gradually occur during the aging process. Despite these limitations, this investigation does provide additional insights into myocardial and renal fibrosis due to aging in a parallel process.
5. CONCLUSION
Our findings in the aging Fischer rat demonstrates that cardiorenal fibrosis is characterized by an imbalance between the anti-fibrotic NPS and pro-fibrotic RAAS/TGF-β1 pathways, as well as a shift in the MMP-9 and TIMP-1 profiles, that favor a decline in collagen degradation, rather than increased production. These age-mediated changes in the cardiorenal ECM may, in part, contribute cardiorenal dysfunction including diastolic impairment and proteinuria. This study lays the foundation for further investigations in the aged heart and kidney, to define whether enhancing collagen degradation by augmenting the NPS, inhibiting the RAAS and/or other novel molecular pathways including direct manipulation of TGF-β1, the MMPs or TIMPs will delay fibrosis and reduce the risk for cardiorenal dysfunction.
Highlights.
Experimental aging is associated with a parallel increase in cardiorenal fibrosis
An imbalance between the NPS, RAAS and TGF-β1 pathways favors fibrogenesis
Cardiorenal fibrosis is correlated with a decline in collagen degradation with aging
ACKNOWLEDGEMENTS
This study was supported by grants from the National Heart, Lung and Blood Institute (R01-HL083231 and P01-HL076611) awarded to Professor John C. Burnett Jr, the National Health and Medical Research Council of Australia (program ID 546272) awarded to Professor Henry Krum, the American Heart Association Scientist Development Grant (13SDG16910051) awarded to Dr. S. Jeson Sangaralingham, a Career Development Award in Cardiovascular Research – St. Jude Medical Foundation awarded to Dr. S. Jeson Sangaralingham, the Mayo Clinic Center for Clinical and Translational Science grant (UL1 TR000135) and the Mayo Foundation. We greatly appreciate the technical assistance of Gerald E. Harders, Denise M. Heublein, Sharon M. Sandberg and Elise A. Oehler.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Volpe M. Natriuretic peptides and cardio-renal disease. Int J Cardiol. 2014;176:630–9. doi: 10.1016/j.ijcard.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Zakeri R, Burnett JC, Jr., Sangaralingham SJ. Urinary C-type natriuretic peptide: an emerging biomarker for heart failure and renal remodeling. Clin Chim Acta. 2015;443:108–13. doi: 10.1016/j.cca.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Lueder TG, Sangaralingham SJ, Wang BH, Kompa AR, Atar D, Burnett JC, Jr., et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail. 2013;6:594–605. doi: 10.1161/CIRCHEARTFAILURE.112.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging Dis. 2011;2:158–73. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG. The aging kidney. Kidney Int. 2008;74:710–20. doi: 10.1038/ki.2008.319. [DOI] [PubMed] [Google Scholar]
- 6.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–8. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 7.Cao L, Gardner DG. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension. 1995;25:227–34. doi: 10.1161/01.hyp.25.2.227. [DOI] [PubMed] [Google Scholar]
- 8.Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, et al. Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185–92. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 9.Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, et al. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–84. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- 10.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–76. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Zhang J, Zhang JQ, Ramires FJ. Local angiotensin II and transforming growth factor-beta1 in renal fibrosis of rats. Hypertension. 2000;35:1078–84. doi: 10.1161/01.hyp.35.5.1078. [DOI] [PubMed] [Google Scholar]
- 12.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–71. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–30. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 15.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–13. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, et al. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J. 2006;151:1101 e1–8. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Eddy AA, Kim H, Lopez-Guisa J, Oda T, Soloway PD. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney Int. 2000;58:618–28. doi: 10.1046/j.1523-1755.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 18.Yan AT, Yan RT, Spinale FG, Afzal R, Gunasinghe HR, Arnold M, et al. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. J Card Fail. 2006;12:514–9. doi: 10.1016/j.cardfail.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Moshal KS, Tyagi N, Moss V, Henderson B, Steed M, Ovechkin A, et al. Early induction of matrix metalloproteinase-9 transduces signaling in human heart end stage failure. J Cell Mol Med. 2005;9:704–13. doi: 10.1111/j.1582-4934.2005.tb00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–71. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 22.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–42. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 23.Sprott RL, Ramirez I. Current Inbred and Hybrid Rat and Mouse Models for Gereontological Research. ILAR J. 1997;38:104–9. doi: 10.1093/ilar.38.3.104. [DOI] [PubMed] [Google Scholar]
- 24.Abrass CK, Adcox MJ, Raugi GJ. Aging-associated changes in renal extracellular matrix. Am J Pathol. 1995;146:742–52. [PMC free article] [PubMed] [Google Scholar]
- 25.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–85. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 26.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, et al. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2132–7. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razzaque MS, Shimokawa I, Nazneen A, Higami Y, Taguchi T. Age-related nephropathy in the Fischer 344 rat is associated with overexpression of collagens and collagen-binding heat shock protein 47. Cell Tissue Res. 1998;293:471–8. doi: 10.1007/s004410051139. [DOI] [PubMed] [Google Scholar]
- 28.Sangaralingham SJ, Heublein DM, Grande JP, Cataliotti A, Rule AD, McKie PM, et al. Urinary C-type natriuretic peptide excretion: a potential novel biomarker for renal fibrosis during aging. Am J Physiol Renal Physiol. 2011;301:F943–52. doi: 10.1152/ajprenal.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, et al. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic Peptide. Hypertension. 2011;57:201–7. doi: 10.1161/HYPERTENSIONAHA.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sangaralingham SJ, Ritman EL, McKie PM, Ichiki T, Lerman A, Scott CG, et al. Cardiac micro-computed tomography imaging of the aging coronary vasculature. Circ Cardiovasc Imaging. 2012;5:518–24. doi: 10.1161/CIRCIMAGING.112.973057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtani T, Mohammed SF, Yamamoto K, Dunlay SM, Weston SA, Sakata Y, et al. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1742–9. doi: 10.1093/eurheartj/ehs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8:71–8. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- 33.Burnett JC, Jr., Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–7. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 34.Lisy O, Redfield MM, Jovanovic S, Jougasaki M, Jovanovic A, Leskinen H, et al. Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function In vivo. Circulation. 2000;102:338–43. doi: 10.1161/01.cir.102.3.338. [DOI] [PubMed] [Google Scholar]
- 35.Sancho J, Haber E. A direct microassay for aldosterone in plasma extracts. J Clin Endocrinol Metab. 1978;47:391–6. doi: 10.1210/jcem-47-2-391. [DOI] [PubMed] [Google Scholar]
- 36.Lekawanvijit S, Kompa AR, Zhang Y, Wang BH, Kelly DJ, Krum H. Myocardial infarction impairs renal function, induces renal interstitial fibrosis, and increases renal KIM-1 expression: implications for cardiorenal syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1884–93. doi: 10.1152/ajpheart.00967.2011. [DOI] [PubMed] [Google Scholar]
- 37.Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res. 1989;23:723–9. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- 38.Gagliano N, Arosio B, Santambrogio D, Balestrieri MR, Padoani G, Tagliabue J, et al. Age-dependent expression of fibrosis-related genes and collagen deposition in rat kidney cortex. J Gerontol A Biol Sci Med Sci. 2000;55:B365–72. doi: 10.1093/gerona/55.8.b365. [DOI] [PubMed] [Google Scholar]
- 39.Volpe M, Rubattu S, Burnett J., Jr Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–25. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou G, Kandala JC, Tyagi SC, Katwa LC, Weber KT. Effects of angiotensin II and aldosterone on collagen gene expression and protein turnover in cardiac fibroblasts. Mol Cell Biochem. 1996;154:171–8. doi: 10.1007/BF00226785. [DOI] [PubMed] [Google Scholar]
- 41.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J J Mol Cell Cardiol. 1997;29:1947–58. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 42.Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. Angiotensin II stimulates the autocrine production of transforming growth factor-beta 1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:2347–57. doi: 10.1016/s0022-2828(95)91983-x. [DOI] [PubMed] [Google Scholar]
- 43.Campbell SE, Janicki JS, Weber KT. Temporal differences in fibroblast proliferation and phenotype expression in response to chronic administration of angiotensin II or aldosterone. J Mol Cell Cardiol. 1995;27:1545–60. doi: 10.1016/s0022-2828(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 44.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–10. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 45.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–95. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fragiadaki M, Ikeda T, Witherden A, Mason RM, Abraham D, Bou-Gharios G. High doses of TGF-beta potently suppress type I collagen via the transcription factor CUX1. Mol Biol Cell. 2011;22:1836–44. doi: 10.1091/mbc.E10-08-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudo T, Baird A. Inhibition of aldosterone production in the adrenal glomerulosa by atrial natriuretic factor. Nature. 1984;312:756–7. doi: 10.1038/312756a0. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa H, Oberwinkler H, Nikolaev VO, Gassner B, Umbenhauer S, Wagner H, et al. Atrial natriuretic peptide locally counteracts the deleterious effects of cardiomyocyte mineralocorticoid receptor activation. Circ Heart Fail. 2014;7:814–21. doi: 10.1161/CIRCHEARTFAILURE.113.000885. [DOI] [PubMed] [Google Scholar]
- 49.Burnett JC, Jr., Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247:F863–6. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 50.Kilic A, Bubikat A, Gassner B, Baba HA, Kuhn M. Local actions of atrial natriuretic peptide counteract angiotensin II stimulated cardiac remodeling. Endocrinology. 2007;148:4162–9. doi: 10.1210/en.2007-0182. [DOI] [PubMed] [Google Scholar]
- 51.Nishikimi T, Inaba-Iemura C, Ishimura K, Tadokoro K, Koshikawa S, Ishikawa K, et al. Natriuretic peptide/natriuretic peptide receptor-A (NPR-A) system has inhibitory effects in renal fibrosis in mice. Regul Pept. 2009;154:44–53. doi: 10.1016/j.regpep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Ishigaki N, Yamamoto N, Jin H, Uchida K, Terai S, Sakaida I. Continuos intravenous infusion of atrial natriuretic peptide (ANP) prevented liver fibrosis in rat. Biochem Biophys Res Commun. 2009;378:354–9. doi: 10.1016/j.bbrc.2008.10.154. [DOI] [PubMed] [Google Scholar]
- 53.Pollack JA, Skvorak JP, Nazian SJ, Landon CS, Dietz JR. Alterations in atrial natriuretic peptide (ANP) secretion and renal effects in aging. J Gerontol A Biol Sci Med Sci. 1997;52:B196–202. doi: 10.1093/gerona/52a.4.b196. [DOI] [PubMed] [Google Scholar]
- 54.Davis KM, Fish LC, Minaker KL, Elahi D. Atrial natriuretic peptide levels in the elderly: differentiating normal aging changes from disease. J Gerontol A Biol Sci Med Sci. 1996;51:M95–101. doi: 10.1093/gerona/51a.3.m95. [DOI] [PubMed] [Google Scholar]
- 55.Wu SQ, Kwan CY, Tang F. The effect of aging on ANP levels in the plasma, heart, and brain of rats. J Gerontol A Biol Sci Med Sci. 1997;52:B250–4. doi: 10.1093/gerona/52a.5.b250. [DOI] [PubMed] [Google Scholar]
- 56.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–22. [PubMed] [Google Scholar]
- 57.Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, et al. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail. 2007;13:530–40. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]