SUMMARY
The aim of this study was to establish the incidence, risk factors, and the management of pharyngocutaneous fistula (PCF) after primary and salvage total laryngectomy. A retrospective, match-paired analysis of 86 patients who developed fistula after total laryngectomy was carried out and compared with a control group of 86 patients without fistula, randomly selected from a pool of 352 total laryngectomies, performed between January 1999 to October 2014. The overall incidence of PCF in the series was 24.4%; we recorded rates of 19.0%, 28.6% and 30.3% following primary total laryngectomy (PTL), salvage laryngectomy post-radiotherapy (RT-STL) and salvage laryngectomy postchemoradiotherapy (CRT-STL), respectively. Multivariate analysis revealed that the relative risk of fistula was respectively 2.47, 3.09 and 7.69 for hypoalbuminaemia ≤3.5 g/dL, RT-STL and CRT-STL. An early onset of PCF within 10 postoperative days was recorded in case of salvage total laryngectomy. The management of PCF significantly differed between PTL, RT-STL and CTRT-STL, with exclusive conservative treatment for PTL (93.55%), while in the CRT-STL group surgical closure with regional flaps (58.82%) prevailed. Conservative management, adjuvant hyperbaric oxygen therapy and surgical closure were equally distributed in the RT-STL group. Thorough knowledge of patient-related risk factors and its prognostic value, allows the surgeon to better evaluate preventive strategies with the aim of minimising fistula formation, hospitalisation times and related costs.
KEY WORDS: Pharyngocutaneous fistula, Salvage total laryngectomy, Risk factors, Chemoradiotherapy
RIASSUNTO
In questo studio sono state valutate l'incidenza, i fattori di rischio e le modalità di trattamento in pazienti con fistola faringocutanea dopo laringectomia totale primaria e di salvataggio. Nel periodo compreso tra gennaio 1999 e ottobre 2014, 352 pazienti affetti da carcinoma squamocellulare della laringe sono stati sottoposti a laringectomia totale. Il decosro postoperatorio di 86 pazienti è stato complicato dall'insorgenza di fistola fainrogcutanea. Questi sono stati comparati in uno studio caso-controllo con 86 pazienti selezionati tramite software, fra quelli che non avevano sviluppato la fistola salivare. L'incidenza globale di fistola dopo laringectomia totale è stata del 24,4%, rispettivamente abbiamo registrato incidenze del 19,0%, del 28,6% e del 30,3% dopo laringectomia totale primaria, dopo radioterapia e dopo radiochemioterapia. L'analisi multivariata ha rivelato che per ipoalbuminemia ≤3,5 g/dL, per pregressa radioterapia e radiochemioterapia il rischio relativo di sviluppo di fistola è stato rispettivamente 2,47, 3,09 e 7,69. In caso di laringectomia totale di salvataggio abbiamo registrato una comparsa precoce della fistola entro i primi 10 giorni postoperatori. Le modalità di trattamento della fistola faringocutanea sono risultate essere significativamente differenti in caso di laringectomia totale primaria, dopo radioterapia e dopo radiochemioterapia. Infatti, mentre nel primo caso è stato sufficiente un trattamento di tipo conservativo (93,55%), dopo chemioradioterapia ha prevalso il ricorso a tecniche chirurgiche ricostruttive con lembi regionali (58,82%). Nel caso dei pazienti radiotrattati, le opzioni terapeutiche della fistola sono risultate essere equamente distribuite tra quella medica, eventualmente con l'aggiunta dell'ossigenoterapia iperbarica, e quella chirurgica ricostruttiva. La conoscenza dei fattori di rischio soggettivi e il loro valore prognostico, permettono al chirurgo di pianificare le strategie preventive al fine di ridurre il rischio di formazione della complicanza e, conseguentemente, dei tempi di degenza e dei relativi costi.
Introduction
The development of a pharyngocutaneous fistula represents a complication of total laryngectomy that is usually self-limiting. Its management is thus mainly based on careful conservative treatment; however, at times, this complication can carry potentially devastating sequelae, and further surgery is required.
The incidence of pharyngocutaneous fistula has been reported to be 14.3% (95% CI 11-7-17.0) after primary total laryngectomy, increasing significantly to 22.8% (95% CI 18.3-27.4) and 34.1% (95% CI 22.6-45.6) in case of previous radiotherapy or previous chemoradiotherapy 1.
Numerous risk factors, such as pre-existing comorbidities, smoke and alcohol abuse, pre- and postoperative haemoglobin and albumin values, tumour site and stage, concurrent neck dissection etc., have been implicated in fistula formation 2-13, although, the actual impact of these remains debatable.
In this retrospective study, we analysed potential risk factors for the development of postoperative pharyngocutaneous fistula after total laryngectomy and describe our systematic approach in the management of this complication.
Materials and methods
Between January 1999 and October 2014, 352 consecutive total laryngectomies for laryngeal squamous cell carcinoma were performed at the Otolaryngology Clinic of the Department of Surgery and Translational Medicine of the University of Florence. The search was restricted to laryngeal SCC and to total laryngectomies without flap reconstruction.
One hundred and sixty-three patients (46.3%) underwent primary total laryngectomy (PTL), 133 (37.8%) underwent salvage total laryngectomy after radiotherapy (RTSTL), and 56 (15.9%) received salvage total laryngectomy after chemoradiotherapy (CRT-STL). In 31 patients, the procedure was extended to the pharynx (piriform sinus and/or base of tongue), and in PTL we also included salvage procedures after previous transoral laser or previous partial laryngectomy.
Institutional Review Board permission was obtained by the local committee.
Pretreatment staging was corrected using the (UICC) TNM 7th edition based on the clinical charts 14.
The postoperative course of 86 patients was complicated by the onset of a pharyngocutaneous fistula; this group was matched and compared with a control cohort group of 86 patients without PCF, randomly selected within our series. Groups were homogeneous for age and gender.
Between groups we matched and compared risk factors for PCF, including smoke and alcohol habits, comorbidities, previous radiotherapy and chemoradiotherapy, preand postoperative haemoglobin (≤ 12.5 mg/dL) and albumin (≤ 3.5 g/dL), tumour site and T stage (T1-3 vs. T4a), N stage (N0 vs. N+), uni- or bilateral neck dissection and type of neck dissection [selective neck dissection (SND), modified radical neck dissection (mRND), radical neck dissection (RND)], neck level VI dissection.
The surgical technique was standardised among all surgeons. Laryngectomy included standard removal of the hyoid bone and infrahyoid muscles, pharyngeal closure was always performed in a single layer, with a "T" shaped suture line, using Vicryl (polyglactin 910) 3/4-0 sutures. Two suction drains were applied and generally removed when producing less than 15 cc in 24 hours. Antibiotic therapy with amoxicillin/clavulanate was generally started one day before surgery and continued for the following seven days. Skin sutures were removed on postoperative day 9. Enteral feeding through the nasogastric tube was started on the third postoperative day, and oral feeding was started on postoperative day 14 if contrast radiographic study showed no signs of fistula.
Statistical analysis
Randomisation was carried out with the Intel® Digital Random Number Generator. A chi-square test was used to compare discrete variables. A p value < 0.05 was considered statistically significant. Analysis was conducted using Graph Pad software (Graph Pad, San Diego, CA). A logistic regression model was used on the significant risk factors to estimate the relative impact of fistula formation. McNemar's test was used to assess time interval significance between variables within a cut-off time (10 days after intervention). Data regarding fistula treatment were also collected and an ANOVA test was employed to compare different treatment modalities in the three patient groups (PTL, RT-STL, and CRT-STL). These tests were all conducted using STATA (Stata Corporation, College Station, TX).
Results
In this series, the incidence of PCF was 19% among patients receiving primary total laryngectomy, while it increased to 28.6% and 30.3% for patients receiving salvage total laryngectomy after radiotherapy and chemoradiotherapy.
The match-paired analysis for factors predisposing to PCF formation showed a statistical significant difference regarding comorbidities, salvage laryngectomy and postoperative albumin values in the two groups (Tab. I).
Table I.
Patient, disease, treatment and pathology specimen-related factors predisposing to PCF formation.
| Variables | Fistula Group (n = 86) |
Control Group (n = 86) |
p value |
|---|---|---|---|
| Smoke | |||
| No | 18 | 27 | p = ns |
| Yes | 68 | 59 | p = ns |
| Alcohol | |||
| No | 50 | 47 | p = ns |
| Yes | 36 | 39 | p = ns |
| Alcohol+Smoke | 34 | 29 | p = ns |
| Comorbidities | 42 | 28 | p = 0.0433 |
| Previous RT | 38 | 22 | p = 0.0161 |
| Previous CRT | 17 | 4 | p = 0.0042 |
| Pharyngolaryngectomy | 4 | 3 | p = ns |
| Neck dissection (type) | 46 | 43 | p = ns |
| RND | 7 | 4 | p = ns |
| mRND | 18 | 15 | p = ns |
| SND | 21 | 24 | p = ns |
| Neck dissection (side) | |||
| Monolateral | 29 | 31 | p = ns |
| Bilateral | 17 | 12 | p = ns |
| Neck dissection+ VI level | |||
| Monolateral | 10 | 8 | p = ns |
| Bilateral | 7 | 1 | p = 0.0640 |
| Preoperative haemoglobin ≤12.5 mg/dL | 34 | 22 | p = 0.0730 |
| Postoperative haemoglobin ≤12.5 mg/dL | 58 | 46 | p = 0.0859 |
| Preoperative albumin ≤3.5 g/dL | 21 | 25 | p = ns |
| Postoperative albumin ≤3.5 g/dL | 28 | 14 | p = 0.0204 |
| T Stage II,III | 55 | 48 | p = ns |
| T Stage IV | 31 | 38 | p = ns |
| N Stage | |||
| N0 | 42 | 37 | p = ns |
| N+ | 44 | 49 | p = ns |
| T site | |||
| Supraglottic | 30 | 26 | p = ns |
| Pharyngolarynx | 4 | 6 | p = ns |
| Glottic | 44 | 51 | p = ns |
| Transglottic | 3 | 2 | p = ns |
| Subglottic | 5 | 1 | p = ns |
ns; not significant.
Furthermore, pre- and postoperative haemoglobin values below 12.5 mg/dL and bilateral neck dissection with inclusion of level VI showed a trend towards statistical significance.
The assessment of the relative impact of independent variables was established using logistic regression analysis on the most relevant risk factors: odds ratios for PCF development were 2.478 for postoperative albumin values below 3.5g/dL (p=0.023), 3.072 for salvage TL after radiotherapy (p=0.002) and 7.694 for salvage TL after chemoradiotherapy (p=0.001) (Tab. II).
Table II.
Multivariate analysis of PCF risk factors.
| Risk factors | p value | Odds ratio (95% CI) |
|---|---|---|
| Comorbidities | 0.191 | 1.584 (0.794-3.158) |
| Postoperative albumin ≤3.5 g/dL | 0.023 | 2.478 (1.131-5.428) |
| Previous RT | 0.002 | 3.072 (1.485-6.353) |
| Previous CRT | 0.001 | 7.694 (2.338-25.323) |
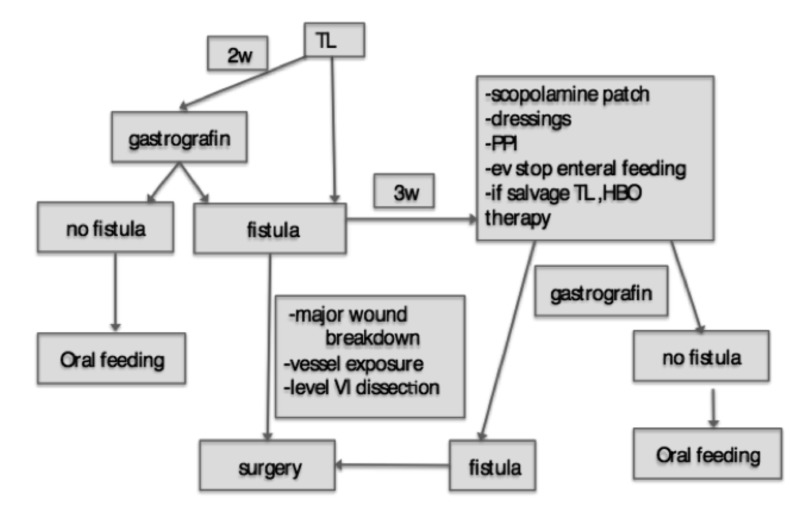
Mean hospitalisation time for fistula group and control group was respectively 51.6 (19-250) and 19.8 (15-22) days, (p=0.0034). The mean time interval between total laryngectomy and fistula formation was 18.33 (3-180) days; patients submitted to primary total laryngectomy showed a mean time of PCF formation of 21.43 days, while for those who developed PCF after salvage procedures was 15.19 days. Risk factors that showed significance at multivariate analysis were analysed with a Mc- Nemar test for early PCF formation, with a cut-off time of 10 days after surgery, radiotherapy and chemoradiotherapy tested positive for early fistula formation (Tab. III). Fifty-four fistula patients (62.8%) were treated conservatively (neck dressing, antibiotic therapy, PPI etc.), 15 patients (27.7%) received hyperbaric oxygen therapy and 32 patients (37.2%) needed further surgery: of these, in 17 cases (53%) surgical revision and primary closure was sufficient, while in the remaining 15 patients (47%) surgical repair was performed using pectoralis major myofascial or myocutaneous flap transposition. Figure 1 displays our flowchart in the management of PCF.
Table III.
McNemar test for the risk factors related time interval impact on PCF formation.
| Risk factors | Exact McNemar significance probability | Odds ratio | 95% CI |
|---|---|---|---|
| Postoperative albumin ≤3.5 g/dL | 0.7283 | 0.8333 | 0.3909-1.7509 |
| Radiotherapy | 0.0008 | 3.0909 | 1.5292-6.7626 |
| Radiochemotherapy | 0.00004 | 9.8 | 3.9260-31.5173 |
Fig. 1.
Fistula treatment flow-chart.
Conservative treatment for PCF was the most frequent option for patients after PTL, CRT-STL patients were mostly treated with pectoralis major flap transposition, RT-STL patients were equally treated either with conservative treatment or conservative treatment plus hyperbaric oxygen therapy and surgical revision.
Discussion
With the evolution of non-surgical organ preservation protocols for treatment of laryngeal and hypopharyngeal squamous cell carcinomas, total laryngectomy is increasingly performed as salvage procedure. Wound complications and pharyngocutaneous fistula have become an increasing concern. Wound healing complications can have a multi-factorial origin including previous chemoradiotherapy, hypoalbuminaemia, hypohemoglobinaemia, neck dissection, tumour stage and site 2-13.
Our study showed that postoperative albumin level below 3.5 g/dL, previous radiotherapy and chemoradiotherapy were significantly correlated with PCF formation. Because of the retrospective setting, we were unable to assess the impact of nutritional status in multivariate analysis. In fact, the albumin level in itself is an insufficient parameter, and the prognostic nutritional index, which is a combination of biochemical factors (serum albumin, transferrin), immune competence (total lymphocyte count, hypersensitivity skin tests) and anthropometric measurements (BMI, arm muscle circumference, and skin fold thickness), would be more appropriate. Moreover, in head and neck carcinoma, nutritional status deteriorates during chemoradiotherapy or hyperfractionated RT 15 and this has been shown to have a negative effect on morbidity, mortality and survival 16 17. It is likely that the increased morbidity of surgery in patients receiving CRT-STL compared with patients of comparable T classification receiving PTL is due to impaired nutritional status as well as the direct toxic effects of chemoradiation on tissue healing.
The role of radiotherapy in the genesis of pharyngocutaneous fistula has been extensively described, and some authors report that there is no significant associations 18-22. In a recent meta-analysis, Paydarfar et al. 23 showed that, although preoperative radiotherapy represented a significant relative risk (RR) of PCF formation, there was also heterogeneity of effects among studies; in fact, other radiotherapy-associated variables such as radiotherapy dose and time frame between the end of radiation and surgery, did not demonstrate an increased RR. At multivariate analysis, the odds ratio for PCF formation after radiation was 3.072 (p=0.002), and fistula seemed to occur early (OR=3.0909; within 10 days).
Similar results have been reported by a small number of authors 19 24. Chemoradiotherapy showed an odds ratio of 7.694 for fistula formation (p=0.001) with a high OR=9.8 for early onset.
Assessment of fistula formation was proven by X-ray swallow study, after two weeks postoperatively (Fig. 1). Some authors advocate the scintigraphic analysis as an objective and non-invasive tool to precisely identify the presence of pharyngocutaneous fistula and location of its internal orifice and to monitor its spontaneous closure 25. Our study is in agreement with the findings from the metaanalysis of Sayles et al., where chemoradiotherapy seems to increase the risk of major wound complications more than radiotherapy alone. Furthermore, concurrent chemoradiotherapy protocols offers superior locoregional control at the expense of more frequent wound complications in the event of salvage surgery than induction chemotherapy followed by radiotherapy 1. Newman reported similar rates of PCF between patients receiving RT alone or more intensive therapy with either sequential or concomitant chemoradiotherapy 26. Aires et al. found no significant differences between PTL and CRT-STL in terms of PCF development 27. Regarding PCF treatment, the results from our observational study pointed out that in PTL patients conservative therapy is almost exclusive, while pectoralis major flap transposition prevails in CRT-STL patients who develop fistula. In our series, the management of PCF in RT-STL was equally distributed among conservative treatment +/- hyperbaric oxygen therapy and revision surgery (Tab. IV). Hyperbaric oxygen therapy improves wound healing, although the data do not suggest that it promotes short-term growth of cancer 28, and thus its use remains controversial. We believe that for salvage patients who cannot receive further adjuvant radiotherapy its use is well justified and highly effective since in all cases it eventually promotes complete healing.
Table IV.
ANOVA for fistula treatments in PTL, RT-STL and CRT-STL groups. Fisher's exact= 0.000.
| Med | Med+HbO | Surgical revision |
PM flap | Total | |
|---|---|---|---|---|---|
| PTL | 29 | 1 | 1 | 0 | 31 |
| % | 93.55 | 3.23 | 3.23 | 0.00 | 100.00 |
| STL-RT | 7 | 14 | 12 | 5 | 38 |
| % | 18.42 | 36.84 | 31.58 | 13.16 | 100.00 |
| STL-CTRT | 2 | 1 | 4 | 10 | 17 |
| % | 11.76 | 5.88 | 23.53 | 58.82 | 100.00 |
| Total | 38 | 16 | 17 | 15 | 86 |
| % | 44.19 | 18.60 | 19.77 | 17.44 | 100.00 |
MED = medication, HbO = Hyperbaric Oxygen, PM = pectoralis major
Early revision surgery with pectoralis major flap transposition was employed in case of major wound breakdown with vessel exposure and in case of evidence of fistula progression in CRT-STL patients, otherwise surgical revision with or without flap transposition was undertaken after persistence of the fistula despite 3 weeks of conservative therapy. The pectoralis major flap can be harvested as a myofascial or myocutaneous flap 29 30. In general, at re-exploration, the pharyngeal mucosa is oedematous and fragile so that primary closure is feasible and wise only when the defect is minimal and direct suture will not produce excessive tension. In all other cases, the myofascial transposition of the flap is the preferred method, and the muscle is sutured inlay around the defect ensuring a tight closure. The myocutaneous transposition is used only in rare cases of large pharyngeal breakdown with major tissue loss/necrosis of the pharyngeal mucosa. In these cases, the skin paddle, sutured inlay, will prevent pharyngeal stenosis that could occur more easily with a large myofascial inlay placement. If the necrosis also involves the central portion of the skin of the neck, we recommend to address the pharyngeal closure as described above and to provide outer skin reconstruction using a split thickness skin graft over the pectoralis major muscle that will be sutured inlay to the outer skin defect. In our opinion, pectoralis major flap transposition is the most efficient option since it is easy and quick to harvest and rotate, and has been demonstrated to be reliable even in hostile recipient sites 31 32. Furthermore, it does not require postoperative monitoring 33 and is cost effective 29.
One minor disadvantage is the morbidity associated with loss of the pectoralis major muscle function especially in case of concomitant shoulder dysfunction following neck dissection 30 34-36.
Based on our retrospective analysis, the incidence of PCF after previous RT or CRT was 29.1% (55 of 189), and the incidence of salvage laryngectomies requiring a flap transposition was 7.9% (15 of 189). We do not suggest to routinely harvest flaps in case of salvage total laryngectomies, although this seems reasonable after previous chemoradiation in patients with comorbidities and low preoperative albumin values. In these cases, we would recommend a myofascial transposition of the pectoralis major flap overlay that is placed over the suture line of the primary pharyngeal closure to protect the suture and overlying skin of the neck. Some authors recently described the use of the myofascial infrahyoid flap for defects following total laryngectomy 37, despite our enthusiastic experience with this reconstructive method 38-42, we always remove the infrahyoid muscles during total laryngectomy for oncologic reasons. Furthermore, we do not believe that this flap can represent a valid solution in the standard management of PCF because of the relative contraindication represented by previous radiotherapy, and because this flap always needs to be harvested during ablative surgery and not when PCF appears in the postoperative course.
Unfortunately, there is no evidence that mechanical stapler pharyngeal closure might reduce fistula formation in high risk cases 43.
We currently continue to assess serum albumin, transferrin, total lymphocyte count and BMI and we are testing other anthropometric parameters to better establish the time for surgery and identify easy-to-obtain parameters during follow-up consultations. We are also trying to improve the preoperative nutritional status by integrating with Oral Impact® (Nestlé, Vevey, Switzerland) 1000 ml (1000 kcal) per day plus usual diet for 5-7 days prior to surgery, as suggested by our nutritionists. However, we have data in this regard at the time of writing.
The limitations of this study are mainly represented by its retrospective setting. Even if the cohort of patients with PCF is relatively small (86 cases), this represents a single institution experience over the last 15 years. Using software, we tried to avoid other biases by random assignment of patients to the control group.
Conclusions
This study confirms that postoperative albumin values below 3.5 g/dL, previous radiotherapy and chemoradiotherapy are significant predictors of PCF. Conservative treatment is advisable for PCF developing in non-(chemo) radiated patients, hyperbaric oxygen therapy is effective as adjuvant treatment after previous radiotherapy, and most PCF developing in chemoradiated patients tend to progress and need flap coverage to avoid devastating outcomes.
References
- 1.Sayles M, Grant, DG Preventing pharyngo-cutaneous fistula in total laryngectomy: a systematic review and metaanalysis. Laryngoscope. 2014;124:1150–1163. doi: 10.1002/lary.24448. [DOI] [PubMed] [Google Scholar]
- 2.Boscolo-Rizzo P, Cillis G, Marchiori C, et al. Multivariate analysis of risk factors for pharyngocutaneous fistula after total laryngectomy. Eur Arch Otorhinolaryngol. 2008;265:929–936. doi: 10.1007/s00405-007-0562-z. [DOI] [PubMed] [Google Scholar]
- 3.Chee N, Siow JK. Pharyngocutaneous fistula after laryngectomy-- incidence, predisposing factors and outcome. Singapore Med J. 1999;40:130–132. [PubMed] [Google Scholar]
- 4.Dedivitis RA, Ribeiro KC, Castro MA, et al. Pharyngocutaneous fistula following total laryngectomy. Acta Otorhinolaryngol Ital. 2007;27:2–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Erdag MA, Arsianoglu S, Onal K, et al. Pharyngocutaneous fistula following total laryngectomy: multivariate analysis of risk factors. Eur Arch Otorhinolaryngol. 2013;270:173–179. doi: 10.1007/s00405-012-2111-7. [DOI] [PubMed] [Google Scholar]
- 6.Galli J, Corso E, Volante M, et al. Postlaryngectomy pharyngocutaneous fistula: incidence, predisposing factors, and therapy. Otolaryngol Head Neck Surg. 2005;133:689–694. doi: 10.1016/j.otohns.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Hier M, Black MJ, Lafond G, et al. Pharyngo-cutaneous fistulas after total laryngectomy: incidence, etiology and outcome analysis. J Otolaryngol. 1993;22:164–166. [PubMed] [Google Scholar]
- 8.Iteld L, Yu P. Pharyngocutaneous fistula repair after radiotherapy and salvage total laryngectomy. J Reconstr Microsurg. 2007;23:339–345. doi: 10.1055/s-2007-992343. [DOI] [PubMed] [Google Scholar]
- 9.Papazoglou G, Doundoulakis G, Terzakis G, et al. Pharyngocutaneous fistula after total laryngectomy: incidence, cause, and treatment. Ann Otol Rhinol Laryngol. 1994;103:801–805. doi: 10.1177/000348949410301010. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi SS, Chaturvedi P, Pai PS, et al. A prospective study of pharyngocutaneous fistulas following total laryngectomy. J Cancer Res Ther. 2005;1:51–56. doi: 10.4103/0973-1482.16092. [DOI] [PubMed] [Google Scholar]
- 11.Saki N, Nikakhlagh S, Kazemi M,, et al. Pharyngocutaneous fistula after laryngectomy: incidence, predisposing factors, and outcome. Arch Iran Med. 2008;11:314–317. [PubMed] [Google Scholar]
- 12.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129:44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Fiorini FR, Deganello A, Larotonda G, et al. Tobacco exposure and complications in conservative laryngeal surgery. Cancers (Basel) 2014;6:1727–1735. doi: 10.3390/cancers6031727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Munshi A, Pandey MB, Durga T, et al. Weight loss during radiotherapy for head and neck malignancies: what factors impact it? Nutr Cancer. 2003;47:136–140. doi: 10.1207/s15327914nc4702_5. [DOI] [PubMed] [Google Scholar]
- 16.Bokhorst-de van der S, Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86:519–527. [PubMed] [Google Scholar]
- 17.Gallo O, Deganello A, Gitti G, et al. Prognostic role of pneumonia in supracricoid and supraglottic laryngectomies. Oral Oncol. 2009;45:30–38. doi: 10.1016/j.oraloncology.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Saydam L, Kalcioglu T, Kizilay A, et al. Early oral feeding following total laryngectomy. Am J Otolaryngol. 2002;23:277–281. doi: 10.1053/ajot.2002.126321. [DOI] [PubMed] [Google Scholar]
- 19.Virtaniemi JA, Kumpulainen EJ, Hirvikoski PP, et al. The incidence and etiology of postlaryngectomy pharyngocutaneous fistulae. Head Neck. 2001;23:29–33. [PubMed] [Google Scholar]
- 20.Soylu L, Kiroglu M, Aydogan B, et al. Pharyngocutaneous fistula following laryngectomy. Head Neck. 1998;20:22–25. doi: 10.1002/(sici)1097-0347(199801)20:1<22::aid-hed4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Ikiz AO, Uca M, Guneri EA, et al. Pharyngocutaneous fistula and total laryngectomy: possible predisposing factors, with emphasis on pharyngeal myotomy. J Laryngol Otol. 2000;114:768–771. doi: 10.1258/0022215001904112. [DOI] [PubMed] [Google Scholar]
- 22.Deganello A, Gallo O, Cesare JM, et al. Supracricoid partial laryngectomy as salvage surgery for radiation therapy failure. Head Neck. 2008;30:1064–1071. doi: 10.1002/hed.20837. [DOI] [PubMed] [Google Scholar]
- 23.Paydarfar JA, Birkmeyer NJ. Complications in head and neck surgery: a meta-analysis of postlaryngectomy pharyngocutaneous fistula. Arch Otolaryngol Head Neck Surg. 2006;132:67–72. doi: 10.1001/archotol.132.1.67. [DOI] [PubMed] [Google Scholar]
- 24.Briant TD. Spontaneous pharyngeal fistula and wound infection following laryngectomy. Laryngoscope. 1975;85:829–834. doi: 10.1288/00005537-197505000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Galli J, Valenza V, Parrilla C, et al. Pharyngocutaneous fistula onset after total laryngectomy: scintigraphic analysis. Acta Otorhinolaryngol Ital. 2009;29:242–244. [PMC free article] [PubMed] [Google Scholar]
- 26.Newman JP, Terris DJ, Pinto HA, et al. Surgical morbidity of neck dissection after chemoradiotherapy in advanced head and neck cancer. Ann Otol Rhinol Laryngol. 1997;106:117–122. doi: 10.1177/000348949710600205. [DOI] [PubMed] [Google Scholar]
- 27.Aires FT, Dedivitis RA, Castro MA, et al. Pharyngocutaneous fistula following total laryngectomy. Braz J Otorhinolaryngol. 2012;78:94–98. doi: 10.5935/1808-8694.20120040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y, Lee CS, Wu J, et al. Effects of hyperbaric oxygen exposure on experimental head and neck tumor growth, oxygenation, and vasculature. Head Neck. 2005;27:362–369. doi: 10.1002/hed.20169. [DOI] [PubMed] [Google Scholar]
- 29.Deganello A, Gitti G, Parrinello G, et al. Cost analysis in oral cavity and oropharyngeal reconstructions with microvascular and pedicled flaps. Acta Otorhinolaryngol Ital. 2013;33:380–387. [PMC free article] [PubMed] [Google Scholar]
- 30.Leite AK, Matos LL, Belli M, et al. Pectoralis major myocutaneous flap for head and neck reconstruction: risk factors for fistula formation. Acta Otorhinolaryngol Ital. 2014;34:389–393. [PMC free article] [PubMed] [Google Scholar]
- 31.Deganello A, Gitti G, Struijs B, et al. Palliative combined treatment for unresectable cutaneous basosquamous cell carcinoma of the head and neck. Acta Otorhinolaryngol Ital. 2013;33:353–356. [PMC free article] [PubMed] [Google Scholar]
- 32.Deganello A, Gallo O, Cesare JM, et al. Surgical management of surgery and radiation induced peristomal neck ulcerations. B-ENT. 2008;4:169–174. [PubMed] [Google Scholar]
- 33.Pellini R, Pichi B, Marchesi P, et al. External monitor for buried free flaps in head and neck reconstructions. Acta Otorhinolaryngol Ital. 2006;26:1–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Deganello A, Meccariello G, Bini B, et al. Is elective neck dissection necessary in cases of laryngeal recurrence after previous radiotherapy for early glottic cancer? J Laryngol Otol. 2014;128:1089–1094. doi: 10.1017/S0022215114002709. [DOI] [PubMed] [Google Scholar]
- 35.Deganello A, Gitti G, Meccariello G, et al. Effectiveness and pitfalls of elective neck dissection in N0 laryngeal cancer. Acta Otorhinolaryngol Ital. 2011;31:216–221. [PMC free article] [PubMed] [Google Scholar]
- 36.Gallo O, Deganello A, Scala J, et al. Evolution of elective neck dissection in N0 laryngeal cancer. Acta Otorhinolaryngol Ital. 2006;26:335–344. [PMC free article] [PubMed] [Google Scholar]
- 37.Kadota H, Fukushima J, Kamizono K, et al. A minimally invasive method to prevent postlaryngectomy major pharyngocutaneous fistula using infrahyoid myofascial flap. J Plast Reconstr Aesthet Surg. 2013;66:906–911. doi: 10.1016/j.bjps.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Deganello A, Leemans R. The infrahyoid flap: a comprehensive review of an often overlooked reconstructive method. Oral Oncol. 2014;50:704–710. doi: 10.1016/j.oraloncology.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Deganello A, Gitti G, Parrinello G, et al. Infrahyoid flap reconstruction of oral cavity and oropharyngeal defects in elderly patients with severe general comorbidities. Head Neck. 2012;34:1299–1305. doi: 10.1002/hed.21913. [DOI] [PubMed] [Google Scholar]
- 40.Deganello A, Manciocco V, Dolivet G, et al. Infrahyoid fascio-myocutaneous flap as an alternative to free radial forearm flap in head and neck reconstruction. Head Neck. 2007;29:285–291. doi: 10.1002/hed.20512. [DOI] [PubMed] [Google Scholar]
- 41.Gangloff P, Deganello A, Lacave ML. Use of the infra hyoid musculo-cutaneous flap in soft palate reconstruction. Eur J Surg Oncol. 2006;32:1165–1169. doi: 10.1016/j.ejso.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Deganello A, Bree R, Dolivet G, et al. Infrahyoid myocutaneous flap reconstruction after wide local excision of a Merkel cell carcinoma. Acta Otorhinolaryngol Ital. 2005;25:50–53. [PMC free article] [PubMed] [Google Scholar]
- 43.Dedivitis RA, Aires FT, Pfuetzenreiter EG, Jr, et al. Stapler suture of the pharynx after total laryngectomy. Acta Otorhinolaryngol Ital. 2014;34:94–98. [PMC free article] [PubMed] [Google Scholar]