SUMMARY
Nowadays oral appliance therapy is recognised as an effective therapy for many patients with primary snoring and mild to moderate obstructive sleep apnoea (OSA), as well as those with more severe OSA who cannot tolerate positive airway pressure (PAP) therapies. For this reason, it is important to focus on objective criteria to indicate which subjects may benefit from treatment with a mandibular advancement device (MAD). Various anthropometric and polysomnographic predictors have been described in the literature, whereas there are still controversies about the role of drug-induced sleep endoscopy (DISE) and advancement bimanual manoeuvre as predictor factors of treatment outcome by oral device. Herein, we report our experience in treatment of mild moderate OSA by oral appliance selected by DISE. We performed a single institution, longitudinal prospective evaluation of a consecutive group of mild moderate patients with obstructive sleep apnoea syndrome who underwent DISE. During sleep endoscopy, gentle manoeuvre of mandibular advancement less than 5 mm was performed. In 30 of 65 patients (46.2%) we obtained an unsuccessful improvement of airway patency whereas in 35 of 65 patients (53.8%) the improvement was successful and patients were considered suitable for oral device application. Because 7 of 35 patients were excluded due to conditions interfering with oral appliance therapy, we finally treated 28 patients. After 3 months of treatment, we observed a significant improvement in the Epworth medium index [(7.35 ± 2.8 versus 4.1 ± 2.2 (p < 0.05)], in mean AHI [(21.4 ± 6 events per hour versus 8.85 ± 6.9 (p < 0.05)] and in mean ODI [(18.6 ± 8 events per hour to 7 ± 5.8 (p < 0.05)]. We observed that the apnoea/hypopnoea index (AHI) improved by up to 50% from baseline in 71.4% of patients selected after DISE for MAD therapy. In the current study, mandibular advancement splint therapy was successfully prescribed on the basis not only of severity of disease, as determined by the subject's initial AHI, but also by DISE findings combined with results of gentle mandibular advancement manoeuvre allowing direct view of the effects of mandibular protrusion on breathing spaces in obstruction sites, and showing good optimisation of selection of patients for oral device treatment.
KEY WORDS: Obstructive sleep apnoea syndrome, Mandibular advancement device, Drug-induced sleep endoscopy, AHI, BMI
RIASSUNTO
Il trattamento con dispositivi di avanzamento mandibolare (MAD) rappresenta un'efficace alternativa terapeutica per i pazienti affetti da roncopatia semplice, OSAS di grado lieve/moderato e in casi selezionati di OSAS grave con scarsa tollerabilità alla terapia ventilatoria con C-PAP. Pertanto è importante identificare dei criteri oggettivi per selezionare i pazienti che possono beneficiare del trattamento con i sistemi di avanzamento mandibolare (MAD). In letteratura sono stati descritti vari fattori predittivi sia antropometrici che polisonnografici, mentre esistono ancora controversie circa il ruolo della Sleep Endoscopy e della manovra di avanzamento mandibolare bimanuale durante lo stesso esame come fattori predittivi del successo terapeutico con MAD. In questo studio descriviamo la nostra esperienza nel management di pazienti affetti da OSAS lieve/moderata trattati con MAD e selezionati mediante "sleep endoscopy". Abbiamo eseguito una valutazione prospettica longitudinale di una serie consecutiva di pazienti giunti alla nostra osservazione con diagnosi di OSAS lieve/moderata e sottoposti a sleependoscopy. Durante il sonno indotto farmacologicamente è stata eseguita una delicata manovra di avanzamento mandibolare con escursione inferiore ai 5 mm e abbiamo riscontrato che in 30 dei 65 pazienti (46,2%) lo spazio respiratorio non migliorava in modo significativo a livello dei siti di ostruzione osservati, mentre in 35 dei 65 pazienti (53,8%) si osservava un miglioramento significativo tale da poter indicare terapia con MAD. In 7 dei 35 pazienti venivano riscontrate condizioni che ostacolavano l'applicazione del MAD per cui 28 dei 35 pazienti sono stati sottoposti a terapia con MAD. Dopo 3 mesi di trattamento abbiamo documentato un miglioramento significativo dell'indice di Epworth medio [(7,35 ± 2,8 vs 4,1 ± 2,2 (p < 0.05)], dell'AHI medio [(21.4 ± 6 eventi per ora verso 8,85 ± 6,9 (p < 0.05) ] e dell'ODI medio [(18.6 ± 8 eventi per ora versus 7 ± 5.8 (p < 0.05)]. Abbiamo inoltre osservato che l'AHI migliorava di almeno il 50% rispetto al basale nel 71.4% dei pazienti selezionati mediante sleep endoscopy. In questo studio, la terapia con i dispositivi di avanzamento mandibolare è stata prescritta con successo sulla base non soltanto dell'indice di apnea/ipopnea, ma anche dei reperti della sleep endoscopy e della manovra di avanzamento mandibolare, ottenendo una visione diretta degli effetti della protrusione mandibolare sullo spazio respiratorio in corrispondenza dei siti di ostruzione, e ottenendo una buona ottimizzazione della selezione dei pazienti per il trattamento con MAD.
Introduction
Obstructive sleep apnoea syndrome (OSAS) is characterised by recurrent episodes of apnoea and hypopnoea during sleep caused by repetitive upper airway (UA) collapse and often resulting in decreased oxygen blood levels and arousal from sleep 1. It is also associated with excessive daytime sleepiness and an increased risk of cardiovascular and cerebrovascular complications 2-4 and metabolic comorbidities 5 6. Its prevalence in the general population is around 3%. However, its distribution varies widely according to gender, age and race. The incidence in men increases with a direct proportion to age from 40 to 65 years 7 8 and it is considered an important and independent risk factor for several systemic diseases such as hypertension, obesity and diabetes 6-9. The aetiological role of OSAS in severe hemodynamic or pulmonary function disorders is now recognised 10.
Treatment of sleep-disordered breathing (i.e. snoring, upper airway resistance syndrome, sleep apnoea syndrome) may include lifestyle modification, i.e. weight loss, cessation of evening alcohol ingestion, sleep position training, upper airway surgery, oral appliances and continuous positive airway pressure (CPAP). The latter is the most widely suggested method because it provides the most reliable therapeutic modality, especially in severe OSAS. Nevertheless, there is poor compliance of patients who often consider it a cumbersome therapy that is difficult to tolerate and unacceptable. For these reasons, several surgical 11-13 and non-surgical procedures have been proposed as an alternative, particularly in young non-apnoeic snorers, mild to moderate OSAS and in selected patients with severe OSAS. Among non-surgical techniques, good results have been obtained with the use of oral appliances 14 15, which are intended to protrude and stabilize the mandible maintaining a patent airway during sleep and producing favourable results within a short time 15 16.
Herein, we report our experience in treatment of mild moderate OSAS by oral appliance selected by drug induced sleep endoscopy (DISE) focusing on the following outcomes: sleep apnoea [i.e. reduction in the apnoea/ hypopnoea index (AHI) or respiratory disturbance index], ability of oral appliances to reduce snoring and effect on daytime function which are predictive parameters of good efficacy of the device.
Materials and methods
From September 2014 to February 2015 we evaluated 65 patients who underwent sleep endoscopy at our institution (Department of Head and Neck Surgery, Institute of Otorhinolaryngology "A. Gemelli Hospital", Catholic University School of Medicine and Surgery, Rome), and polysomnographically diagnosed as mild-to-moderate OSAS (AHI ≥ 5 to ≤ 30/h of sleep), 55 males and 10 females, aged between 22 and 68 years (mean age 44.26 years). BMI ranged from 22.6 to 30.4 kg/m2 (mean BMI 27.2±3.04). Mean Epworth index was 9.8±2.75. Study design: single institution, longitudinal prospective evaluation of a consecutive group of mild moderate OSAS patients who underwent DISE. All patients were willing to participate and consented to their inclusion.
All patients successfully underwent DISE. The procedure was performed in the operating theatre under the presence of an anaesthesiologist, neuroelectrophysiology technician and otolaryngologist. All patients, already prepared for polygraphic intraoperative recording, received sedation with administration of increasing doses of propofol (3 mg/ kg/h). The mean duration of the procedure was 25±18 min. The degree of sedation was under continuous monitoring by bispectral index (BIS) monitoring (Apect Medical Systems, Newton, MA). When BIS was between 50 and 70, generally during snoring, we introduced a 3.4 mm flexible nasopharyngoscope into the nasal cavity to visualise and record the pattern and degree of obstruction (nasopharynx, oropharynx, hypopharynx and larynx). During DISE, the level (palate, oropharynx, tongue base, hypopharynx/ epiglottis), the direction (anteroposterior [AP], concentric, lateral) and degree of upper airway collapse (none, partial, or complete) were scored in a standard fashion 17. Finally, we evaluated the degree of enlargement of the space in the sites of respiratory obstruction through a bimanual mandibular advancement manoeuvre. The mandible was gently advanced 4 to 5 mm by the anaesthetist and its effect on airway obstruction and snoring were noted. Subjects were considered eligible to utilise an oral appliance and were referred to the orthodontic department of our hospital in case of successful mandibular advancement manoeuvre. (Definition of success: obstructive events better or absent for at least 3 min, associated with endoscopic evidence of improved airway patency at one or more sites of obstruction by at least 50%). Within 30 days of the indication, the SomnoDent (Somnomed ® Ltd, Australia), a customised mandibular advancement device made of a rigid material Bflex, was applied.
All treated patients were evaluated at baseline and after an acclimatisation period of 3 months. We used the Epworth sleepiness scale to assess the severity of daytime symptoms, and the Berlin Questionnaire 18 to assess the degree and frequency of snoring, impact on daytime activities and degree of sleepiness. All subjects underwent polysomnography (Somntè, Compumedics Australia) before and after 3 months from application of device. We collected the main reference parameters of number and degree of pathological respiratory events: apnoea hypopnea index (AHI), oxygen desaturation index (ODI) and oxyhaemoglobin saturation performance (mean SpO2, minimal SpO2 and mean desaturation).
The data were analysed using Microsoft Excel and the SSPS statistical software package. Qualitative data were compared using Wilcoxon signed rank and T-tests. We considered an AHI value < 5 or a reduction of AHI ≥ 50% from baseline as criteria for successful treatment. Differences were considered statistically significant if the p value was less than 0.05. Results were presented as means and standard deviations.
Results
During DISE, 30 of 65 patients (46.2%) had a unsuccessful mandibular advancement manoeuvre, and were considered as "not suitable" for treatment with an oral device and other treatment options could be offered. On the other hand, 35 of 65 (53.8%) patients had a successful manoeuvre and were considered suitable for oral device application. Nevertheless, 7 of 35 patients were excluded because of a condition interfering with an oral appliance (edentulous or an insufficient number of healthy teeth in one or both dental arches, evidence or history of temporomandibular joint disease, inability to tolerate the device throughout the night, choking sensations, dry mouth, claustrophobia during MAD wear) and were referred for surgical procedures. Finally, 28 patients (6 females, 22 males) of 35 were recommended for an oral device.
An overview of the distribution of the levels of upper airway collapse at baseline for patients treated with an oral appliance based on DISE scoring is shown in Table I. The majority of patients had multilevel collapse [26/28 (92.8%)], predominantly at the palatal and tongue base levels and rarely at the oropharyngeal and hypopharynx/epiglottis levels. The most common upper airway collapse patterns noted were AP collapse at the levels of the palate [28/28 patients (100%)] and the tongue base [20/28 (71.4%)]. In none of the locations considered was a latero-lateral closing pattern observed. In agreement with literature data, most of our mild/moderate OSAS patients were without serious comorbidities [hypertension was seen in 10/28 patients (35.7%); no patient had diabetes, ischaemic heart disease, cerebrovascular disease, or atrial fibrillation)].
Table I.
Distribution of the levels of upper airway collapse including the corresponding direction of upper airway collapse based on DISE scoring in the 28 treated patients.
| Palate | Tongue base | Oropharynx | Larynx | |
|---|---|---|---|---|
| Anterior-posterior | 14/28 (50%) | 18/20 (90%) | 8/10 (80%) | 2/2 (100%) |
| Concentric | 14/28 (50%) | 2/20 (10%) | 2/10 (20%) | 0/2 (0%) |
| Latero-lateral | 0/28 (0%) | 0/20 (0%) | 0/10 (0%) | 0/2 (0%) |
The results for each question of the Berlin questionnaire before and after therapy are shown in Table II. Clustering the Berlin questionnaire answers by category, we observed a positive category 1 in 28/28 (100%) and a positive category 2 in 18/28 (64.2%) before treatment. After treatment, both were significantly reduced to 8/28 (28.6%) and 0/28 (0%), respectively. Answers related to category 3 were always negative.
Table II.
Berlin questionnaire results before and after treatment.
| Pre-treatment | Post-treatment | |
|---|---|---|
| CATEGORY 1 | ||
| Do you snore? | ||
| a. Yes | 28/28 (100%) | 8/28 (28.6%) |
| b. No | 0/28 (0%) | 15/28 (53.5%) |
| c. Don't know | 0/28 (0%) | 5/28 (17.9%) |
| Your snoring is: | ||
| a. Slightly louder than breathing | 0/28 (0%) | 20/28 (71.4%) |
| b. As loud as talking | 0/28 (0%) | 8/28 (28.6%) |
| c. Louder than talking | 14/28 (50%) | 0/28 (0%) |
| d. Very loud. Can be heard in adjacent rooms. | 14/28 (50%) | 0/28 (0%) |
| How often do you snore? | ||
| a. Almost every day | 26/28 (92.86%) | 0/28 (0%) |
| b. 3-4 times per week | 2/28 (7.14%) | 8/28 (28.6%) |
| c. 1-2 times per week | 0/28 (0%) | 14/28 (50%) |
| d. 1-2 times per month | 0/28 (0%) | 2/28 (7.14%) |
| e. Rarely or never | 0/28 (0%) | 4/28 (14.3%) |
| Has your snoring ever bothered other people? | ||
| a. Yes | 15/28 (53.5%) | 4/28 (14.3%) |
| b. No | 3/28 (10.9%) | 8/28 (28.6%) |
| c. Don't know | 10/28 (35.6%) | 16/28 (57.1%) |
| Has anyone noticed that you stop breathing during your sleep? | ||
| a. Almost every day | 16/28 (57.1%) | 0/28 (0%) |
| b. 3-4 times per week | 8/28 (28.6%) | 0/28 (0%) |
| c. 1-2 times per week | 4/28 (14.3%) | 0/28 (0%) |
| d. 1-2 times per month | 0/28 (0%) | 25/28 (89.3%) |
| e. Rarely or never | 0/28 (0%) | 3/28 (10.7%) |
| CATEGORY 2 | ||
| How often do you feel tired or fatigued after your sleep? | ||
| a. Almost every day | 10/28 (35.6%) | 0/28 (0%) |
| b. 3-4 times per week | 8/28 (28.6%) | 0/28 (0%) |
| c. 1-2 times per week | 4/28 (14.3%) | 10/28 (35.6%) |
| d. 1-2 times per month | 2/28 (7.14%) | 10/28 (35.6%) |
| e. Rarely or never | 4/28 (14.3%) | 8/28 (28.6%) |
| During your waking time, do you feel tired, fatigued or not up to par? | ||
| a. Almost every day | 10/28 (35.6%) | 0/28 (0%) |
| b. 3-4 times per week | 8/28 (28.6%) | 0/28 (0%) |
| c. 1-2 times per week | 4/28 (14.3%) | 10/28 (35.6%) |
| d. 1-2 times per month | 2/28 (7.14%) | 10/28 (35.6%) |
| e. Rarely or never | 4/28 (14.3%) | 8/28 (28.6%) |
| Have you ever nodded off or fallen asleep while driving a vehicle? | ||
| a. Yes | 4/28 (14.3%) | 0/28 (0%) |
| b. No | 24/28 (85.7%) | 28/28 (100%) |
| How often does this occur? | ||
| a. Almost every day | 0/4 (0%) | 0 (0%) |
| b. 3-4 times per week | 1/4 (25%) | 0 (0%) |
| c. 1-2 times per week | 2/4 (50%) | 0 (0%) |
| d. 1-2 times per month | 1/4 (25%) | 0 (0%) |
| e. Rarely or never | 0/4 (0%) | 0 (0%) |
| CATEGORY 3 | ||
| Do you have high blood pressure? | ||
| a. Yes | 10/28 (35.6%) | 10/28 (35.6%) |
| b. No | 18/28 (64.3%) | 18/28 (64.3%) |
| c. Don't know | 0/28 (0%) | 0/28 (0%) |
| BMI | always <30 | always <30 |
Scoring Questions: Any answer in bold is a positive response; Scoring categories: Category 1 is positive with 2 or more positive responses to questions 1-5. Category 2 is positive with 2 or more positive responses to questions 6-9. Category 3 is positive with 1 positive response and/or a BMI > 30.
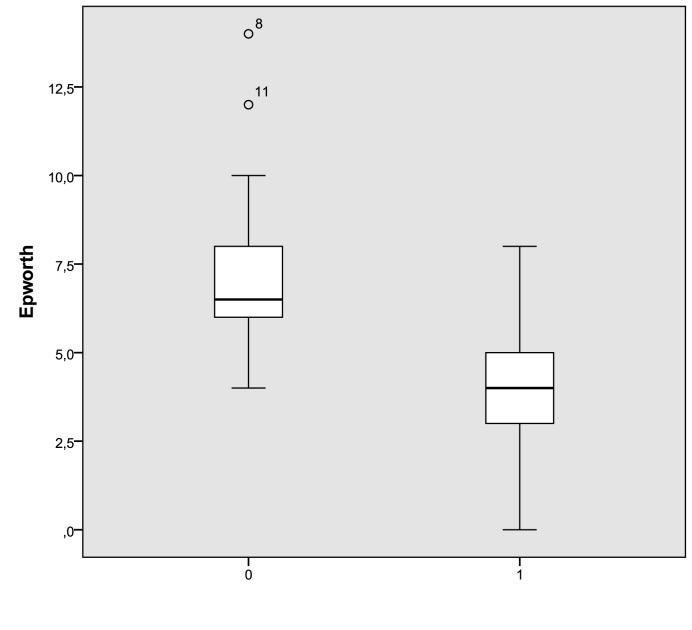
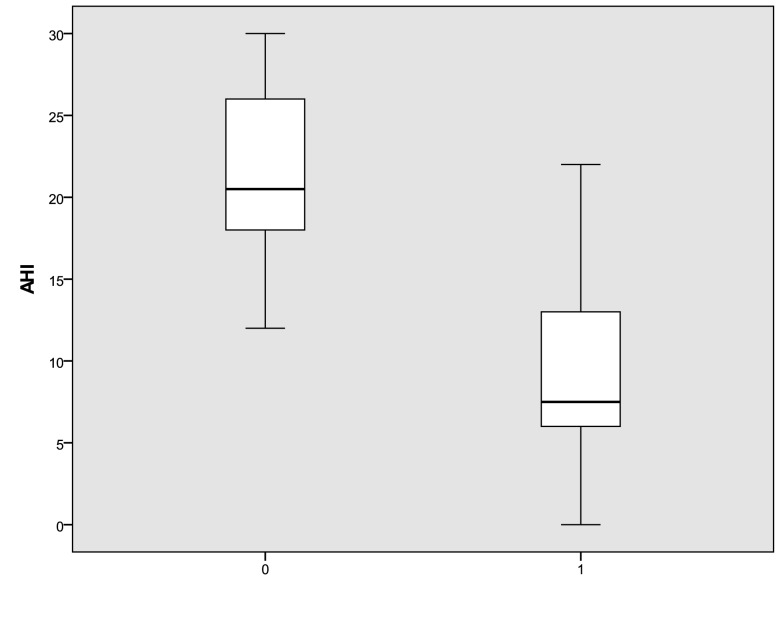
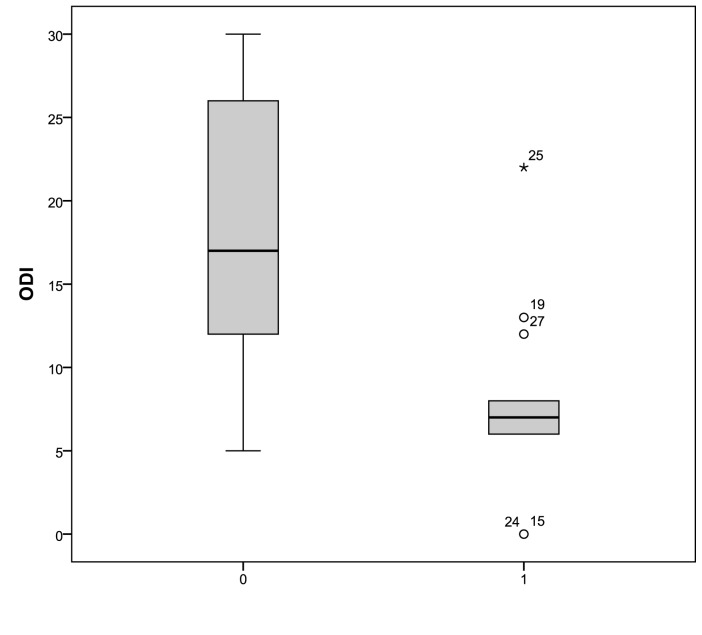
In treated patients, the Epworth medium index 19 significantly decreased after application of an oral device from 7.35 ± 2.8 to 4.1 ± 2.2 (p < 0.05). The distribution of Epworth scores in treated patients are shown in Figure 1. The mean AHI decreased significantly from 21.4 ± 6 events per hour at baseline to 8.85±6.9 events per hour (p < 0.05) after 12 weeks of treatment. In addition, the mean ODI decreased significantly from 18.6 ± 8 events per hour at baseline to 7 ± 5.8 (p < 0.05), as shown in Figures 2 and 3. We did not observe a significant variation of the mean values of desaturation maximum (respectively 85% versus 84.5%), desaturation average (91% versus 90.4%) and average saturation (94% versus 93.5%) before and after treatment. None of the treated patients referred complications with the oral device.
Fig. 1.
Epworth score distribution at baseline and after three months of treatment with an oral device (0: pre; 1: post). The box plots show the median and inter-quartile range and the error bars show the 5th and 95th percentiles.
Fig. 2.
AHI distribution at baseline and after three months of treatment with an oral device (0: pre; 1: post). The box plots show the median and inter-quartile range and the error bars show the 5th and 95th percentiles.
Fig. 3.
ODI distribution at baseline and after three months of treatment with an oral device (0: pre; 1: post). The box plots show the median and inter-quartile range and the error bars show the 5th and 95th percentiles.
Analysing the success rate of treatment at baseline 4 out of 28 (14.3%) patients showed polysomnography AHI > 5 and < 15 and 24 out of 28 (85.7%) patients showed an AHI > 15 and < 30. After treatment, 6 out of 28 (21.4%) patients achieved an AHI < 5, whereas in 18 out of 28 (57.1%) cases AHI values decreased by ≥ 50%. In Table III the distribution of patients according to the degree of OSAS before and after treatment is presented.
Table III.
Distribution of patients according to the degree of OSAS before and after treatment.
| AHI | Pre | Post | p value |
|---|---|---|---|
| AHI<5 | 0/28 (0%) | 6/28 (21.4%) | <0.005 |
| 5<AHI<15 | 4/28 (14.3%) | 16/28 (57.1%) | <0.005 |
| 15<AHI<30 | 24/28 (85.7%) | 6/28 (21.4%) | <0.005 |
Discussion
Nowadays oral appliance therapy (OAT) 20 is recognised as an effective therapy for many patients with primary snoring and mild to moderate OSAS, as well as those with more severe OSAS who cannot tolerate positive airway pressure (PAP) therapies 21. It has been presumed that OAT may prevent upper airway collapse and increase the cross-sectional airway dimensions, thereby reduce snoring and obstructive sleep apnoeas 22. Advancement devices (MADs) are currently the most common class of oral appliances used to treat OSAS, and custom-made MADs are preferred and recommended over prefabricated devices 23. Bearing in mind the wide use of mandibular advancement appliances in the management of sleep-related breathing disorders, it is very important to focus on objective criteria to indicate which subjects may benefit from such treatment by trying to look for predictors of treatment outcome and selecting patients for MAD treatment 24.
Various anthropometric and polysomnographic predictors have been described in the literature, including lower AHI, lower BMI, lower age, female gender and supine-dependent OSA 25, whereas there are still controversies about the role of DISE and in particular about mandibular advancement bimanual manoeuvre during OSA as predictor factors of treatment outcome by an oral device. In the literature, the effect of a mandibular protrusion less than 5 mm was suggested to improve patient selection for MAD treatment 26. Johal et al. 27 suggested that DISE with concomitant gently mandibular advancement manoeuvre to mimic the treatment effect may be of prognostic value in determining the likelihood of successful mandibular advancement splint therapy in subjects with sleep-related breathing disorders. In the literature, other authors have supported the concept of DISE with the addition of a simulation bite 28. Vroegop et al. 29 demonstrated that patients in whom upper airway patency improved substantially with the presence of the simulation bite in maximal comfortable protrusion during DISE are more likely to be treated successfully with MAD treatment. Furthermore, the author concluded that a manoeuvre of hyperprotrusion/maximal protrusion of the mandible had no predictive value on oral device therapy outcomes. The authors suggested that the chin-lift manoeuvre in maximal protrusion may be clinically less relevant for therapeutic decision-making than simulated advancement because each oral appliance inherently causes a certain amount of vertical mouth opening and the manoeuvre is not reproducible in terms of the degree of mandibular advancement. Finally, manoeuvres can be disturbing stimuli during DISE, potentially provoking arousal by awakening of the patient during the procedure.
In the current study, mandibular advancement splint therapy was prescribed on the basis not only of severity of disease, as determined from the subject's initial AHI, but also by DISE findings and results of the gentle mandibular advancement manoeuvre during DISE allowing a direct view of effects of mandibular protrusion on breathing spaces in obstruction sites 30 31. All data were considered and subjects who demonstrated improved airway patency and/or reduced snoring as a result of a gentle mandibular advancement manoeuvre were referred for splint therapy. In treated patients, a significant improvement of Epworth index [(7.35 ± 2.8 versus 4.1 ± 2.2 (p < 0.05)], mean AHI [(21.4 ± 6 events per hour versus 8.85 ± 6.9 (p < 0.05)] and mean ODI [(18.6 ± 8 events per hour to 7±5.8 (p < 0.05)]. Furthermore, the results of this study indicate that the presence of antero-posterior pattern of closure and absence of the latero-lateral one at the level of the palate as documented during pre-treatment DISE are associated with therapeutic success in mild/moderate OSA patients treated with custom-made MADs.
The results of our study suggest that the mandibular advancement manoeuvre during DISE could help to optimise the selection of patients for oral device treatment. In fact, we observed that AHI improved up to 50% from baseline in 71.4% of patients selected after DISE for MAD therapy. This result is consistent with data reported in the literature. In 2013, Doff MJ et al. 32, by comparing the results obtained with MAD vs. CPAP in two groups homogeneous for age, sex, BMI, degree of OSAS and symptoms, confirmed the efficacy of MAD in mild/moderate diseases (64% of success at one year) and CPAP in patients with severe disease. Other studies have already indicated such a trend 33 34. Finally, Vanderveken V et al. 35, using a simulation byte, demonstrated that the successful rate may increase to 83.3%.
We believe that several factors may affect the interpretation of data about mandibular advancement manoeuvre during DISE including: subjective nature of the observations during DISE, DISE scoring system used, grade of protrusion (hyper or gentle protrusion), criterion adopted for successful manoeuvre (complete resolution of snoring and obstructive breathing events) and complete or partial resolution of apnoeic events > 50% from baseline); extent that upper airway patency needs to change in order to counteract upper airway collapse effectively. Further studies should help to clarify these points. We believe that it is also very important to look for predictive factors of tolerability of the MADs 36 based on the grade of mandibular protrusion during DISE.
Based on our data, we believe that even though the best setting should be obtained using a simulation costumed - byte during DISE, if it is not available gentle manoeuvre of mandibular advancement should be performed because it may optimise selection of patients for MAD therapy. Since it has been recently demonstrated that DISE, completed with a jaw thrust manoeuvre, has a relevant influence on the location of treatment recommendations, especially when considering MAD treatment 37 38, future studied should focus on the most simple and reliable procedure with the best cost effectiveness ratio that should be applied during sleep endoscopy to predict the best outcomes with oral appliance therapy.
References
- 1. American Academy of Sleep Medicine Task Force , author. Sleeprelated breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Avaheri S, Wexler L. Prevalence and treatment of breathing disorders during sleep in patients with heart failure. Treat Options Cardiovasc Med. 2005;7:295–306. doi: 10.1007/s11936-005-0040-0. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Fusetti M, Fioretti AB, Valenti M, et al. Cardiovascular and metabolic comorbidities in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2012;32:320–325. [PMC free article] [PubMed] [Google Scholar]
- 6.Passàli D, Tatti P, Toraldo M, et al. OSAS and metabolic diseases: Round Table, 99(th) SIO National Congress, Bari 2012. Acta Otorhinolaryngol Ital. 2014;34:158–166. [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, et al. The occurrence of sleepdisordered breathing among middle-aged adults. N Engl J Med. 1993;32:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Duran J, Esnaola S, Rubio R, et al. Obstructive sleep apnoea hypopnoea and related clinical features in a population based sample of subjects aged 30 to 70 yr. Am J Respir Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 9.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: lung cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 10.Khan F, Walsh C, Lane SJ, et al. Sleep apnoea and its relationship with cardiovascular, pulmonary, metabolic and other morbidities. Ir Med J. 2014;107:6–8. [PubMed] [Google Scholar]
- 11.Mantovani M, Minetti A, Torretta S, et al. The "Barbed Roman Blinds" technique: a step forward. Acta Otorhinolaryngol Ital. 2013;33:128–128. [PMC free article] [PubMed] [Google Scholar]
- 12.Salamanca F, Costantini F, Mantovani M, et al. Barbed anterior pharyngoplasty: an evolution of anterior palatoplasty. Acta Otorhinolaryngol Ital. 2014;34:434–438. [PMC free article] [PubMed] [Google Scholar]
- 13.Scarano E, Marca G, Corso E, et al. Hyoid myotomy without suspension: a surgical approach to obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2014;34:362–367. [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnadoux F, Fleury B, Vielle B, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34:914–920. doi: 10.1183/09031936.00148208. [DOI] [PubMed] [Google Scholar]
- 15.Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea mandibular advancement device therapy. Eur Respir J. 2012;39:1241–1247. doi: 10.1183/09031936.00144711. [DOI] [PubMed] [Google Scholar]
- 16.SC DL, Almeida FR, Bennett KM, et al. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: a report of the American Academy of Dental Sleep Medicine. J Dent Sleep Med. 2014;1:39–50. [Google Scholar]
- 17.Vito A, Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE) Sleep Breath. 2014;18:453–465. doi: 10.1007/s11325-014-0989-6. [DOI] [PubMed] [Google Scholar]
- 18.Karakoc O, Akcam T, Genc H, et al. Use of the Berlin Questionnaire to screen at-risk patients for obstructive sleep apnea. B-ENT. 2014;10:21–25. [PubMed] [Google Scholar]
- 19.Vignatelli L, Plazzi G, Barbato A, et al. GINSEN (Gruppo Italiano Narcolessia Studio Epidemiologico Nazionale). Italian version of the Epworth sleepiness scale: external validity. Neurol Sci. 2003;23:295–300. doi: 10.1007/s100720300004. [DOI] [PubMed] [Google Scholar]
- 20.Hoffstein V. Review of oral appliances for treatment of sleepdisordered breathing. Sleep Breath. 2007;11:1–22. doi: 10.1007/s11325-006-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milano F, Mondini S, Billi MC, et al. The impact of a multidisciplinary approach on response rate of mandibular advancing device therapy in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:337–342. [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuiki S, Lowe AA, Almeida FR, et al. Effects of mandibular advancement on airway curvature and obstructive sleep apnoea severity. Eur Respir J. 2004;23:263–268. doi: 10.1183/09031936.04.00094304. [DOI] [PubMed] [Google Scholar]
- 23.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178:197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 24.Petri N, Svanholt P, Solow B, et al. Mandibular advancement appliance for obstructive sleep apnoea: results of a randomised placebo controlled trial using parallel group design. J Sleep Res. 2008;17:221–229. doi: 10.1111/j.1365-2869.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 25.Marklund M, Stenlund H, Franklin KA. Mandibular advance- ment devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–1278. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 26.Battagel JM, Johal A, Kotecha BT. Sleep nasendoscopy as a predictor of treatment success in snorers using mandibular advancement splints. J Laryngol Otol. 2005;119:106–112. doi: 10.1258/0022215053419916. [DOI] [PubMed] [Google Scholar]
- 27.Johal A, Hector MP, Battagel JM, et al. Impact of sleep nasendoscopy on the outcome of mandibular advancement splint therapy in subjects with sleep-related breathing disorders. J Laryngol Otol. 2007;121:668–675. doi: 10.1017/S0022215106003203. [DOI] [PubMed] [Google Scholar]
- 28.Vanderveken OM, Vroegop AV, Heyning PH, et al. Drug-induced sleep endoscopy completed with a simulation bite approach for the prediction of the outcome of treatment of obstructive sleep apnea with mandibular repositioning appliances. Op Techn Otolaryngol Head Neck Surg. 2011;22:175–182. [Google Scholar]
- 29.Vroegop AV, Vanderveken OM, Dieltjens M, et al. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res. 2013;22:348–355. doi: 10.1111/jsr.12008. [DOI] [PubMed] [Google Scholar]
- 30.Salamanca F, Costantini F, Bianchi A, et al. Identification of obstructive sites and patterns in obstructive sleep apnoea syndrome by sleep endoscopy in 614 patients. Acta Otorhinolaryngol Ital. 2013;33:261–266. [PMC free article] [PubMed] [Google Scholar]
- 31.Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;9:433–438. doi: 10.5664/jcsm.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doff MH, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep. 2013;36:1289–1296. doi: 10.5665/sleep.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Canadian Agency for Drugs and Technologies in Health , author. Oral appliances for treatment of snoring and obstructive sleep apnea: a review of clinical effectiveness. CADTH Technol Overv. 2010;1:e0107–e0107. [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson KA, Ono T, Lowe AA, et al. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52:362–368. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderveken OM, Vroegop AV, Heyning PH, et al. Drug-induced sleep endoscopy completed with a simulation bite approach for the prediction of the outcome of treatment of obstructive sleep apnea with mandibular repositioning appliances. Op Techn Otolaryngol Head Neck Surg. 2011;22:175–182. [Google Scholar]
- 36.Marklund M, Franklin KA. Long-term effects of mandibular repositioning appliances on symptoms of sleep apnoea. J Sleep Res. 2007;16:414–420. doi: 10.1111/j.1365-2869.2007.00615.x. [DOI] [PubMed] [Google Scholar]
- 37.Corso E, Fiorita A, Rizzotto G, et al. The role of druginduced sleep endoscopy in the diagnosis and management of obstructive sleep apnoea syndrome: our personal experience. Acta Otorhinolaryngol Ital. 2013;33:405–413. [PMC free article] [PubMed] [Google Scholar]
- 38. Eichler C, Sommer JU, Stuck BA, et al. Does drug-induced sleep endoscopy change the treatment concept of patients with snoring and obstructive sleep apnea? Sleep Breath. 2012 doi: 10.1007/s11325-012-0647-9. DOI: 10.1007/s11325-012-0647-9. [DOI] [PubMed] [Google Scholar]