Abstract
The aggregation of the 37-amino acid polypeptide islet amyloid polypeptide (IAPP, amylin), as either insoluble amyloid or as small oligomers, appears to play a direct role in the death of pancreatic β-islet cells in type 2 diabetes. It is believed that inhibiting the aggregation of IAPP may slow down, if not prevent entirely, the progression of this disease. Extracts of thirteen different common fruits were analyzed for their ability to prevent the aggregation of amyloidogenic IAPP. Thioflavin T binding, immuno-detection and circular dichroism assays were performed to test the in vitro inhibitory potential of each extract. Atomic force microscopy was used to visualize the formation of amyloid fibrils with and without each fruit extract. Finally, extracts were tested for their ability to protect living mammalian cells from the toxic effects of amyloid IAPP. Several fruits showed substantial ability to inhibit IAPP aggregation and protect living cells from toxic IAPP amyloid.
Keywords: Amyloid Inhibition, Diabetes, Natural Product Therapeutics
1. Introduction
The natural products isolated from fruit extracts have been associated with many positive health benefits. These health benefits span a variety of fields, showing beneficial functionalities for anti-cancer (Chia-Hung, Chi-Chou, Hsu, Kao, & Wang, 2015; Olsson, Gustavsson, Andersson, Nilsson, & Duan, 2004; Zunino, Zhang, Seeram, & Storms, 2010), anti-aging (Shukitt-Hale, Carey, Jenkins, Rabin, & Joseph, 2007), and anti-obesity (Chaudhary et al., 2014) to name a few. Previous studies have indicated that several individual fruits are capable of inhibiting the formation of protein aggregates associated with amyloid-linked diseases, such as Alzheimer’s disease and type 2 diabetes (Li, Jang, Sun, & Surh, 2004; Park; Zhu, Bickford, Sanberg, Giunta, & Tan, 2008). However, to the best of our knowledge, no study has been performed to directly compare the efficacy of multiple fruits, spanning several fruit families, at inhibiting amyloid formation. In this work, thirteen common fruits were analyzed for their ability to prevent the aggregation of amyloidogenic islet amyloid polypeptide (IAPP), the amyloid protein associated with the progression of type 2 diabetes. The natural propensity of IAPP to aggregate into toxic amyloidogenic species is believed to occur in a similar fashion as other amyloidogenic proteins, such as Aβ42 in Alzheimer’s disease and α-synuclein in Parkinson’s disease. Fruit extracts capable of inhibiting the aggregation of IAPP, considered one of the most amyloidogenic protein known (Cao, Abedini, & Raleigh, 2013a), are likely to function as inhibitors of other amyloid proteins as well (Montane, Klimek-Abercrombie, Potter, Westwell-Roper, & Verchere, 2012).
The incidence of type 2 diabetes within developed nations continues to increase. The American Diabetes Association estimates that 25.8 million Americans have diabetes (approximately 8.3% of the population ("Type 2 Diabetes Facts and Figures," 2011)), with nearly 2 million new cases diagnosed each year. While obesity is a factor in the development of type 2 diabetes, over 70% of obese people do not have diabetes, suggesting that other factors influence the progression of this disease (cdc-national center for health statistics, National Data Brief, No. 82, Jan 2012) (Ogden, Carroll, Kit, & Flegal; Ogden, Carroll, Kit, & Flegal).
One possible factor linked to the progression of type 2 diabetes is the aggregation of the amyloidogenic peptide islet amyloid polypeptide (IAPP) (Montane et al., 2012). This 37-amino acid polypeptide is co-secreted from pancreatic β-islet cells with insulin. During the progression of type 2 diabetes, IAPP aggregates into a variety of different amyloidogenic states (Abedini & Schmidt, 2013; Apostolidou, Jayasinghe, & Langen, 2008; Hull, Westermark, Westermark, & Kahn, 2004; Kahn, Andrikopoulos, & Verchere, 1999). While the exact structure and nature of these aggregates remains to be determined, it is clear that some of these aggregates are highly toxic to cells (Andrews, Inayathullah, Jayakumar, & Malar, 2009; Cao et al., 2013b; Nanga, Brender, Xu, Veglia, & Ramamoorthy, 2008). For example, Eisenberg and coworkers recently suggested that the most toxic form of IAPP may self-assemble into a cylindrin structure (Laganowsky et al., 2012; Liu et al.). These oligomeric structures seem to be the toxic form of the amyloid proteins. While the exact mechanism of toxicity remains unknown, two theories suggest that the oligomers either (1) form pores in the cellular membranes or (2) disrupt cellular membranes through membrane fragmentation (Brender, Salamekh, & Ramamoorthy, 2012). Ramamoorthy and coworkers have used NMR to solve the structures of several human and rat IAPP peptides under a variety of conditions, including within micelles (Brender, Durr, Heyl, Budarapu, & Ramamoorthy, 2007; Brender et al., 2008; Nanga et al., 2009; 2008; Soong, Brender, Macdonald, & Ramamoorthy, 2009). Regardless of the exact structure of the IAPP aggregates, their toxicity to cells may be the root cause of the progression of type 2 diabetes (Ritzel, Meier, Lin, Veldhuis, & Butler, 2007). Likewise, the toxicity of these aggregates appears to preclude the use of β-cell transplantation therapies (Potter et al.). The possibility exists that preventing the formation of toxic IAPP aggregates could slow, if not prevent entirely, the progression of type 2 diabetes. Additionally, inhibiting the formation of these toxic aggregates may make future pancreatic β-cell transplantation therapies a reality.
The fruits described in this study were chosen for analysis because of their abundant availability within the United States and their distribution across several different fruit families. Because of the considerable levels of carbohydrates within these fruits (a fact that prevents many diabetic patients from ingesting large amounts of these fruits on a regular basis), we chose an extraction method that removed over 99% of the carbohydrates from the samples. The resulting fruit extracts were tested for their in vitro ability to prevent the formation of amyloid aggregates and their ex vivo ability to protect living mammalian cells from toxic IAPP amyloid.
2. Materials and Methods
2.1 Preparation of Fruit Extracts
For each individual whole fruit, 100 grams was ground using a mortar and pestle in the presence of 100 mL of ethyl acetate. The resulting slurry was filtered and the ethyl acetate layer isolated. The ethyl acetate was removed via speed vacuum yielding approximately 8 aliquots of dehydrated solid extracts. These dehydrated extracts were stored at −20°C. Extracts were rehydrated by resuspending each aliquot in 150 µL 20 mM tris buffer pH 7.40 (standard solution) or 1.50 mL of the same buffer (yielding samples 10× diluted).
2.2 Preparation of IAPP stock solutions
IAPP stock solutions were prepared by dissolving 1 mg of synthetic amylin (Anaspec Corp. Fremont, CA, USA) in 8 mL of hexafluorisopropanol (HFIP, Sigma Aldrich, St. Louis, MO, USA). To fully disaggregate the IAPP, the sample was placed in a sonicating water bath for 5 min. This stock solution was stored at −80°C for up to two months.
2.3 Thioflavin T binding (ThT) assays
HFIP was removed from the IAPP stock solution under speed-vacuum. The dry IAPP sample was resuspended in 20 mM tris buffer pH 7.40 containing each individual fruit extract. The final in-solution concentration of IAPP was 106 µM. Aggregation was initiated by incubating samples at 37°C with shaking (200 rpm). At designated time points, a 17 µL aliquot of each sample was removed and mixed with 663 µL of 50.0 µM thioflavin T in 20 mM Tris buffer pH 7.40. The IAPP/thioflavin T mixture was incubated at room temperature in the dark for 2 min before recording the thioflavin T fluorescence emission spectrum (Ex450nm) using a Hitatchi F-7000 fluorescence spectrophotometer.
2.4 Atomic Force Microscopy (AFM)
Synthetic IAPP was prepared as described above and incubated with shaking in the presence of individual fruit extracts. After 40 min of incubation, 20 µL of each sample were deposited onto freshly cleaved mica. After incubation at room temperature for 5 min, the mica was washed with 200 µL sterile-filtered water and allowed to air dry. The samples were scanned using an MFP-3D atomic force microscope (Asylum Research, Santa Barbara, CA, USA) set on A/C mode and a 240 µm silicon cantilever (Olympus Corporation of the Americas, PA, USA). Images shown are the raw data with no flattening.
2.5 Circular Dichroism (CD)
CD spectroscopy was performed using a J-815 CD Spectrometer (Jasco Inc, Easton, MD, USA) with quartz cuvettes of 1 mm pathlength. Samples were scanned from 190 nm to 250 nm. In order to reduce interference by the fruit extracts, 10× diluted fruit samples were mixed with IAPP. The final in-solution IAPP concentration was 76.8 µM. IAPP, in the presence and absence of each fruit sample, was incubated for 40 min at 37°C with shaking (200 rpm). These rigorous conditions reproducibly yielded IAPP fibrils (visible with Atomic Force Microscopy) with CD spectra having a single minimum at 218 nm.
2.6 Immunoblot dot test
Samples of IAPP were incubated with individual fruit extracts as described in the thioflavin T assay above. At designated time intervals, 2uL of each IAPP sample (with or without a fruit extract) were placed on a nitrocellulose membrane. The resulting membrane was allowed to air dry before blocking in 2.5% BSA in TBST buffer for 1 h at room temperature. After blocking, the membrane was cut in half, with one half probed with Millipore’s A11 antibody (AB9234) and the other half probed with Millipore’s Fibril OC antibody (AB2286) (Millipore, Billerica, MA, USA). Both antibodies were applied at a 1:1000 (v,v) dilution for 1 h at room temperature. The membranes were washed three times for 5 min each with TBST buffer. The membranes were probed with donkey anti-rabbit secondary antibody conjugated to HRP (1:2000 v,v) for 1 h at room temperature. The membranes were washed three times with TBST buffer. A final wash with TBS for 5 min was conducted before developing using GE’s Amersham ECL reagent with a 10 min exposure time. Images were taken using the Bio-Rad Imaging System.
2.7 HeLa cell rescue
HeLa cells were incubated with 15 µM of synthetic IAPP prepared as described above. Equal amounts of HeLa cells were plated in triplicate and incubated overnight in 96-well plates. After 24 h, F-12K media with phenol red was replaced by DMEM/F12 (1:1 v,v) without phenol red. Hexafluoroisopropanol (HFIP) was evaporated from IAPP using a centrivap concentrator (LabOnco). IAPP was re-suspended in DMEM/F12 media, the pellets were then mixed into the media by vortexing (Vortex Genie Mixer) for 2 min. Stock solutions of each fruit extract were diluted with 25 mM Tris Buffer (pH 7.4) and added to the appropriate wells. The treated cells were incubated for 22 h at 37°C. Post-incubation, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide) was added into each well and incubated at 37°C for 2 h. Formazan crystals, formed on the bottom of the wells, were re-suspended in a solubilization buffer (20% SDS and 50% dimethylformamide). The absorbance in each well was taken at 570 nm using a BioRad 550 microplate reader. HeLa cells and media were obtained from ATCC. All incubations took place in a water jacketed incubator in 5% carbon dioxide at 37°C (Shell Lab). The average percentage of cell viability was calculated for each of the conditions.
3. Results
The ethyl-acetate extracts of several common fruits were analyzed to identify those having the greatest potential for inhibiting the aggregation of amyloidogenic IAPP. Each fruit extract was initially analyzed for its ability to prevent thioflavin-T (ThT) binding to IAPP (Figure 1). ThT binding is a facile method for detecting and quantifying amyloid formation. ThT shows little fluorescence emission when solubilized in an aqueous solution. However, ThT fluorescence increases in a concentration-dependent manner when in the presence of amyloid (LeVine, 1993).
Figure 1.
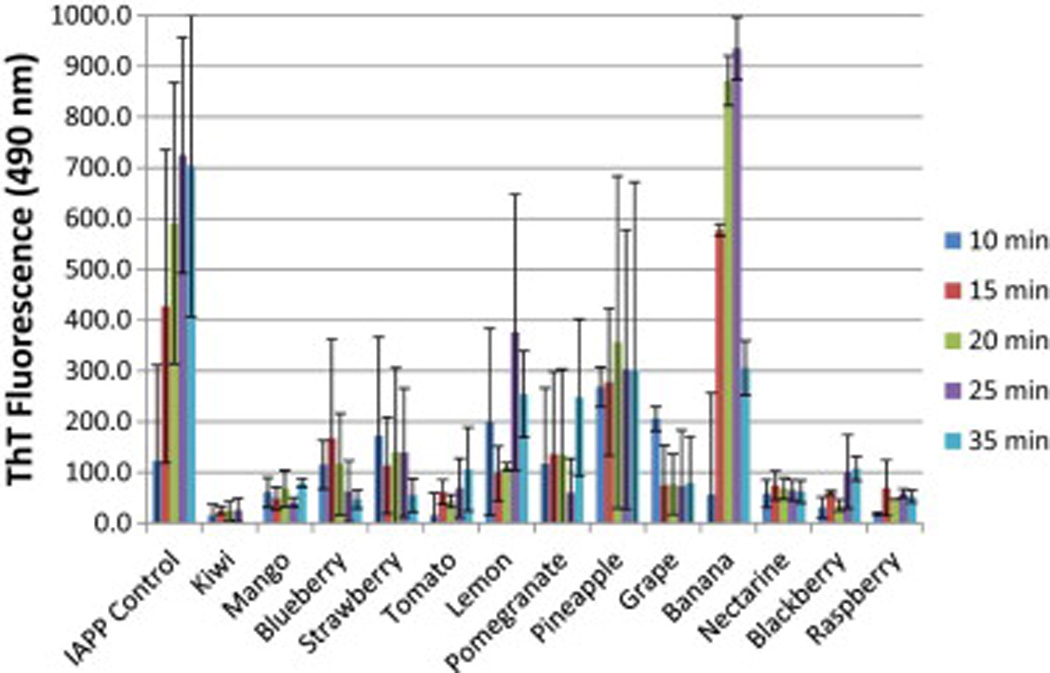
Thioflavin T fluorescence of IAPP alone and IAPP in the presence of each fruit extract. Fluorescence was measured with an excitation wavelength of 450 nm and an emission wavelength of 488 nm. The solution concentration of IAPP was 106 µM for each sample. Data is the average of at least three data sets.
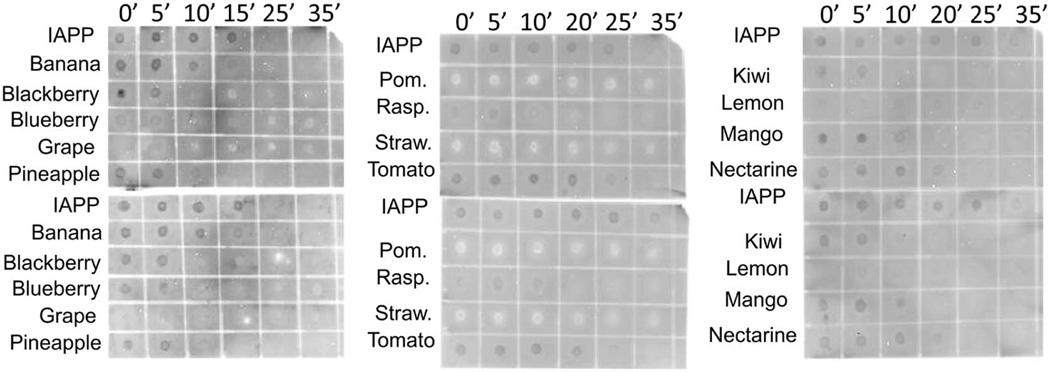
IAPP is believed to form a variety of different amyloidogenic aggregates with varying degrees of cellular toxicity (Andrews et al., 2009; Gazit, 2005; Haataja, Gurlo, Huang, & Butler, 2008). IAPP with and without each fruit extract was probed with the A11 antibody and the Fibril OC antibody (Figure 2). The A11 antibody is an anti-oligomer antibody that specifically binds to small soluble oligomers, believed to be the more toxic form of IAPP amyloid. The Fibril OC antibody is an anti-fibril antibody that specifically binds to the fibrillar form of IAPP. Dark spots on the immuno-detection assays indicated the presence of the amyloidogenic species, while colorless spots and white spots both indicated the absence of amyloidogenic species.
Figure 2.
Immunoblot dot test. Samples containing IAPP alone or IAPP with each fruit extract were incubated at 37°C with shaking (200 rpm). 2 µL of each sample was dotted onto nitrocellulose membrane and probed with A11 antibody (top panels) or Fibril OC antibody (bottom panels). Dark spots indicate where the amyloid-binding antibodies detected amyloid deposits. Blank and light spots indicate where amyloid was not present.
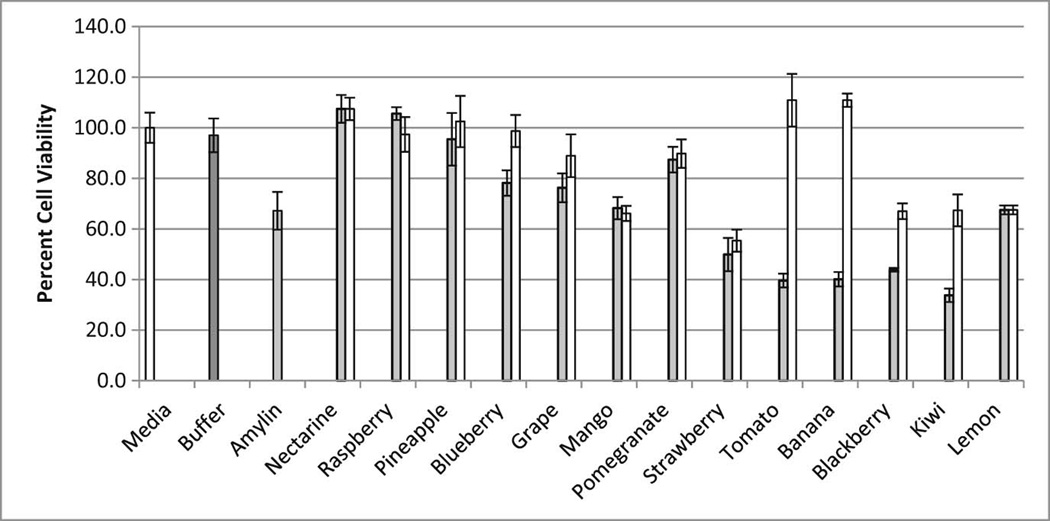
A more direct method for assessing amyloid inhibition potential is through the visualization of amyloidogenic fibrils. Atomic force microscopy (AFM) was used to visualize the formation of amyloid fibrils in the presence and absence of each fruit extract (Figures 3 and 4). The use of AFM allows for a direct detection of an amyloidogenic species in the presence and absence of each fruit extract. Likewise, circular dichroism (CD) spectroscopy was used to detect the conversion of unstructured IAPP peptides into the β-sheet-rich amyloidogenic species of IAPP (Figure 5). Finally, to test the ability of each fruit extract to protect living cells from IAPP, the MTT assay was performed using HeLa cells (Figure 6). The MTT levels of HeLa cells alone were set as 100%. The addition of IAPP (labeled as amylin) to the HeLa cells decreased the cell viability to 67% in less than 24 h. The 25mm Tris Buffer was tested to ensure that there was no conflict with cell viability.
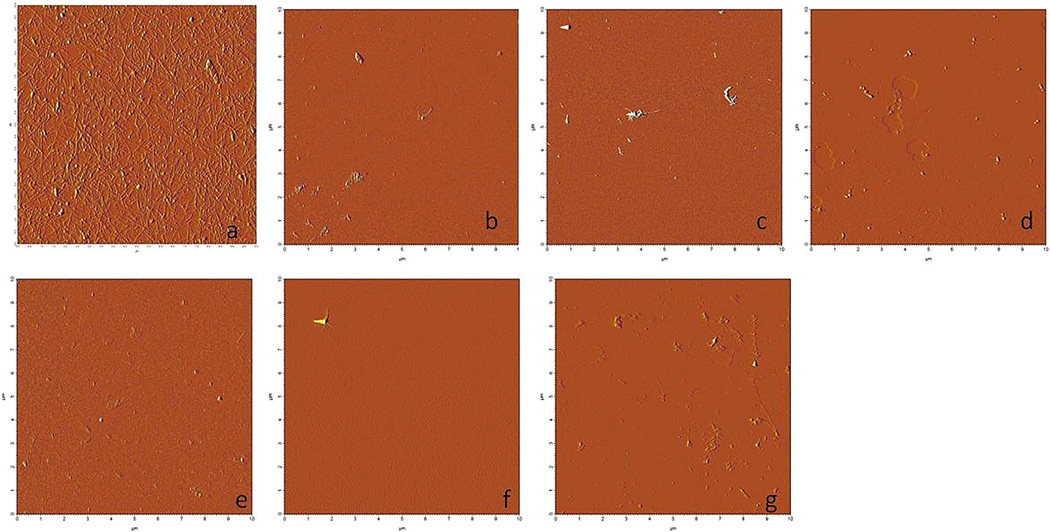
Figure 3.
Atomic force microsope images of fruits showing modest to negligible ability to inhibit IAPP fibril formation. a) IAPP alone b) banana c) kiwi d) lemon e) mango f) nectarine g) strawberry h) tomato.
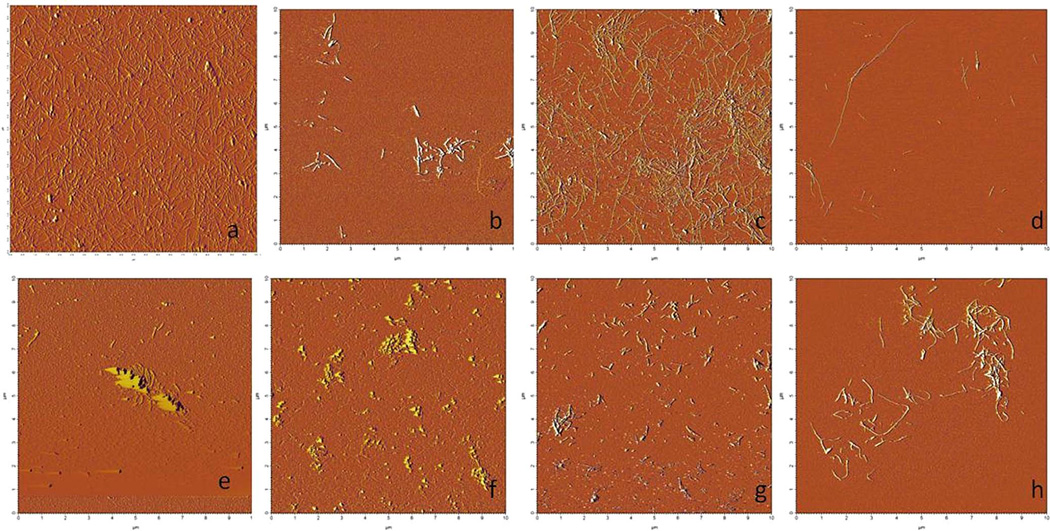
Figure 4.
Atomic force microsope images of fruits showing significant ability to inhibit IAPP fibril formation. a) IAPP alone b) blackberry c) blueberry d) grape e) pineapple f) pomegranate g) raspberry.
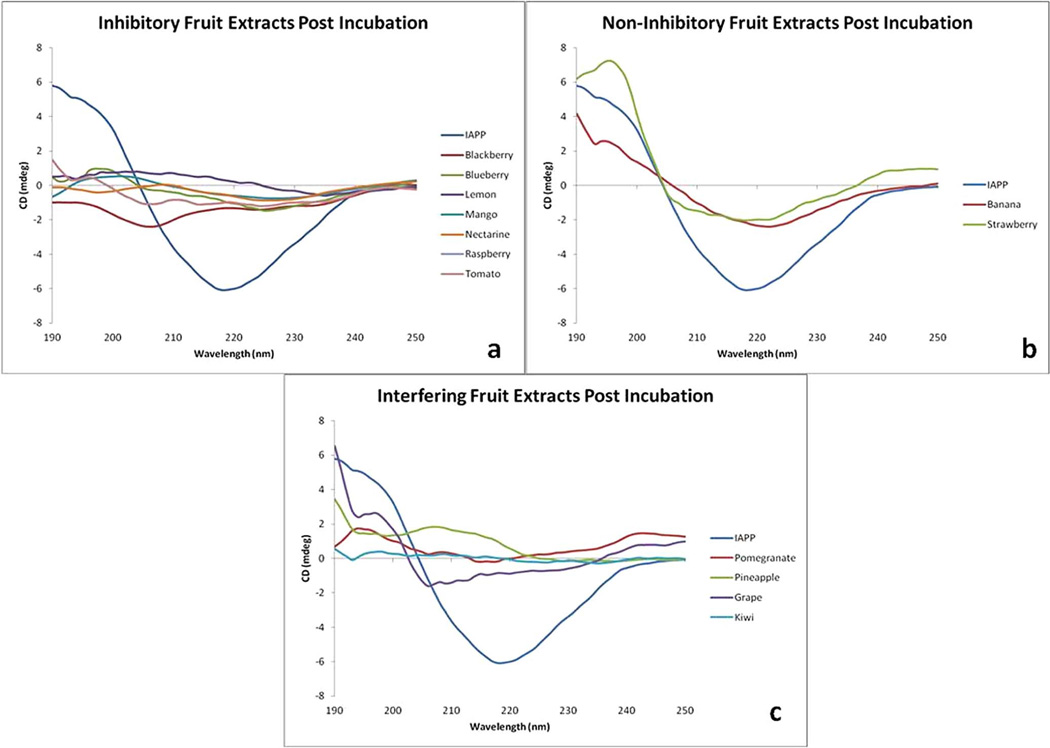
Figure 5.
Circular Dichroism of IAPP after 40 minute incubation. (a) Fruits showing significant ability to prevent the conversion of IAPP to β-sheet conformation (b) Fruits showing negligible ability to prevent the conversion of IAPP to β-sheet conformation and (c) Fruits with UV-active extracts that interfered with monitoring of the IAPP β-sheet transition.
Figure 6.
Relative cell viability (%) in the presence of IAPP and/or fruit extracts. The “Media” and “Buffer” samples represent cell viability in the presence of media alone and media with Tris Buffer respectively (no IAPP was included in these samples). “Amylin” shows the effect of IAPP on cell viability. Grey bars indicate samples with IAPP and the specified fruit extract. White bars correspond to the effect of each individual fruit extract alone (with no IAPP present) on the viability of cells.
4. Discussion
4.1 Thioflavin T binding
The time course data for ThT binding to IAPP amyloid (Figure 1) indicated that most of the fruit samples were capable of slowing and/or preventing the aggregation of IAPP under rigorous aggregate-forming conditions. The fruits showing the least inhibitory potential according to the ThT results were banana, pineapple, and lemon. The remaining fruit extracts showed significant inhibitory potential. Th T binding by amyloid is a useful diagnostic for gauging the ability of a potential therapeutic to prevent amyloid formation. However, it is possible that the lack of ThT fluorescence may be due to inhibition of ThT binding to the amyloid, rather than preventing amyloid formation (this has been experimentally observed in the case of EGCG competing with ThT for binding to IAPP) (Suzuki, Brender, Hartman, Ramamoorthy, & Marsh, 2012). For this reason, it is important to conduct additional experiments to identify true amyloid inhibitors and potential therapeutic agents.
4.2 Immuno-detection
We chose to perform immuno-detection assays to identify the amyloid species being inhibited by each fruit extract. The results (Figure 2) indicated that several fruits (banana, tomato, mango and nectarine) had little inhibitory potential, while the remaining fruits displayed some ability to prevent IAPP amyloid formation. These results also suggest that both IAPP oligomers and fibrils were inhibited in the presence of most of the fruit extracts. The fruit extracts showing the greatest inhibitory potential from these immuno-detection assays were grape, strawberry, pomegranate and raspberry (all of which were strong inhibitors according to the ThT results in Figure 1).
4.3 Atomic force microscopy
AFM, a direct test that allows the visualization of amyloidogenic fibrils, provided evidence suggesting that some fruit extracts were stronger inhibitors than others. Due to the fact that significant amounts of fibrils were observed, several fruit extracts (banana, kiwi, lemon, mango, nectarine, strawberry, and tomato) showed little ability to inhibit IAPP fibril formation (Figure 3). IAPP fibrils were found on every AFM sample in the presence of these non-inhibiting fruits. The remaining fruit extracts (blackberry, blueberry, grape, pineapple, pomegranate, and raspberry) showed significant ability to inhibit fibril formation (Figure 4) compared to IAPP in the absence of inhibitors. In every sample tested, high concentrations of the fruit extracts prevented fibril formation (Figure 4). The strongest inhibitors of fibril formation were the pomegranate, raspberry and blueberry extracts. When diluted 100×, these three extracts still prevented fibril formation in all samples tested. Whereas, extracts of the other inhibitor fruits (blackberry, grape and pineapple) began to lose potency after a 10× dilution of the extract (minor amounts of fibrils could be seen – data not shown).
4.4 Circular Dichroism
Like most amyloidogenic proteins, it is believed that the conversion of IAPP to an amyloidogenic state involves the formation of β-sheet secondary structure. It is well documented that the fibrillar form of IAPP forms a cross β-structure perpendicular to the axis of the fibril. This conversion from unstructured peptide to β-sheet was monitored via circular dichroism spectroscopy (CD). As IAPP adopts the β-sheet structure indicative of amyloidogenic aggregates, the CD signal displays a local minima at 218 nm. Fruit extracts found to prevent the conversion of IAPP into β-sheet rich structures are shown in Figure 5A. The CD signal of IAPP after incubation in the presence of inhibitory extracts (blackberry, blueberry, lemon, mango, nectarine, raspberry and tomato) resembles the CD signal of IAPP prior to incubation (data not shown). The fruit extracts of banana and strawberry (Figure 5B) showed little ability to prevent the conversion to β-sheet rich structures. Several fruit extracts (pomegranate, pineapple, grape, and kiwi) possessed CD-active extracts that interfered with the IAPP signal.
4.5 Cell Rescue
To test the potential of fruit extracts to protect living cells from toxic IAPP aggregates, the MTT assay was performed using HeLa cells (Figure 6). The MTT assay, which quantitates the oxidative capacity of HeLa cells after treatment with the respective fruit extract and/or IAPP, indicated that certain fruit extracts had greater protective qualities compared to others. The addition of nectarine, raspberry, and pineapple significantly rescued the HeLa cells (p < 0.05, t-test) from the toxic effects of IAPP. Tomato and banana extracts demonstrated the least potential for rescuing cells from IAPP toxicity. Several fruit extracts (strawberry, blackberry, lemon and kiwi) seemed to hinder cellular viability even in the absence of IAPP.
5. Conclusions
This comparative study evaluated the ability of common fruit extracts to inhibit the aggregation of IAPP into toxic amyloid species. These results indicate that many fruits possess the potential to inhibit IAPP aggregation, but several fruits were found to have significantly greater inhibitory activity. The fruit extracts with the greatest overall ability to prevent amyloid aggregation and simultaneously protect living cells from IAPP toxicity were the berries, raspberry and blueberry. Also showing substantial inhibitory activity were the pomegranate and grape extracts. Conversely, the banana and kiwi extracts showed virtually no inhibitory activity. The remaining fruits tested possessed moderate activity, showing mixed levels of aggregation inhibition in the various experiments performed.
The use of ethyl acetate as the extraction solvent yielded two beneficial outcomes. First, over 99% of the total carbohydrate of each extract remained within the aqueous phase (data not shown). Therefore, this choice of extraction solvent removed over 99% of the total sugar content. Because individuals suffering from type 2 diabetes must control their sugar intake, this extraction process could be used to produce a therapeutically viable form of each fruit. Second, the use of ethyl acetate pulls out and concentrates polyhydroxylated phenols and catechins, classes of compounds believed to possess a range of positive health benefits, including inhibition of amyloid formation.
From these results, we believe it is unlikely that the inhibitory component(s) for each fruit extract are the same. In our studies, the extracts from raspberry, blueberry and blackberry all performed well and were considered strong inhibitors of amyloid formation. Both raspberry and blackberry contain a substantial amount of flavanols such as EGCG, a known inhibitor of IAPP aggregation (Cao & Raleigh, 2012; Tang, 2007). Conversely, blueberry contains very little EGCG and flavanol content, yet was found to be an equally good inhibitor compared to raspberry and blackberry. Likewise, the amyloid inhibitory potential of each fruit extract was not necessarily linked to the anti-oxidant content of each fruit. Grapes, one of the strongest inhibitors of IAPP aggregation, have roughly the same antioxidant content as nectarines, bananas, and kiwis, the worst performers in this study.
The folding (or misfolding) process proteins undergo in their transition into toxic amyloid is not yet fully understood. However, it is believed that despite their differences in amino acid sequences and compositions, the majority of amyloid proteins undergo a similar aggregation pathway that leads to structurally similar amyloidogenic aggregates. Because of this, it is possible that substances capable of inhibiting the aggregation of one amyloid protein may also be capable of inhibiting others. Because IAPP is considered to be one of the most amyloidogenic proteins known, substances found to inhibit this amyloid protein may be potential therapeutics for other amyloid proteins, such as Aβ42 aggregation in Alzheimer’s disease or α-synuclein in Parkinson’s disease (Fox et al., 2010).
Highlights.
Comparative study to identify fruits having the greatest potential for preventing amyloid.
Extraction system described for producing bioactive samples lacking carbohydrates.
Several fruit extracts protected living cells from high doses of toxic IAPP.
Acknowledgments
Funding provided by the National Institutes of Health, NIDDKD (#R15DK094273-01) and the generosity of the LMU Women’s Leadership Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abedini A, Schmidt AM. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013;587:1119–1127. doi: 10.1016/j.febslet.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ME, Inayathullah NM, Jayakumar R, Malar EJ. Conformational polymorphism and cellular toxicity of IAPP and beta AP domains. J Struct Biol. 2009;166:116–125. doi: 10.1016/j.jsb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Apostolidou M, Jayasinghe SA, Langen R. Structure of alpha-helical membrane-bound human islet amyloid polypeptide and its implications for membrane-mediated misfolding. J Biol Chem. 2008;283:17205–17210. doi: 10.1074/jbc.M801383200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Durr UH, Heyl D, Budarapu MB, Ramamoorthy A. Membrane fragmentation by an amyloidogenic fragment of human Islet Amyloid Polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. Biochimica et Biophysica Acta. 2007;1768:2026–2029. doi: 10.1016/j.bbamem.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Lee EL, Cavitt MA, Gafni A, Steel DG, Ramamoorthy A. Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide. J Am Chem Soc. 2008;130:6424–6429. doi: 10.1021/ja710484d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Salamekh S, Ramamoorthy A. Membrane disruption and early events in the aggregation of the diabetes related peptide IAPP from a molecular perspective. Acc Chem Res. 2012;45:454–462. doi: 10.1021/ar200189b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Abedini A, Raleigh DP. Aggregation of islet amyloid polypeptide: from physical chemistry to cell biology. Curr Opin Struct Biol. 2013a;23:82–89. doi: 10.1016/j.sbi.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Abedini A, Wang H, Tu LH, Zhang X, Schmidt AM, Raleigh DP. Islet amyloid polypeptide toxicity and membrane interactions. Proc Natl Acad Sci U S A. 2013b;110:19279–19284. doi: 10.1073/pnas.1305517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Raleigh DP. Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry. 2012;51:2670–2683. doi: 10.1021/bi2015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Bhardwaj J, Seo HJ, Kim MY, Shin TS, Kim JD. Camellia sinensis fruit peel extract inhibits angiogenesis and ameliorates obesity induced by high-fat diet in rats. Journal of Functional Foods. 2014;7:479–486. [Google Scholar]
- Chia-Hung H, Chi-Chou H, Hsu L-S, Kao S-H, Wang C-J. Apple polyphenol inhibits colon carcinoma metastasis via disrupting snail binding to focal adhesion kinase. Journal of Functional Foods. 2015;12:80–91. [Google Scholar]
- Fox A, Snollaerts T, Casanova CE, Calciano A, Nogaj LA, Moffet DA. Selection for Nonamyloidogenic Mutants of Islet Amyloid Polypeptide (IAPP) Identifies an Extended Region for Amyloidogenicity. Biochemistry. 2010;49:7783–7789. doi: 10.1021/bi100337p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit E. Mechanisms of amyloid fibril self-assembly and inhibition. Febs Journal. 2005;272:5971–5978. doi: 10.1111/j.1742-4658.2005.05022.x. [DOI] [PubMed] [Google Scholar]
- Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: A long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Jang JH, Sun B, Surh YJ. Protective effects of oligomers of grape seed polyphenols against beta-amyloid-induced oxidative cell death. Ann N Y Acad Sci. 2004;1030:317–329. doi: 10.1196/annals.1329.040. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao M, Jiang L, Cheng PN, Park J, Sawaya MR, Pensalfini A, Gou D, Berk AJ, Glabe CG, Nowick J, Eisenberg D. Out-of-register beta-sheets suggest a pathway to toxic amyloid aggregates. Proc Natl Acad Sci U S A. 109:20913–20918. doi: 10.1073/pnas.1218792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montane J, Klimek-Abercrombie A, Potter KJ, Westwell-Roper C, Verchere CB. Metabolic stress, IAPP and islet amyloid. Diabetes Obes Metab. 2012;14(Suppl 3):68–77. doi: 10.1111/j.1463-1326.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- Nanga RP, Brender JR, Xu J, Hartman K, Subramanian V, Ramamoorthy A. Three-dimensional structure and orientation of rat islet amyloid polypeptide protein in a membrane environment by solution NMR spectroscopy. J Am Chem Soc. 2009;131:8252–8261. doi: 10.1021/ja9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanga RP, Brender JR, Xu J, Veglia G, Ramamoorthy A. Structures of rat and human islet amyloid polypeptide IAPP(1–19) in micelles by NMR spectroscopy. Biochemistry. 2008;47:12689–12697. doi: 10.1021/bi8014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. :1–8. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. :1–8. [PubMed] [Google Scholar]
- Olsson ME, Gustavsson KE, Andersson S, Nilsson A, Duan RD. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J Agric Food Chem. 2004;52:7264–7271. doi: 10.1021/jf030479p. [DOI] [PubMed] [Google Scholar]
- Park SY. Potential therapeutic agents against Alzheimer's disease from natural sources. Arch Pharm Res. 33:1589–1609. doi: 10.1007/s12272-010-1010-y. [DOI] [PubMed] [Google Scholar]
- Potter KJ, Abedini A, Marek P, Klimek AM, Butterworth S, Driscoll M, Baker R, Nilsson MR, Warnock GL, Oberholzer J, Bertera S, Trucco M, Korbutt GS, Fraser PE, Raleigh DP, Verchere CB. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci U S A. 107:4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes. 2007;56:65–71. doi: 10.2337/db06-0734. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey AN, Jenkins D, Rabin BM, Joseph JA. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol Aging. 2007;28:1187–1194. doi: 10.1016/j.neurobiolaging.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Soong R, Brender JR, Macdonald PM, Ramamoorthy A. Association of highly compact type II diabetes related islet amyloid polypeptide intermediate species at physiological temperature revealed by diffusion NMR spectroscopy. J Am Chem Soc. 2009;131:7079–7085. doi: 10.1021/ja900285z. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Brender JR, Hartman K, Ramamoorthy A, Marsh EN. Alternative pathways of human islet amyloid polypeptide aggregation distinguished by (19)f nuclear magnetic resonance-detected kinetics of monomer consumption. Biochemistry. 2012;51:8154–8162. doi: 10.1021/bi3012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JLaC. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. [Google Scholar]
- Type 2 Diabetes Facts and Figures. American Diabetes Association. 2011 [Google Scholar]
- Zhu Y, Bickford PC, Sanberg P, Giunta B, Tan J. Blueberry opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activation protein kinase. Rejuvenation Res. 2008;11:891–901. doi: 10.1089/rej.2008.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino SJ, Zhang YJ, Seeram NP, Storms DH. Berry fruit extracts inhibit growth and induce apoptosis of high-risk acute lymphoblastic leukemia cells in vitro. Journal of Functional Foods. 2010;2:187–195. [Google Scholar]