Abstract
Background
We performed a meta-analysis to clarify whether the molecular detection of tumor cells or micrometastases in the lymph node (LN) indicates a high risk of disease recurrence and poor survival in negative pathologic lymph node status non-small cell lung cancer (NSCLC).
Methods
A literature search was performed using relevant keywords. We searched relevant studies from PubMed, Embase, and the Cochrane Library. Direct and indirect meta-estimates were generated using Review Manager software with fixed effects for the study. Study-to-study heterogeneity was summarized using I2 statistics and predictive intervals (PIs).
Results
Our analysis of eight eligible studies revealed that patients with lymph node micrometastases (LNMM) were associated with poor overall survival (OS) (HR, 1.98; 95 % CI, 1.50 to 2.62; p < 0.00001) and disease-free survival (DFS) (HR, 2.34; 95 % CI, 1.67-3.27; p < 0.00001).
Conclusion
LNMM is associated with an increased risk of disease recurrence and poor survival in patients with negative pathologic node negative NSCLC. Thus, these patients need to be carefully followed up after the initial pulmonary resection.
Keywords: Non–small-cell lung cancer, Lymph node micrometastases, Meta-analysis
Background
Lung cancer is the leading cause of cancer deaths worldwide, constituting 18 % of all cancer-related deaths. Up to 80–85 % of lung cancers are classified as non–small-cell lung cancer (NSCLC) [1]. Surgical treatment is an important therapy for early-stage non-small cell lung cancer (NSCLC). The extent of disease in NSCLC is staged according to the TNM classification [2], and because the stage at diagnosis is the principal prognostic indicator, accurate staging is essential for treatment decisions.
In theory, patients with early-stage non-small cell lung cancer can be cured by surgical resection alone. However, approximately 30 % of patients with pathologic stage 1 NSCLC have a recurrence of the tumor and die, despite complete surgical resection [3, 4]. This poor prognosis suggests the occurrence of micrometastasis, which often occurs before the primary operation and therefore cannot be assessed by routine clinical examinations and conventional histopathologic methods after the cancer has already spread to the regional lymph nodes. The detection of occult disease could help to identify those patients with lymph nodes micrometastases NSCLC who are at high risk for tumor recurrence and who might benefit from adjuvant therapy. Therefore, for an accurate prediction of prognosis, it is necessary to assess the lymph node status and to characterize micrometastasis.
The prognostic value of molecular tumor cell detection in patients with lymph node micrometastases NSCLC remains uncertain. To clarify this issue, we conducted a systematic review with meta-analyses of studies that evaluated the prognostic significance of molecular tumor cell detection in lymph nodes.
Methods
Search strategy
We performed a systematic search to identify articles published after 1996 that described the association between lymph node micrometastases of NSCLC and postoperative survival. Publications were included if they provided the most up-to-date analysis of at least one of the above-mentioned end points of interest. A literature search of Medline was performed using PubMed to identify published studies using the search terms (“lymph nodes micrometastases” OR “micrometastases”) AND (“non–small-cell lung cancer” OR “adenocarcinoma” OR “lung cancer”).
Before the beginning of this study, a rigorous protocol was developed according to the recommendations of the Cochrane Collaboration. The content of the documents identified by the search were then scrutinized by two researchers to determine eligibility for inclusion in the meta-analysis. All documents were included in our meta-analysis that evaluated the association of LNMM with disease-free survival (DFS) and overall survival (OS) of patients with negative pathologic node-negative NSCLC. The exclusion criteria were as follows: case report, clinical trials, letters to the editor and reviews without evaluation of the association of LNMM with overall survival (OS), and disease-free survival (DFS) of patients. Data from the included documents were entered into an electronic spreadsheet for analysis.
Statistical analysis
Synchronized extraction results were pooled statistically as effect estimates in meta-analyses. HRs and their corresponding SEs were extracted for individual time-to-event outcome parameters of each primary study. If an HR and the associated SE or CIs were not reported, we approximated the HR by using the statistical data provided in the article [5, 6].
The extracted HRs were pooled by using the generic inverse-variance method available in the Review Manager software 5.2.9 (Cochrane Collaboration, Oxford, UK). Statistical heterogeneity among the trials was evaluated using Cochran’s Q test (χ2 test) and the Higgins I2 statistic to determine the percentage of total variation among studies resulting from heterogeneity. If the I2 statistic was ≤ 50 % the fixed effect model was used to pool studies; if not, the random effects model was used. Publication bias was assessed with an Egger’s regression intercept [7].
Results
Search results
Eight eligible articles were analyzed [8–15]. All included documents were published between 1996 and 2011 (Tables 1 and 2). The details of the search are provided in Fig. 1.
Table 1.
The basic information about the included studies
| References | Definition of tumor-cell positivity | Detection method | Target gene/antigen | Follow-up (months) | Outcomes reported | stage | Multivariate analysis | |
|---|---|---|---|---|---|---|---|---|
| Median | Range | |||||||
| Chun-Dong Gu | LNMM | IHC | CK(AbAE1/A) | 44 | 4 –60 | OS | I | Yes |
| Kazuhito Dobashi | LNMM | IHC | CK(AbAE1/A) | 13.6 | 1-60 | OS | pN0 | Yes |
| B. Passlick | LNMM | IHC | CK | NR | NR | OS | pN0 | Yes |
| Jakob R. Izbicki, | LNMM | IHC/FCM | Ber-Ep-4 | NR | 3-40 | DFS | pN0 | Yes |
| By Toshihiro Osaki | LNMM | IHC | CK(AbAE1/A) | 35.8 | 0.1-90.6 | OS | I | Yes |
| Kosei Yasumoto | LNMM | IHC | CK2 | 48.2 | 1-64 | OS/DFS | I | Yes |
| Valerie W. Rusch | LNMM | IHC | CK(AE-1) | NR | 0-60 | OS/DFS | pN0 | Yes |
| Wang Zhou | LNMM | RT-PCR | MUC1 | NR | 0-36 | OS | pN0 | Yes |
Abbreviations: LMMM lymph node micrometastases, IHC immunohistochemistry, CK cytokeratin, DFS disease- free survival, OS overall survival, NR not reported, RT-PCR Reverse transcription-polymerase chain reaction. MUC1, polymorphic epithelial mucin 1
Table 2.
Demographic data
| Author | Year | LNM+ | LNM- | HR(LNM+ VS LNM-) | 95 %CI(Lower-upper) | In(HR) | SE(InHR) | Outcomes reported |
|---|---|---|---|---|---|---|---|---|
| Chun-Dong Gu | 2002 | 22 | 27 | 2.12 | 0.53-8.41 | 0.75 | 0.7 | OS |
| Kazuhito Dobashi | 1997 | 14 | 17 | 5.1 | 0.61-42.66 | 1.63 | 1.08 | OS |
| B. Passlick | 2001 | 27 | 125 | 2.5 | 1.4-4.6 | 0.9 | 0.3 | OS |
| Jakob R. Izbicki | 1996 | 16 | 51 | 4.96 | 1.65-14.87 | 1.6 | 0.56 | DFS |
| By Toshihiro Osaki | 2002 | 32 | 83 | 1.7 | 0.57-5.09 | 0.53 | 0.56 | OS |
| Kosei Yasumoto | 2003 | 34 | 182 | 2.77 | 0.94-8.17 | 1.02 | 0.55 | OS |
| Kosei Yasumoto | 2003 | 34 | 180 | 3.06 | 1.38-6.78 | 1.12 | 0.41 | DFS |
| Wang Zhou | 2003 | 16 | 42 | 1.84 | 0.68-4.93 | 0.61 | 0.5 | OS |
| Valerie W. Rusch | 2011 | 130 | 450 | 1.63 | 1.13-2.36 | 0.49 | 0.19 | DFS |
| Valerie W. Rusch | 2011 | 130 | 450 | 1.59 | 1.13-2.23 | 0.46 | 0.17 | OS |
Fig. 1.
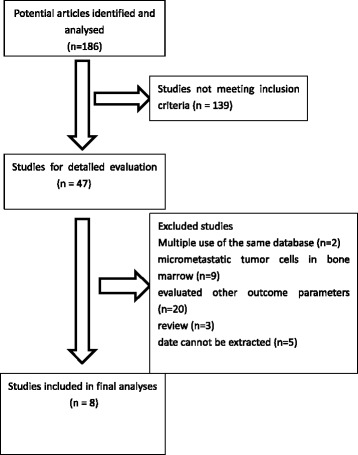
Selection diagram for studies
Impact of LNMM status on OS
The impact of LNMM status on OS was assessed with the generic inverse-variance method available in the Review Manager software in five documents. The analysis identified a significantly different positive correlation between the presence of isolated tumor cells in LN at the time of diagnosis and a shortened overall survival duration (HR, 1.98; 95 % CI, 1.50 to 2.62; p < 0.00001). There was no evidence of statistical heterogeneity (I2 = 0 %, χ2 = 2.28, df = 6, P = 0.89) (Fig. 2).
Fig. 2.
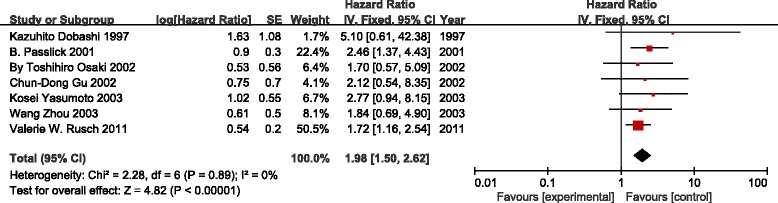
Meta-analyses on the association of molecular tumor cell detection in regional lymph nodes with overall survival
Impact of LNMM status on DFS
Only three documents reported the incidence of disease-free recurrence. Meta-analyses confirmed the prognostic significance of molecular tumor-cell detection in LN for the outcomes of DFS (HR, 2.34; 95 % CI, 1.67-3.27; p < 0.00001). Statistical heterogeneity was not detected (I2 = 30 %, χ2 = 2.87, df = 2, P = 0.24) (Fig. 3).
Fig. 3.
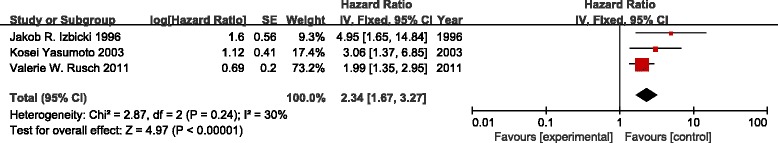
Meta-analysis on the association of molecular tumor cell detection in regional lymph nodes with disease-free survival
Discussion
This systematic review and meta-analysis showed that the molecular detection of tumor cells in regional lymph nodes is associated with an increased risk of disease recurrence and poor survival in patients with negative pathologic lymph node status NSCLC.
Lung cancer remains the most common cancer in the world, both in terms of new cases (1.8 million cases, 12.9 % of all cancers) and deaths (1.6 million deaths, 19.4 %) because of the high number of fatal cases [16]. The TNM staging system of lung cancer is widely used as a guide for predicting the prognosis. The presence of lymph node metastases along with T and M status represents the most accurate factor that is currently available for the prediction of prognosis in patients who undergo complete surgical resection. However, a significant proportion of patients with early stage NSCLC who undergo potentially curative resections subsequently relapse and die. This suggests that occult micrometastasis can exist at the time of surgery; the rate is clearly underestimated by current clinical staging examinations and conventional histopathologic methods. A nodal micrometastasis was defined according to the definition of the International Union Against Cancer (UICC; i.e. as a single or small number of tumor cells that are 0.2 mm in dimension [ITCs] and as tumor deposits < 2.0 mm but greater than 0.2 mm in dimension [MMs]) [17]. If the UICC definition was not applied or mentioned explicitly, single or clustered cells that were detected in molecular analyses were also considered as occult disease. In this study, we identified occult micrometastatic tumor cells in pN0 lymph nodes in half of the patients with completely resected stage 1 NSCLC using an immunohistochemical staining assay. The patients with lymph node micrometastasis had a poorer prognosis than the patients without micrometastasis by univariate or multivariate analyses; the prognostic impact was independent from the TNM staging system [8].
For this meta-analysis, we attempted to closely follow the recommendations presented by the Cochrane Collaboration. We pre-specified a strict study protocol, and we searched documents from several international conferences, electronic databases and reference research for relevant trials. The language was not restricted. Eight documents, which were published worldwide from 1996 to 2011, were included in the meta-analysis. Despite the number of documents that have been reported and the well-explained risk of patients with LNMM, the prognostic significance of LNMM of patients with NSCLC has remained highly undetermined. Therefore, we pooled the analysis of all documents that indicated a strong evidence of the independent, adverse prognostic impact of LNMM regarding OS and DFS at the time of the initial diagnosis of operable NSCLC.
Micrometastases disease is the significant cause of mortality from solid tumors. Tumor cells that are detected molecularly in the LN of patients with NSCLC are subjected to one of three fates: death, dormancy, or proliferation [18]. Most metastatic cells die in this hostile microenvironment [19, 20], or they balance the growth rates with the death rates [21]. Other cells adapt by responding to stimuli from the microenvironment.
The poor prognostic significance of LNMM regarding OS and DFS likely occurs because the tumor cells that survive in the LN can completely escape dormancy and begin to proliferate as soon as they reach their destination. Alternately, some of the tumor cells begin to proliferate later after an unknown situation that permits them to overcome inhibitory effects at the metastatic site [18]. Therefore, these tumor cells become viable micrometastases rather than shed tumor cells with a limited life span. Lymph node micrometastasis, as well as overt lymph node metastasis, does not necessarily reflect lymphogenous spread, but it can signal the early phase of hematogenous systemic tumor cell dissemination.
This meta-analysis has shown that the detection of lymph node occult micrometastatic tumor cells is helpful in predicting recurrence and the prognosis of patients with early stage NSCLC. It would also be possible to select patients who might benefit from adjuvant chemotherapy., postoperative adjuvant chemotherapy is not a routine standard therapy for patients with completely resected NSCLC because of its unreliable results for the improvement of prognosis. However, numerous trials, including two randomized trials [22, 23], of induction chemotherapy for stage 3A NSCLC have shown that it was feasible and indicated higher response rates and a survival advantage. Additionally, LNMM patients with a minimal amount of residual tumor could respond better to chemotherapy.
This study has limitations because of the sample size. Although we contacted each author to supplement the missing data, the number of included studies was insufficient to perform some of the preplanned subgroup analyses, and only 3 subgroup studies were included in our analysis. Consequently, we could not identify the effect modifiers that could be attributable to the low statistical power.
Conclusions
In conclusion, there is evidence that molecular tumor cell detection in regional lymph nodes indicates poor prognosis in node-negative NSCLC. Despite a prognostic impact of tumor cell detection across the applied detection assays, standardized protocols should be used in future studies that use standardized end points and adhere to the UICC definition of tumor cells and micrometastasis. By using such protocols, it should be clarified within prospective randomized trials whether patients with occult lymph node disease benefit from adjuvant chemotherapy. Furthermore, the findings of this study encourage efforts to identify subpopulations of disseminated cells that put patients at a particular risk of disease recurrence and poor survival.
Footnotes
Xu Feng Deng and Li Jiang are co-first authors.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
JM and JD contributed substantially to the conception and design of this meta-analysis and gave final approval of the version of the analysis to be published. XD, QL and DZ contributed to the analysis and interpretation of all data and drafted the article. BH critically reviewed and revised the article for important intellectual content. LJ and KC reviewed the article in the final reviewed process. All authors read and approved the final manuscript.
References
- 1.Haaland B, Tan PS, de Castro GJ, Lopes G. Meta-analysis of first-line therapies in advanced non-small-cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol. 2014;9(6):805–11. [DOI] [PMC free article] [PubMed]
- 2.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111(6):1710–7. [DOI] [PubMed]
- 3.Martini N, Bains MS, Burt ME, Zakowski M F, McCormack P, Rusch V W, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109(1):120–9. [DOI] [PubMed]
- 4.Strauss GM, Kwiatkowski DJ, Harpole DH, Lynch TJ, Skarin AT, Sugarbaker DJ. Molecular and pathologic markers in stage I non-small-cell carcinoma of the lung. J Clin Oncol. 1995;13(5):1265–79. [DOI] [PubMed]
- 5.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. [DOI] [PubMed]
- 6.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed]
- 7.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed]
- 8.Gu CD, Osaki T, Oyama T, Inoue M, Kodate M, Dobashi K, et al. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer: impact on recurrence and Survival. Ann Surg. 2002;235(1):133–9. [DOI] [PMC free article] [PubMed]
- 9.Passlick B. Micrometastases in non-small cell lung cancer (NSCLC). Lung Cancer-J Iaslc. 2001;34 Suppl 3:S25-9. [DOI] [PubMed]
- 10.Dobashi K, Sugio K, Osaki T, Oka T, Yasumoto K. Micrometastatic P53-positive cells in the lymph nodes of non-small-cell lung cancer: prognostic significance. J Thorac Cardiovasc Surg. 1997;114(3):339–46. [DOI] [PubMed]
- 11.Izbicki JR, Passlick B, Hosch SB, Kubuschock B, Schneider C, Busch C, et al. Mode of spread in the early phase of lymphatic metastasis in non-small-cell lung cancer: significance of nodal micrometastasis. J Thorac Cardiovasc Surg. 1996;112(3):623–30. [DOI] [PubMed]
- 12.Osaki T, Oyama T, Gu CD, Yamashita T, So T, Takenoyama M, et al. Prognostic impact of micrometastatic tumor cells in the lymph nodes and bone marrow of patients with completely resected stage I non-small-cell lung cancer. J Clin Oncol. 2002;20(13):2930–6. [DOI] [PubMed]
- 13.Yasumoto K, Osaki T, Watanabe Y, Kato H, Yoshimura T. Prognostic value of cytokeratin-positive cells in the bone marrow and lymph nodes of patients with resected nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg. 2003;76(1):194–201, 202. [DOI] [PubMed]
- 14.Rusch VW, Hawes D, Decker PA, Martin S E, Abati A, Landreneau R J, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol. 2011;29(32):4313–9. [DOI] [PMC free article] [PubMed]
- 15.Wang Z, Liu XY, Liu FY, Chen JH. Lymph Node Occult Micrometastasis in Patients with Non- Small Cell Lung Carcinoma: Genetic Diagnosis and Its Impact on Prognosis. Cancer. 2003;22(11):1204–1208. [PubMed] [Google Scholar]
- 16.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. [DOI] [PubMed]
- 17.LH S, MK G, C W. UICC;TNM Classification of Malignant Tumours, 7th Edition: Wiley-Blackwell; 2009. 336 p.
- 18.Wieder R. Insurgent micrometastases: sleeper cells and harboring the enemy. J Surg Oncol. 2005;89(4):207–10. [DOI] [PubMed]
- 19.Wong CW, Lee A, Shientag L, Yu J, Dong Y, Kao G, et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 2001;61(1):333–8. [PubMed]
- 20.Larmonier N, Ghiringhelli F, Larmonier CB, Moutet M, Fromentin A, Baulot E et al. Freshly isolated bone marrow cells induce death of various carcinoma cell lines. Int J Cancer. 2003;107(5):747–56. [DOI] [PubMed]
- 21.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1(2):149–53. [DOI] [PubMed]
- 22.Roth JA, Fossella F, Komaki R, Ryan M B, Putnam JB Jr, Lee J S, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86(9):673–80. [DOI] [PubMed]
- 23.Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, Canto A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330(3):153–8. [DOI] [PubMed]


