Abstract
Tremor is a common side effect of tacrolimus correlated with peak‐dose drug concentration. LCPT, a novel, once‐daily, extended‐release formulation of tacrolimus, has a reduced C max with comparable AUC exposure, requiring a ~30% dose reduction vs. immediate‐release tacrolimus. In this phase 3b study, kidney transplant recipients (KTR) on a stable dose of tacrolimus and with a reported clinically significant tremor were offered a switch to LCPT. Tremor pre‐ and seven d post‐conversion was evaluated by independent, blinded movement disorder neurologists using the Fahn–Tolosa–Marin (FTM) scale and by an accelerometry device; patients completed the QUEST (quality of life in essential tremor) and the Patient Global Impression of Change. There were 38 patients in the mITT population. A statistically and clinically significant improvement in tremor (FTM score, amplitude as measured by the accelerometry device and QOL [p‐values < 0.05]) resulted post‐conversion. Change in QUEST was significantly (p = 0.006) correlated (R = 0.44) with change in FTM; 78.9% of patients reported an improvement after switching to LCPT (p < 0.0005). To our knowledge this is the first trial in KTR that utilizes a sophisticated and reproducible measurement of tremor. Results suggest LCPT is associated with clinically meaningful improvement of hand tremor and may be an alternative management approach in lieu of further dose reduction of immediate‐release tacrolimus for patients experiencing tremor.
Keywords: adverse events, Envarsus, extended‐release, kidney transplantation, LCP‐Tacro, MeltDose, Prograf, tacrolimus, tremor
Tacrolimus twice‐daily capsules (Prograf®; Astellas Pharma US, Inc., Northbrook, IL, USA) is the most widely used calcineurin inhibitor in contemporary kidney transplantation, due to its effectiveness in preventing acute rejection 1, 2. However, tacrolimus possesses a narrow therapeutic index that requires individual dose titration to achieve satisfactory efficacy while minimizing dose‐related toxicities 3. One of the most common tacrolimus‐induced side effects is tremor, occurring in 34%–54% of kidney transplant recipients 4, 5. Other neurologic side effects reported with tacrolimus include headaches, insomnia, nightmares, vertigo, dysesthesia, photophobia, and mood disturbances, as well as more severe manifestations such as akinetic mutism, seizures, cortical blindness, focal deficits, psychosis, encephalopathy, and reduced cognitive ability 5, 6, 7, 8. The exact mechanism by which tacrolimus induces neurological adverse events (AEs) remains unknown; however, it has been observed that many symptoms occur or are most pronounced at peak serum tacrolimus blood concentrations and symptoms generally improve when the tacrolimus dose is reduced or when tacrolimus is withdrawn 6, 9, 10. Tremor is associated with a significant decrease in the quality of life (QOL) of transplant patients 11, 12. Furthermore, side effects of immunosuppressant drugs are associated with non‐adherence to medication regimens in transplant patients 11, 13, 14.
LCP‐Tacro tablets (Envarsus XR™; Veloxis Pharmaceuticals, Hørsholm, Denmark) is an extended‐release formulation of tacrolimus designed for once‐daily administration. LCP‐Tacro is associated with consistent tacrolimus exposure (AUC) at an approximately 30% lower dose compared to twice‐daily, immediate‐release tacrolimus 15 Tacrolimus peak (C max), the C max/C min ratio, and percent fluctuation and swing are significantly lower for LCP‐Tacro vs. twice‐daily tacrolimus, and T max is significantly prolonged 15. There is a robust correlation between LCP‐Tacro tacrolimus exposure and trough levels, with AUC24 and C min correlation coefficients after seven and 14 d of therapy of ≥0.86 15. A hallmark difference between LCP‐Tacro and other forms of once‐ and twice‐daily tacrolimus products is the unique, proprietary MeltDose® drug delivery technology (Veloxis Pharmaceuticals), which reduces tacrolimus's particle size to a molecular level 16. The decreased surface area of the drug particles results in complete absorption and increased bioavailability. Studies in de novo and stable renal 15 and liver recipients 17, 18, 19, 20 have shown that the bioavailability of tacrolimus is 20%–30% higher for LCP‐Tacro compared to traditional twice‐daily tacrolimus capsules. The increased bioavailability of LCP‐Tacro allows for lower doses of LCP‐Tacro (approximately 30%; 15% for black patients) compared to tacrolimus twice‐daily capsules, with non‐inferior efficacy and similar safety between the two formulations 18, 21, 22.
This study's primary objective was to determine the change in tremor severity after switching from tacrolimus twice‐daily capsules to LCP‐Tacro once‐daily tablets, in stable kidney transplant recipients experiencing clinically significant pre‐conversion tremors.
Materials and methods
Patient population
Eligible patients were adult (≥18 yr) recipients of a living or deceased donor kidney transplant who had received their kidney transplant between one month and five yr prior to screening and were on a stable dose of oral twice‐daily tacrolimus capsules for at least seven consecutive days at targeted trough levels. A clinically significant tremor was either initially observed by a health care provider or apprised by patient complaint. A formal examination needed to display amplitude postural or action tremor (finger to nose) characterized by a score of at least two (moderate in intensity) on any of the four upper extremity (UE) postural or action and intention assessments of the Fahn–Tolosa–Marin (FTM) tremor rating scale. Patients who had a history of tremor prior to transplantation or with a family history of tremor were excluded from enrollment.
Other exclusionary criteria included: recipients of any extra‐renal organ except for bone marrow transplant; an estimated glomerular filtration rate (eGFR) (based on MDRD7) <30 mL/min at screening; receiving treatment with an investigational agent within three months prior to screening; unstable dosing and concomitant use of medications known to affect the metabolism of or affect the pharmacokinetic (PK) profile of tacrolimus; a diagnosis of parkinsonism, or tremor from any cause other than tacrolimus including medications known to induce tremors or dopamine blocking agents within the past six months; patients who were taking unstable dosing of drugs known to reduce tremor; and patients who had a rejection episode within three months of screening.
Study design and conduct
This was a 2‐sequence, open‐label, prospective phase 3b, multicenter, clinical study. Stable kidney transplant patients with tremor were converted from twice‐daily tacrolimus to once‐daily LCP‐Tacro (Fig. 1).
Figure 1.
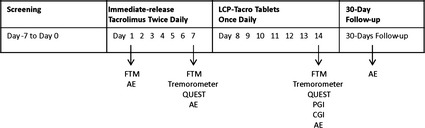
Study design. AE, adverse event; FTM, Fahn–Tolosa–Marin rating scale; CGI, clinician global impression of change; PGI, patient global impression of change; QUEST, quality of life in essential tremor scale.
Following the screening (day 0) and enrollment visits (day 1), scheduled study visits were conducted on day 7 and day 14. Subjects were assessed and videotaped two h after tacrolimus dosing. On days 1 through 7, patients continued their pre‐study twice‐daily tacrolimus regimen to prove stability in dosing and tacrolimus trough levels. On day 8, patients were switched to once‐daily LCP‐Tacro for a total of seven d. For safety assurance, all patients who received at least a single dose of LCP‐Tacro received either a follow‐up telephone call or study visit 30 d after their last dose.
Patients who completed the two‐wk study period were offered to participate in the extension phase of the study and continue treatment with LCP‐Tacro for an additional two yr. Patients who declined participation in the extension phase returned to their prior tacrolimus regimen.
Institutional Review Board approval was obtained at each participating center, and informed consent was obtained from all patients. The study was undertaken in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice and conformed to the Declaration of Helsinki.
Materials
Tremor was evaluated by independent, blinded movement disorder neurologists applying the FTM tremor rating scale to videotaped examinations obtained pre‐ and seven d post‐conversion. If the FTM scores differed significantly between the two neurologists, a third neurologist was enlisted to adjudicate the disparate scores for the ratings in question. The FTM score is derived from 21 elements within three subscales: (i) tremor location/severity rating – four elements on upper limb postural and action tremor severity based on tremor amplitude; (ii) specific motor tasks/functions of writing, pouring liquids and drawing (nine elements); and (iii) subject‐reported functional disabilities resulting from tremor (i.e., eating, dressing, drinking, writing; eight elements). Each subscale and the overall score is converted to a 0–100 scale (higher = worse) 23, 24.
To provide a quantitative measure of tremor, a Tremorometer™ (FlexAble Systems; Fountain Hills, AZ, USA) was utilized. A Tremorometer™ is an accelerometry device that measures frequency and amplitude of tremor. Subjects were studied in three positions while wearing the tremorometer device on each hand: posture‐holding (posture), with movement on finger to nose (move) and with a 135 gram weight in the hand studied (load). Typically tacrolimus‐induced tremor is in the 7–8 Hz range 25.
Lastly, the patients made self‐assessments by completing the Quality of Life in Essential Tremor (QUEST) scale 26 and Patient Global Impression of Change (PGI). Physicians completed the Clinical Global Impression of Improvement (CGI) scale. The PGI and CGI are 7‐point scales assessing tremor change ranging from most improved to the most significant worsening of symptoms.
Training and blinding procedures
Staff from participating centers who were responsible for enrolling patients into the study and conducting study visits underwent a formal training and certification process. The video‐taped FTM instrument administration was sent to independent neurologists in a blinded fashion (i.e., neurologists were not able to differentiate whether the FTM instrument was taken on day 7 or day 14).
Study drug dosing
Following screening, the two‐wk treatment period consisted of one wk of twice‐daily tacrolimus (days 1 through 7) with conversion to once‐daily LCP‐Tacro based on a conversion factor from twice‐daily tacrolimus to LCP‐Tacro of 0.7 for non‐black patients and 0.85 for black patients. The goal was to maintain tacrolimus trough levels between 3 and 12 ng/mL.
Patients were maintained on LCP‐Tacro for one wk (days 8 through 14). All patients continued their pre‐conversion antimetabolite and prednisone without dosage changes.
Study endpoints
Primary efficacy
The primary efficacy endpoint was mean change from baseline in the total FTM score seven d after LCP‐Tacro conversion.
Secondary efficacy
The secondary efficacy endpoints were percent change in FTM overall and subscale tremor scores from baseline to seven d after LCP‐Tacro conversion, FTM subscale scores, tremorometer measurements, PGI, QUEST, and CGI scores.
Safety
The safety evaluation included reporting AEs and serious adverse events (SAEs), performing physical examinations and obtaining laboratory assessments.
Statistical analyses
The efficacy analyses were conducted using the modified intent‐to‐treat (mITT) analysis set which included all patients who received at least one investigational treatment (i.e., LCP‐Tacro) and had evaluable baseline (pre‐conversion) and post‐conversion FTM scores. The safety analysis set included all patients who were enrolled in the study.
The primary efficacy analysis was based on evaluating mean change (i.e., absolute change) from baseline (day 7, pre‐conversion) on FTM overall score to day 14 (post‐conversion) using paired t‐test (at 0.05 significance level). A 95% confidence interval (CI) was constructed for the mean change from baseline.
The secondary efficacy analyses included evaluating percent change from baseline on FTM overall score to day 14; the correlation between FTM subscale scores/overall score and CGI scales; descriptive summary of tremorometer measurements by treatment phases; descriptive summary of CGI for each score with additional categorized presentation (favorable and unfavorable categories); one‐sample binomial test with 95% CI to evaluate LCP‐Tacro improvement on CGI scale; improvement in PGI was treated similarly as for CGI scale; summarized presentation of change from baseline in five components of the QUEST questionnaire (Physical, Psychosocial, Communication, Hobbies/Leisure, and Work/Finance) as well as the summary index to evaluate overall patient quality of life with tremor.
Spearman's correlation was used to examine the relationship between tacrolimus trough and tremor. Analyses of Safety included descriptive summary of AEs, SAEs, and other safety endpoints.
Results
The study took place from 20 December 2011 to 18 April 2013 at 12 US sites.
Patient disposition and baseline characteristics
Forty‐four patients were included in the ITT/safety population and 40 (90.9%) completed the study period. Four (9.1%) patients withdrew prematurely from the treatment period. No patients withdrew from study due to an AE. Of the 40 patients who completed the study period, 38 (86.4%) patients were evaluable for efficacy evaluation and were included in the mITT population.
In the ITT population, the majority of patients were male (77.3%) and white (77.3%), with a median age of 50.5 yr. The mean (SD) tacrolimus trough on day 1 for the mITT population was 6.70 (1.83) ng/mL. The mean time from kidney transplantation to study enrollment was 16.85 months. Eleven of 44 (25.0%) patients had a previous kidney transplant (Table 1). All demographics and baseline characteristics were similar in the mITT population.
Table 1.
Patient demographics and baseline characteristics – ITT and mITT population
| ITT population twice‐daily tacrolimus → LCP‐Tacro (N = 44) | mITT population twice‐daily tacrolimus → LCP‐Tacro (N = 38) | |
|---|---|---|
| Age (yr), mean (SD) | 47.8 (13.68) | 48.3 (13.73) |
| Sex | ||
| Male | 34 (77.3) | 29 (76.3) |
| Female | 10 (22.7) | 9 (23.7) |
| Race | ||
| White | 34 (77.3) | 31 (81.6) |
| Black | 6 (13.6) | 4 (10.5) |
| Asian | 1 (2.3) | 0 |
| Other | 3 (6.8) | 3 (7.9) |
| Mean (SD) months from current kidney transplant to enrollment | 16.85 (14.45) | 15.63 (13.72) |
| Donor type, n (%) | ||
| Living | 15 (34.1) | 14 (36.8) |
| Deceased | 29 (65.9) | 24 (63.2) |
ITT, intent‐to‐treat; mITT, modified intent‐to‐treat.
Primary efficacy endpoint
After the switch from twice‐daily tacrolimus and completion of treatment with LCP‐Tacro for one wk, the mean (SD) absolute change (improvement) in FTM total tremor score (TTS) from baseline (day 7) was −5.35 ([7.50]; p < 0.0001) on day 14 (Table 2 and Fig. 2).
Table 2.
Summary of change in FTM scores: mITT population
| FTM category – visit | FTM scores (N = 38) | ||
|---|---|---|---|
| Original value | Absolute change | Percent (%) change | |
| Total score – day 7 | |||
| Mean (SD) | 25.30 (9.472) | ||
| Total score – day 14 | |||
| Mean (SD) | 19.96 (7.613) | −5.35 (7.501) | −15.59 (32.004) |
| 95% CI (p‐value)a | −7.81, −2.88 (<0.0001) | −26.11, −5.07 (0.0048) | |
| Part A: tremor location/severity rating – day 7 | |||
| Mean (SD) | 26.32 (13.409) | ||
| Part A: tremor location/severity rating – day 14 | |||
| Mean (SD) | 22.70 (11.107) | −3.62 (11.950) | −5.18 (58.229) |
| 95% CI (p‐value)a | −7.55, 0.31 (0.0699) | −24.59, 14.24 (0.5920) | |
| Part B: specific motor tasks/function rating – day 7 | |||
| Mean (SD) | 26.90 (10.213) | ||
| Part B: specific motor tasks/function rating – day 14 | |||
| Mean (SD) | 23.61 (8.008) | −3.29 (8.165) | −8.61 (24.963) |
| 95% CI (p‐value)a | −5.97, −0.61 (0.0177) | −16.82, −0.40 (0.0402) | |
| Part C: functional disabilities resulting from tremor – day 7 | |||
| Mean (SD) | 22.70 (13.071) | ||
| Part C: functional disabilities resulting from tremor – day 14 | |||
| Mean (SD) | 13.57 (9.701) | −9.13 (10.285) | −36.48 (38.005) |
| 95% CI (p‐value)a | −12.51, −5.75 (<0.0001) | −49.34, −23.62 (<0.0001) | |
FTM, Fahn–Tolosa–Marin; mITT, modified intent‐to‐treat.
Asymptotic 95% confidence interval for the mean absolute changes and p‐value for paired t‐test (absolute change); asymptotic 95% confidence interval for the mean % changes and p‐value using one‐sample t‐test (percent change).
Figure 2.
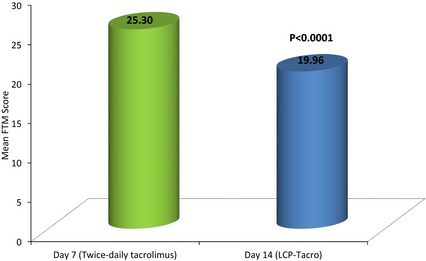
Fahn–Tolosa–Marin (FTM) score reduction (improvement) after conversion to LCP‐Tacro in patients experiencing severe hand tremors.
Secondary efficacy endpoints
Fahn–Tolosa–Marin
After the switch from twice‐daily tacrolimus and completion of treatment with LCP‐Tacro for one wk, there was a significant reduction (improvement) in the mean (SD) percent change FTM TTS (−15.59% (32.00; p = 0.005). For the subscale of tremor location/severity (Part A), the mean (SD) absolute change in total score from baseline was −3.62 (11.95, p = 0.07) on day 14 and the corresponding percent change was −5.18% (58.23, p = 0.59). For specific motor tasks (Part B), the mean (SD) absolute change in total score from baseline was −3.29 (8.17; P = 0.02) on day 14 and the corresponding percent change was −8.61% (24.96; p = 0.04). For functional disabilities resulting from tremor (Part C), the mean (SD) absolute change in total score from baseline was −9.13 (10.30; p < 0.0001) on day 14 and the corresponding percent change was −36.48% (38.01; p < 0.0001) (Table 2 and Fig. 3).
Figure 3.
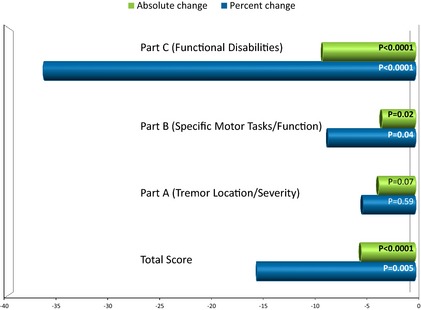
Absolute and percent change (improvement) in Fahn–Tolosa–Marin (FTM) score and subscale scores at day 14.
Tremorometer
Table 3 and Fig. 4 summarizes absolute change at day 14 from original values in tremorometer measures by frequency and amplitude score for the dominant hand. Thirty‐six patients had a mean (SD) absolute improvement (−21.58 milli‐g [58.34]) in measured amplitude for posture position of the dominant hand that was statistically significant (p = 0.03). There was no change in tremor frequency (~8 Hz) post‐conversion in any position (p > 0.50) or amplitude with loading (p = 0.08) the hand with a 135 gram weight.
Table 3.
Summary of tremorometer measurements (dominant hand, absolute change): mITT population
| Visit/Position | Posture | Load | Move | |||
|---|---|---|---|---|---|---|
| Original valuea | Absolute change | Original valuea | Absolute change | Original valuea | Absolute change | |
| Frequency – day 7 | ||||||
| N | 36 | 36 | 35 | |||
| Mean (SD) | 8.48 (0.97) | 8.32 (1.12) | 7.96 (1.59) | |||
| Frequency – day 14 | ||||||
| N | 37 | 36 | 38 | 36 | 37 | 34 |
| Mean (SD) | 8.58 (1.10) | 0.06 (0.77) | 8.42 (1.28) | 0.08 (0.88) | 7.92 (1.55) | −0.06 (2.00) |
| 95% CI (p‐value)b | −0.20, 0.32 (0.63) | −0.21, 0.38 (0.57) | −0.76, 0.64 (0.87) | |||
| Amplitude – day 7 | ||||||
| N | 36 | 36 | 35 | |||
| Mean (SD) | 52.53 (63.02) | 40.86 (38.37) | 150.37 (71.97) | |||
| Amplitude – day 14 | ||||||
| N | 37 | 36 | 38 | 36 | 37 | 34 |
| Mean (SD) | 30.54 (17.11) | −21.58 (58.34) | 30.18 (23.15) | −9.83 (32.50) | 135.57 (75.29) | −18.56 (89.61) |
| 95% CI (p‐value)b | −41.32, −1.84 (0.03) | −20.83, 1.16 (0.08) | −49.83, 12.71 (0.24) | |||
mITT, modified intent‐to‐treat.
Frequency, amplitude, spread, and tremor score value of zero (0) are set to missing for the summary.
Asymptotic 95% confidence interval for the mean change and p‐value using one‐sample t‐test.
Figure 4.
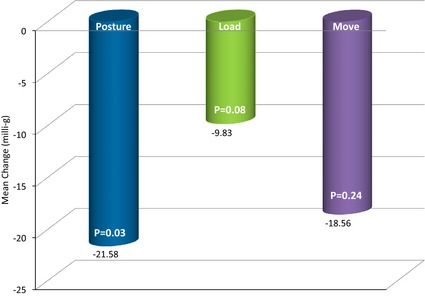
Absolute change from baseline (improvement) in tremorometer amplitude indicators.
Quality of life
There was a significant (p < 0.0001) improvement in the QUEST QOL summary score at day 14. The mean (SD) absolute change from baseline was −7.04 (9.41), and the corresponding mean percent change was −39.08% (39.43). Change in the QUEST score was driven by the change in the physical (mean absolute and percent change from baseline: −11.91, p < 0.0001; −31.80%, p < 0.0001), psychosocial (−7.02, p < 0.0001; −39.10%, p < 0.0001), and work/finance (−6.61, p = 0.02; −53.06%, p = 0.0005) subscales. Mean absolute and percent change from baseline in the other subscales were communication, −3.07 (p = 0.19), −33.46% (p = 0.07); and, hobbies/leisure: −6.58 (p = 0.15), −29.10% (p = 0.23) (Fig. 5).
Figure 5.
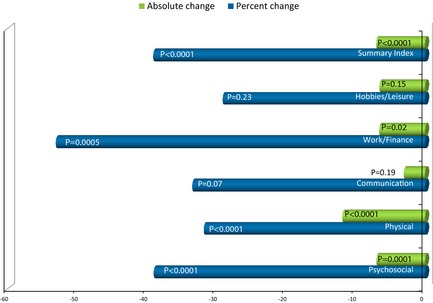
Absolute and percent change (improvement) in QUEST score and subscale scores at day 14.
Change in QUEST score was significantly (p = 0.006) correlated (Pearson coefficient R = 0.44) with change in FTM total score; change in QUEST and change in Part C were also significantly (p < 0.0001) correlated (Pearson coefficient R = 0.68). Most notable, change in physical, (R = 0.48), psychosocial (R = 0.47), and hobbies/leisure (R = 0.41), p‐values ≤0.01, were significantly correlated with change in the functional disabilities component of the FTM.
PGI and CGI
The PGI indicated that 78.9% of patients reported an improvement of “much better” (23.7%) or “a little better” (55.3%) after switching to LCP‐Tacro (p < 0.0005). Similarly, 86.8% of physicians on the CGI reported an improvement of “very much improved” (2.6%), “much improved” (28.9%), or minimally improved” (55.3%) (p < 0.0001) (Fig. 6).
Figure 6.
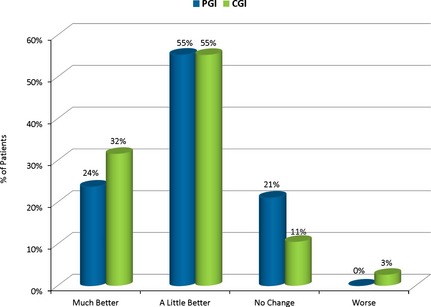
CGI and PGI Scales show improvement after seven d of LCP‐Tacro treatment.
Safety
Mean (SD) (ng/mL) tacrolimus trough levels were similar across all three time points: day 1: 6.70 (1.83); day 7: 6.53 (2.34); and day 14 (LCP‐Tacro): 6.12 (2.64). Tacrolimus trough level was not found to be meaningfully correlated with FTM score (r 2 = 0.07), or with tremorometer measurements (posture, r 2 = 0.04; load, r 2 = 0.03; move, r 2 = 0.05). Likewise, change in tacrolimus trough was not meaningfully correlated with change in FTM score (r 2 = 0.003). There was a reduction in post‐conversion tacrolimus dose levels. Patients enrolled were exposed to a total of 54.82 mg of twice‐daily tacrolimus for a mean duration of nine d and 30.29 mg of LCP‐Tacro for a mean duration of 7.4 d with only one patient requiring a dose adjustment.
During the twice‐daily tacrolimus period (Week 1), there were 10 (22.7%) patients with at least one AE and two (4.5%) patients with a drug‐related AE. During the LCP‐Tacro administration in Week 2, eight (19.5%) patients experienced an AE and one (2.4%) AE was drug related. All AEs were of mild or moderate severity. There were no AEs leading to drug discontinuation.
Adverse events reported in two or more patients while taking twice‐daily tacrolimus included peripheral edema (n = 3), cardiac murmur (n = 2), and hyperkalemia (n = 2). Adverse events reported in two or more patients while taking LCP‐Tacro included nausea (n = 2). There were no patient deaths during the study. One of 44 (2.3%) patients experienced an SAE, cellulitis at the incision site, while taking twice‐daily tacrolimus. No patients in the LCP‐Tacro group experienced an SAE. There were no patients with marked hematological or laboratory abnormalities, AEs causing study drug discontinuation, or events that led to an intervention.
Discussion
Tremor is the most common tacrolimus‐associated side effect experienced by kidney transplant recipients 4 and may significantly affect their quality of life 11, 12. The peak serum concentration (C max) of tacrolimus is associated with the highest amplitude of tremor, typically occurring two h after ingestion. Reducing tacrolimus exposure in an attempt to ameliorate tremor symptoms may increase the risk of developing an acute rejection. The PK profile of LCP‐Tacro reveals a reduction in the peak‐to‐trough ratio and C max yet prolongs the T max of LCP‐Tacro compared to twice‐daily tacrolimus 18, 22. It was hypothesized that the lower concentration maximum experienced with LCP‐Tacro may result in a reduction of tremor severity in kidney transplant recipients previously prescribed twice‐daily tacrolimus.
The results of this phase 3b study reveal that the majority of kidney transplant patients who are experiencing tacrolimus‐induced hand tremors experienced significant improvement after conversion to LCP‐Tacro while maintaining comparable tacrolimus exposure. This translates into LCP‐Tacro‐treated kidney transplant recipients having less difficulty with numerous activities of daily living such as handwriting, eating, drinking, and dressing as well as less psychosocial embarrassment due to their tremor. Open‐label studies such as this are at significant risk of being influenced by a participant placebo effect. In an attempt to reduce this possibility, the protocol was designed to “blind” the rating neurologists. In addition, the tremorometer produces objective data that are reproducible, quantitative, and cannot be falsified by a study subject without detection. One approach to ameliorating tremor is to simply reduce the immediate‐release tacrolimus dose, which would lower the peak and overall exposure of the drug. In some patients, this approach might increase the rate of acute and chronic rejection, especially if their measured trough levels were at the low end of acceptable range to begin with. We did not perform a full hour by hour PK profile on each participant. Although contemplated, we expected that the performance of a full PK profile would hinder study enrollment and increase the complexity of the study likely leading to more errors than benefit.
Although the results show a statistical benefit, the question remains whether the impact is clinically significant to the patient. Three separate (one clinician and two patient reported) questionnaires that were previously vetted as quality of life measures were administered. As all three questionnaires supported each other and were consistent with the results of the objective components of the study, we conclude that the improvements in tremor were true and meaningful to the majority of patients. Another line of evidence that supports switching to LCP‐Tacro and corroborates clinician‐ and patient‐rated meaningful improvement in tacrolimus‐induced tremor comes from a comparison of our data with the essential tremor literature, where the improvements in the FTM and QUEST were similar (on a percentage basis) relative to the improvements we saw in LCP‐Tacro‐treated subjects in this study 27, 28.
Tremorometer results demonstrated that tacrolimus‐induced tremor amplitudes were reduced across all three positions, but significantly in the posture‐holding position. Many drug‐induced tremors (including those caused by tacrolimus) typically represent enhanced physiological tremors that respond with a reduction of amplitude and frequency with loading (weighting) 29. The lack of reduction in tremor frequency with loading we found may indicate that the load we used with the Tremorometer was too light (only 135 g). In another study of tacrolimus‐ and cyclosporine‐induced tremor in liver transplant recipients, a load of 500 g resulted in a reduction of tremor frequency of >1.5 Hz in the majority of patients, indicating an enhanced physiological tremor 25. As previously mentioned, tacrolimus has been implicated as a cause in every imaginable neurologic symptom from tremors to seizures. A possible future study would be to determine whether changing the PK profile of tacrolimus would influence the severity of other neurologic parameters. Certain side effects are easy to measure and quantify such as insomnia and cognitive dysfunction. In addition, these symptoms are widely reported and, similar to tremor, are likely to adversely influence affected patient's quality of life.
There were no new safety concerns with LCP‐Tacro beyond those expected for Prograf/generic tacrolimus. Adverse events reported in the LCP‐Tacro group were mild or moderate in severity. The reduction of dose post‐conversion to LCP‐Tacro yielded trough levels similar to pre‐conversion tacrolimus. In addition, no correlation was found between tacrolimus trough levels and tremor.
The LCP‐Tacro program has demonstrated the ability of the innovative MeltDose formulation to reduce peak and peak‐to‐trough fluctuation without compromising efficacy. Given the narrow therapeutic window of tacrolimus, LCP‐Tacro offers the unique advantage of maintaining blood levels in the therapeutic range while avoiding high peaks that may result in toxicities. The current STRATO study is the first clinical demonstration of this clinical advantage. In the STRATO study, it was demonstrated that LCP‐Tacro can reduce a troubling side effect and improve quality of life over existing therapies in kidney transplant recipients for patients experiencing tremor.
It is possible that the benefit shown in the STRATO study may extend to other troubling side effects as well, including hypertension and diabetes. Further clinical study is worth undertaking to explore these additional potential significant benefits.
Authors' contributions
Anthony Langone participated in the research design, writing of the paper, and in the performance of the research; Steven M. Steinberg participated in the writing of the paper and in the performance of the research; Roberto Gedaly participated in the writing of the paper and in the performance of the research; Laurence K. Chan participated in the writing of the paper and in the performance of the research; Tariq Shah participated in the writing of the paper and in the performance of the research; Kapil D. Sethi participated in the research design, writing of the paper, and in the performance of the research; Vincenza Nigro participated in the research design, writing of the paper, and in the performance of the research; John C. Morgan participated in the research design, writing of the paper, and in the performance of the research.
Acknowledgements
Portions of these data were presented at the European Society of Transplantation (ESOT) 2013 conference in Vienna, the American Transplant Congress (ATC) 2013 conference in Seattle, and the American Academy of Neurology (AAN) 2014 conference in Philadelphia. The authors thank Dr. Shyamal Mehta for serving as an FTM rating adjudicator in the study and to Christine Culkin, FNP‐BC, MSN, and John Morgan MD, PhD, for creating the training video. STRATO Investigators: Richard N. Formica and Antonios Arvelakis, Yale University; Yousri M. Barr, Dallas Transplant Institute; Daniel C. Brennan, Washington University School of Medicine; Laurence K. Chan, University of Colorado Denver; Jose‐Marie Albert El‐Amm, INTEGRIS Baptist Medical Center, Inc.; Roberto Gedaly, University of Kentucky Medical Center; Tomasz Kozlowski, University of North Carolina‐Chapel Hill; Anthony Langone, Vanderbilt University Medical Center; Arthur Jeremy Matas, University of Minnesota Medical Center; Tariq Shah, Transplant Research Institute; Steven M. Steinberg, California Institute of Renal Research; Patricia M. West‐Thielke, University of Illinois Medical Center. Kristin Kistler, PhD, from Evidera provided medical writing support. Barbra Perry from United BioSource Corporation (UBC) managed the study. Wenjiong Zhou, PhD, from UBC provided statistical expertise. Veloxis Pharmaceuticals provided funding for the study.
Langone A, Steinberg SM, Gedaly R, Chan LK, Shah T, Sethi KD, Nigro V, Morgan JC. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP‐TacrO (STRATO): an open‐label, multicenter, prospective phase 3b study.
Conflict of interest: None.
References
- 1. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) . OPTN/SRTR 2012 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, 2014. [Google Scholar]
- 2. Webster AC, Taylor RRS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005(4): Art. No.: CD003961. [DOI] [PubMed] [Google Scholar]
- 3. Draft Guidance on Tacrolimus. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM181006.pdf [Last accessed 2 July 2014].
- 4. Prograf Prescribing Information. Northbrook, IL: Astellas Pharma US, Inc., September 2013. [Google Scholar]
- 5. Bulatova N, Yousef AM, Al‐Khayyat G, Qosa H. Adverse effects of tacrolimus in renal transplant patients from living donors. Curr Drug Saf 2011: 6: 3. [DOI] [PubMed] [Google Scholar]
- 6. Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 2000: 13: 313. [DOI] [PubMed] [Google Scholar]
- 7. Paul LC. Overview of side effects of immunosuppressive therapy. Transplant Proc 2001: 33: 2089. [DOI] [PubMed] [Google Scholar]
- 8. Martínez‐Sanchis S, Bernal MC, Montagud JV et al. Effects of immunosuppressive drugs on the cognitive functioning of renal transplant recipients: a pilot study. J Clin Exp Neuropsychol 2011: 33: 1016. [DOI] [PubMed] [Google Scholar]
- 9. Eidelman BH, Abu‐Elmagd K, Wilson J et al. Neurologic complications of FK 506. Transplant Proc 1991: 23: 3175. [PMC free article] [PubMed] [Google Scholar]
- 10. Abouljoud MS, Kumar MSA, Brayman KL, Emre S, Bynon JS, for the OLNSG . Neoral® rescue therapy in transplant patients with intolerance to tacrolimus. Clin Transplant 2002: 16: 168. [DOI] [PubMed] [Google Scholar]
- 11. Kugler C, Fischer S, Gottlieb J et al. Symptom experience after lung transplantation: impact on quality of life and adherence. Clin Transplant 2007: 21: 590. [DOI] [PubMed] [Google Scholar]
- 12. Kugler C, Geyer S, Gottlieb J, Simon A, Haverich A, Dracup K. Symptom experience after solid organ transplantation. J Psychosom Res 2009: 66: 101. [DOI] [PubMed] [Google Scholar]
- 13. de Barros CT, Cabrita J. Self‐report of symptom frequency and symptom distress in kidney transplant recipients. Pharmacoepidemiol Drug Saf 1999: 8: 395. [DOI] [PubMed] [Google Scholar]
- 14. Drent G, De Geest S, Dobbels F, Kleibeuker JH, Haagsma EB. Symptom experience, nonadherence and quality of life in adult liver transplant recipients. Neth J Med 2009: 67: 161. [PubMed] [Google Scholar]
- 15. Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation 2013: 96: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MeltDose® Technology by the US Patent and Trademark Office, US Patent No. 7,217,431.
- 17. DuBay DA, Alloway RR, Alsina AE et al. A phase 2b, open‐label, multicenter, prospective, randomized study to compare the pharmacokinetics and safety of LCP‐Tacro™ tablets once‐a‐day to Prograf® capsules twice‐a‐day in de novo liver transplant patients. Liver Transpl 2009: 15(Suppl 7): S14. [Google Scholar]
- 18. Alloway RR, Eckhoff DE, Washburn WK, Teperman LW. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): phase II trial of stable liver transplant recipients. Liver Transpl 2014: 20: 564. [DOI] [PubMed] [Google Scholar]
- 19. Alloway R, Mulgaonkar S, Ueda K, Cohen D, Kaplan B. A phase 2 randomized study of the pharmacokinetics, safety and efficacy of LCP‐Tacro tablets once‐a‐day vs Prograf capsules twice‐a‐day in de novo kidney transplants. Am J Transplant 2011: 11(Suppl 2): 355. [Google Scholar]
- 20. Alloway RR, Mulgaonkar S, Bowers VD et al. A phase 2b, open‐label, multi‐center, prospective, randomized study to compare the pharmacokinetics and safety of LCP‐Tacro™ tablets once‐a‐day to Prograf® capsules twice‐a‐day in de novo kidney transplant patients. Am J Transplant 2009: 9(Suppl 2): 414. [Google Scholar]
- 21. Bunnapradist S, Ciechanowski K, West‐Thielke P et al. Conversion from twice‐daily tacrolimus to once‐daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant 2013: 13: 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation 2013: 96: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stacy MA, Elble RJ, Ondo WG, Wu S‐C, Hulihan J. Assessment of interrater and intrarater reliability of the Fahn–Tolosa–Marin Tremor Rating Scale in essential tremor. Mov Disord 2007: 22: 833. [DOI] [PubMed] [Google Scholar]
- 24. Fahn S, Tolosa E, Marı′n C. Clinical rating scale for tremor In: Jankovic J, Tolosa E. eds. Parkinson's Disease and Movement Disorders. Baltimore: Williams & Wilkins, 1993: 225. [Google Scholar]
- 25. Paul F, Müller J, Christe W, Steinmüller T, Poewe W, Wissel J. Postural hand tremor before and following liver transplantation and immunosuppression with cyclosporine or tacrolimus in patients without clinical signs of hepatic encephalopathy. Clin Transplant 2004: 18: 429. [DOI] [PubMed] [Google Scholar]
- 26. Tröster AI, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in Essential Tremor Questionnaire (QUEST): development and initial validation. Parkinsonism Relat Disord 2005: 11: 367. [DOI] [PubMed] [Google Scholar]
- 27. Sandvik U, Hariz G‐M, Blomstedt P. Quality of life following DBS in the caudal zona incerta in patients with essential tremor. Acta Neurochir 2012: 154: 495. [DOI] [PubMed] [Google Scholar]
- 28. Ondo WG, Jandovic J, Connor GS et al. Topiramate in essential tremor: a doubleblind, placebo‐controlled trial. Neurology 2006: 66: 672. [DOI] [PubMed] [Google Scholar]
- 29. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998: 13(Suppl 3): 2. [DOI] [PubMed] [Google Scholar]


