SUMMARY
The aim of this study was to investigate the oncological outcomes in patients affected by oral carcinoma treated with radical compartmental surgery followed by microvascular flap reconstruction. We conducted a retrospective analysis on a cohort of 130 patients. All patients underwent ablative tumour resection (compartmental surgery) followed by immediate reconstruction with free flaps and adjuvant chemoradiotherapy, when necessary according to our tumour board and international guidelines. Disease-specific survival (DSS) curves were obtained using the Kaplan-Meier method. Log-rank test and generalised Wilcoxon test were used to investigate the most important prognostic factors on 5-year DSS. A Cox proportional hazards model was constructed to provide hazard ratios or relative risks for individual variables. 88.5% of patients were affected by SCC. There were 46 (35.4%) women and 84 (64.6%) men in the sample with a mean age of 58.5 years. At the end of the follow-up period, 36 (27.7%) patients died, only 3 of which for other causes. The 5-year DSS rate was 67.8% (S.E. 4.9%). In univariate Kaplan-Meier analysis and in multivariate Cox regression model, seven variables were found to have a significant relationship with DSS: T (p = 0.026) and N (p = 0.0001) status, clinical stage (according to the UICC TNM Sixth Edition) (p = 0.007), margins of resection (p = 0.001), extracapsular spread (p = 0.005), recurrence of disease (p = 0.00002) and treatment modality (evaluated as surgery alone or surgery + RT/CHT) (p = 0.004). Our results confirmed findings already reported in the literature, and allowed us to conclude that compartmental surgery combined with free flap reconstruction can increase survival in oral cancer patients.
KEY WORDS: Oral cavity cancer, Microvascular free flaps, Survival, Compartmental surgery
RIASSUNTO
Obiettivo del presente studio è stato valutare i risultati oncologici della nostra casistica di pazienti affetti da tumore del cavo orale trattati mediante chirurgia compartimentale radicale seguita da ricostruzione mediante lembo microvascolare. Abbiamo condotto un'analisi retrospettiva su 130 casi. Tutti i pazienti sono stati sottoposti ad una resezione chirurgica della neoformazione seguita da una ricostruzione immediata mediante lembo libero e, quando necessario, in accordo con la valutazione espressa dal nostro tumor board e con le linee guida internazionali, ad un trattamento chemioradioterapico adjuvante. Le curve di sopravvivenza specifica per malattia (DSS) sono state ottenute mediante il metodo di Kaplan-Meier. Il test Long Rank e il Wilcoxon sono stati utilizzati per investigare i più importanti fattori influenzanti la sopravvivenza specifica per malattia a 5 anni. Per calcolare l'HR e il RR per le singole variabili è stato utilizzato un modello di Cox. L'88,5% dei pazienti è risultato affetto da una neoplasia a istologia squamocellulare. Il campione è risultato essere composto da 46 (35,4%) donne e 84 (64,6%) uomini con un età media di 58,5 anni. Al termine del periodo di follow up, 36 pazienti (27,7%) erano deceduti, 3 dei quali per altre cause. Il DSS è stato del 67,8% (S.E. = 4,9%). All'analisi univariata secondo Kaplan-Meier ed alla analisi multivariata con regressione di Cox sono state individuate sette differenti variabili aventi una relazione significativa con il DSS: T (p = 0,026) ed N (p = 0,0001), lo staging clinico (UICC TNM Sixth Edition) (p = 0,007), i margini di resezione (p = 0,001), l'extracapsular spread (p = 0.005), la recidiva di malattia (p = 0,00002) e la modalità di trattamento (sola chirurgia o chirurgia + RT/CHT) (p = 0,004). In nostri risultati sono risultati in linea con le osservazioni in letteratura, e ci permettono di sottolineare come la chirurgia ricostruttiva mediante lembo libero microvascolare possa incrementare la sopravvivenza nei pazienti con tumore del cavo orale.
Introduction
Oral cancer is the 8th most common neoplasm worldwide with 300,000 cases annually 1. It presents extensive variability in terms of incidence between different regions and is highest in southeast Asia. In Italy, about 4/100,000 new cases per year are documented and it is most common in areas where voluptuary habits such alcohol consumption and tobacco smoking are more diffuse 2.
Surgery is considered the gold standard to achieve tumour control, but the diagnosis is usually late when the disease has already reached an advanced stage, for this reason, in the majority of cases, neoplasm dimensions, combined with the necessity of clear margins at least 1 cm around the tumour, lead to large resections requiring reconstructive surgery with important functional implications. Today this aim can be achieved through the use of microvascular free flaps that have replaced classical local and regional flaps to ensure oncologic radicality on one hand, better functional and aesthetic results on the other 3.
In fact, quality of life has gained great interest in the last years, and has become a secondary endpoint of care, while survival is the main outcome for these patients 4-8. Starting from this observation, we focused on diseasespecific survival (DSS), because the relatively advanced age and comorbidities of patients can create several problems when basing outcome only on overall survival 9. We analysed a cohort of 130 patients treated with reconstructive surgery affected by oral cancer from 2005 to 2013 and correlated survival to clinical and pathological parameters.
Materials and methods
Patients
The retrospective cohort consisted of 130 patients affected by oral cancer; all underwent surgical treatment between 2005 and 2013 at the Department of Head and Neck Surgery - Otolaryngology/Department of Plastic and Reconstructive Surgery, Catholic University of Sacred Heart. 88% of the patients were affected by oral squamous cell carcinoma (OSCC). The main clinical characteristics of our patients are shown in Table I. Preoperative head and neck CT and MRI (to establish clinical staging and therapeutic planning), total body PET-CT (in case of relapse/ persistence of disease), colour Doppler ultrasound of neck vessels and free-flap donor vessels (to evaluate anatomy and calibre of vessels and perforator anatomy in case of perforator flaps) were performed for each patient.
Table I.
Clinical features.
| Characteristics | No. patients (%) |
|---|---|
| Gender | |
| Male | 84 (64.6%) |
| Female | 46 (35.4%) |
| Age | |
| Mean | 58.5 (±1.05 S.E.) |
| <50 | 36 (27.7%) |
| ≥50 | 94 (72.3%) |
| Primary site | |
| Tongue | 56 (50%) |
| Floor of mouth | 29 (22.3%) |
| Retromolar trigone | 12 (9.2%) |
| Gum | 8 (6.2%) |
| Buccal mucosa | 3 (2.3%) |
| Palate | 7 (5.4%) |
| Mandible | 3 (2.3%) |
| Lip | 3 (2.3%) |
| Histology | |
| Squamous cell carcinoma (SCC) | 115 (88.4) |
| Adenoid cystic carcinoma | 7 (5.4) |
| Other | 8 (6.2) |
| T status | |
| T1 | 10 (7.7%) |
| T2 | 45 (34.6%) |
| T3 | 30 (23.1%) |
| T4 | 45 (34.6%) |
| N status | |
| N0 | 52 (40%) |
| N1 | 31 (23.9%) |
| N2 | 45 (34.6%) |
| N3 | 2 (1.5%) |
| Clinical Stage* | |
| I | 5 (3.8%) |
| II | 20 (15.4%) |
| III | 33 (25.4%) |
| IV | 72 (55.4%) |
| Type of flap | |
| ALT** | 58 (44.6%) |
| Fibula | 28 (21.5%) |
| FFRF** | 18 (13.9%) |
| DIEP** | 14 (10.8%) |
| TRAM** | 6 (4.6%) |
| Chimeric Flap** | 5 (3.8%) |
| VRAM** | 1 (0.8%) |
| Margin | |
| Positive | 19 (14.6%) |
| Close | 14 (10.8%) |
| Negative | 97 (74.6%) |
| Extracapsular spread | |
| Yes | 98 (75.4%) |
| No | 32 (24.6%) |
| Perineural invasion | |
| Yes | 118 (90.8%) |
| No | 12 (9.2%) |
| Mandible/Maxilla involvement | |
| Yes | 48 (36.9%) |
| No | 82 (63.1%) |
| Recurrence | |
| Yes | 47 (36.1%) |
| No | 83 (63.9%) |
| Treatment modality | |
| Surgery alone | 46 (35.4%) |
| Surgery + RT/CT | 84 (64.6%) |
| Lymph node dissection | |
| Unilateral | 14 (10.8%) |
| Bilateral | 114 (87.7%) |
| No | 2 (1.5%) |
| Clinical status at end of follow-up | |
| No evidence of disease (NED) | 79 (60.8%) |
| Alive with disease (AWD) | 15 (11.5%) |
| Dead of disease (DOD) | 36 (27.7%) |
According to UICC TNM Sixth Edition.
ALT: Anterolateral Thigh Perforator flap; FFR: Free Forearm Radial Flap; DIEP: Deep Inferior Epigastric Perforator flap; TRAM: Transverse Rectus Abdominis Myocutaneous flap; VRAM: Vertical Rectus Abdominis Myocutaneous flap; Chimeric flap: combination of two microvascular flaps.
The type of oncologic resection and reconstruction, as well as length of stay in the intensive care unit and recipient/ donor site postoperative complications were also recorded.
All patients underwent radical compartmental surgery followed by immediate microvascular flap reconstruction. Because of the correlation between the thickness of the primary tumour and the risk of nodal metastasis 10, we performed selective bilateral neck dissection in all patients who presented a depth of tumour invasion > 3 mm, or a locally advanced stage (T3-T4) with N+ at presentation. Adjuvant radiotherapy was performed in case of positive margins, T4 status, N status >1 and extracapsular spread. Following the NCCN guidelines, we respected the ≤6 week interval between resection and post-operative RT and used a typical regimen based on a three field method including bilateral parallel opposed fields to the primary site and upper neck. We generally administered 60-66 Gy (2 Gy daily fraction 5 days per week) for irradiation of the primary site and neck in case of involved nodal stations, and 44-64 Gy (1.6-2.0 Gy daily fraction 5 days per week) for the neck in case of uninvolved nodal stations.
Recurrence was evaluated as local (if involving only the oral cavity relative to the primary tumour), regional (if involving only the neck) and loco-regional (if involving both the primary site and neck). To confirm recurrences, we used biopsy, CT or MRI and generally PET-CT.
Statistical analysis
Data were analysed with statistical software (SPSS 21.0 for Windows; SPSS, Inc., Chicago, IL). DSS curves were obtained using the Kaplan-Meier method. Log-rank test and generalised Wilcoxon test were used to investigate the most important prognostic factors on 5-year DSS. A Cox proportional hazards model was constructed to provide hazard ratios for individual variables. A P value <0.05 was considered statistically significant.
Results
There were 46 (35.4%) women and 84 (64.6%) men in the sample with a mean age of 58.5 years ± 12.04 (range 26 to 83 years). On a total 130 patients, 119 received primary surgery in our department; 13 received salvage surgery for persistence/recurrence of disease; 11 underwent primary surgery in different hospitals and were referred to us for treatment of recurrence with reconstructive surgery. As shown in Table I, 58 patients (44.6%) received an ALT flap, 28 (21.5%) a fibula flap, 18 (13.9%) a FFR flap, 14 (10.8%) a DIEP flap, 6 (4.6%) a TRAM flap and 1 (0.8%) a VRAM flap reconstruction. Five patients (3.8%) underwent reconstruction with a chimeric flap, defined as a combined composite flap used in special cases in which we had the need to reconstruct, in addition to a bone defect, an extensive cutaneous or mucosal defect.
At the end of the follow-up period, on June 2013, (average 33.4; range 2 to 205 months) 36 patients had died, 33 for the disease and 3 for other causes. We observed a 5-year DSS of 67.8% (± 4.9% SE) (Fig. 1). Univariate Kaplan-Meier analysis revealed statistically significant relationships between DSS and T (p = 0.026) and N (p = 0.0001) status, clinical stage (p = 0.007), margins of resection (p = 0.001), extracapsular spread (p = 0.005), recurrence of disease (p = 0.00002) and treatment modality (p = 0.004). Results are shown in Table II. Kaplan- Meier survival curves by different variables are shown in Figure 2.
Fig. 1.
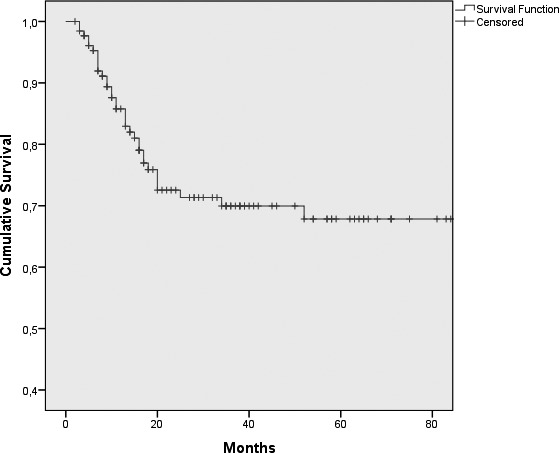
Cumulative disease-specific survival.
Table II.
Kaplan-Meier analysis: relationship between variables and survival.
| Variables | No. patients (%) | 5 year DSS (±S.E.) | Mean survival time (mo) (95% CI) | p value |
|---|---|---|---|---|
| Gender | 0.20 | |||
| Male | 84 (64.6%) | 63.9% (5.9%) | 75.9 (64-87) | |
| Female | 46 (35.4%) | 75.4% (8.4%) | 159.7 (130-188) | |
| Age | 0.88 | |||
| Mean | 58.5 (±1.05 S.E.) | |||
| <50 | 36 (27.7%) | 62.3% (9.6%) | 76.7 (60-93) | |
| ≥50 | 94 (72.3%) | 68.1% (5.4%) | 148.8 (128-269) | |
| Primary site | 0.62 | |||
| Tongue | 56 (50%) | 73% (6.1%) | ||
| Floor of mouth | 29 (22.3%) | 68.5% (9.5%) | ||
| Retromolar trigone | 12 (9.2%) | 56.6% (17%) | ||
| Gum | 8 (6.2%) | 37.5% (17.1%) | ||
| Buccal mucosa | 3 (2.3%) | 50% (34%) | ||
| Palate | 7 (5.4%) | 53.3% (24.8) | ||
| Mandible | 3 (2.3%) | |||
| Lip | 3 (2.3%) | 66.7% (27.2%) | ||
| Histology | 0.36 | |||
| Squamous cell carcinoma (SCC) | 115 (88.4) | 65.2% (5.3%) | ||
| Adenoid cystic carcinoma | 7 (5.4) | 100% | ||
| Other | 8 (6.2) | 83.3% (15.2%) | ||
| T status | 0.026 | |||
| T1 | 10 (7.7%) | 100% | ||
| T2 | 45 (34.6%) | 84.9% (5.7%) | ||
| T3 | 30 (23.1%) | 64.7% (10.6%) | ||
| T4 | 45 (34.6%) | 47.5% (9.2%) | ||
| N status | 0.0001 | |||
| N0 | 52 (40%) | 87% (5.7%) | 98.8 (88-108) | |
| N1 | 31 (23.9%) | 66.2% (10.2%) | 66.9 (53-80) | |
| N2 | 45 (34.6%) | 54% (8.1%) | 115.6 (85-146) | |
| N3 | 2 (1.5%) | 0% | 11.5 (0-28) | |
| Clinical Stage* | 0.007 | |||
| I | 5 (3.8%) | 100% | ||
| II | 20 (15.4%) | 93.3% (6.4%) | ||
| III | 33 (25.4%) | 80.1% (8.3%) | ||
| IV | 72 (55.4%) | 54.2 (6.9%) | ||
| Type of flap | 0.055 | |||
| ALT | 58 (44.6%) | 77.4% (6.1%) | ||
| Fibula | 28 (21.5%) | 68.3% (10.4%) | ||
| FFRF | 18 (13.9%) | 73.3% (17.6%) | ||
| DIEP | 14 (10.8%) | 30.6% (15.7%) | ||
| TRAM | 6 (4.6%) | 66.7% (19.2%) | ||
| Chimeric Flap | 5 (3.8%) | 30% (23.9%) | ||
| VRAM | 1 (0.8%) | 100% | ||
| Margin | 0.001 | |||
| Positive | 19 (14.6%) | 24.2% (10.5%) | 35.3 (17-52) | |
| Close | 14 (10.8%) | 77% (11.5%) | 71.4 (52-89) | |
| Negative | 97 (74.6%) | 76.5 % (5.5%) | 161 (141-180) | |
| Extracapsular spread | 0.005 | |||
| Yes | 98 (75.4%) | 46.7% (9.6%) | 102.7 (66-138) | |
| No | 32 (24.6%) | 75.6% (5.4%) | 88.3 (78-97) | |
| Perineural invasion | 0.22 | |||
| Yes | 118 (90.8%) | 58.3% (17%) | 38.8 (23-54) | |
| No | 12 (9.2%) | 68.9% (5.1%) | 146.3 (127-164) | |
| Mandible/Maxilla involvement | 0.75 | |||
| Yes | 48 (36.9%) | 60.5% (9%) | 68.4 (55-81) | |
| No | 82 (63.1%) | 72.4% (5.5%) | 151.7 (130-172) | |
| Recurrence | 0.00002 | |||
| Yes | 47 (36.1%) | 86.9% (4.1%) | 97.9 (89-105) | |
| No | 83 (63.9%) | 38.4% (8.7%) | 89.2 (58-120) | |
| Treatment modality | ||||
| Surgery alone | 46 (35.4%) | 90.1% (4.7%) | 101 (91-110) | |
| Surgery + RT/CT | 84 (64.6%) | 56.6% (6.4%) | 122.9 (99-146) | |
| Lymph node dissection | 0.63 | |||
| Unilateral | 14 (10.8%) | 57.9% (19.9%) | ||
| Bilateral | 114 (87.7%) | 67.5% (5.2%) | ||
| No | 2 (1.5%) | 100% |
According to UICC's TNM Sixth Edition.
Fig. 2.
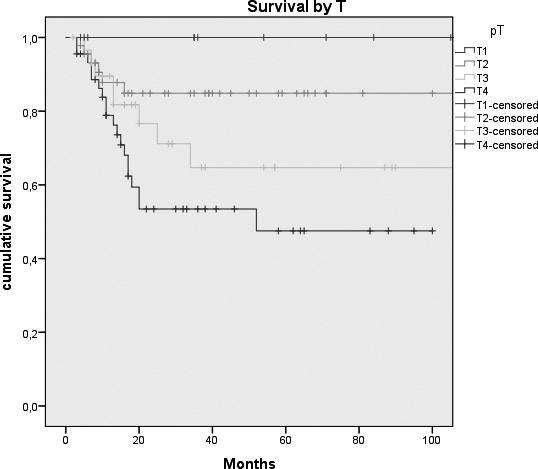
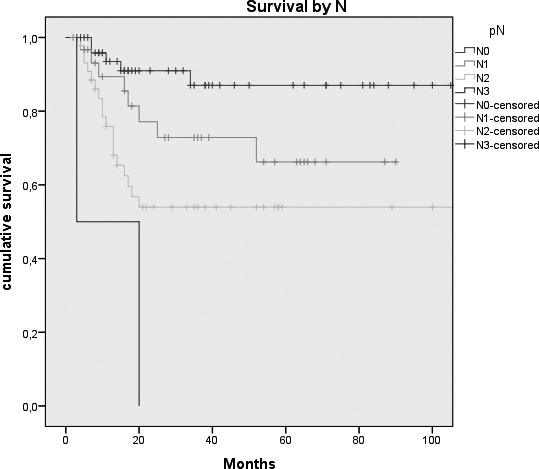
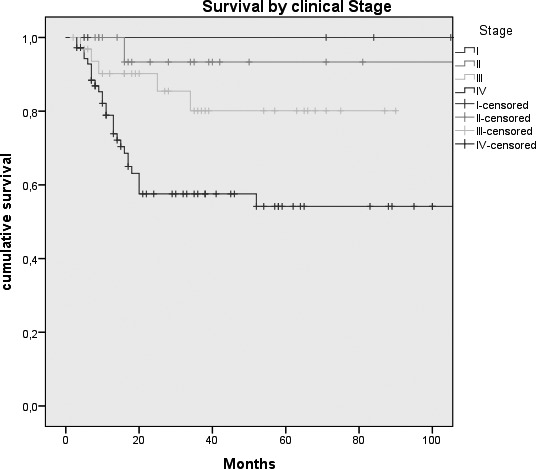
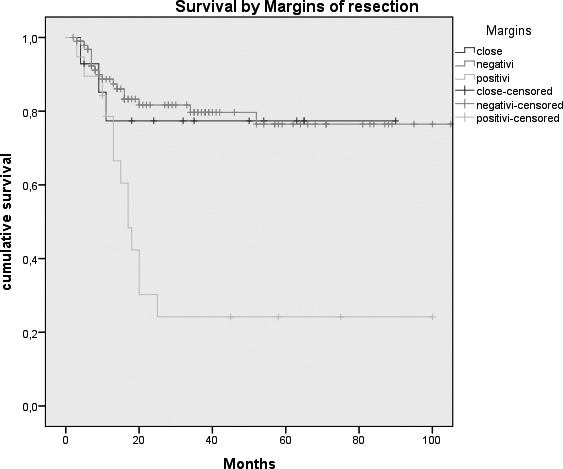
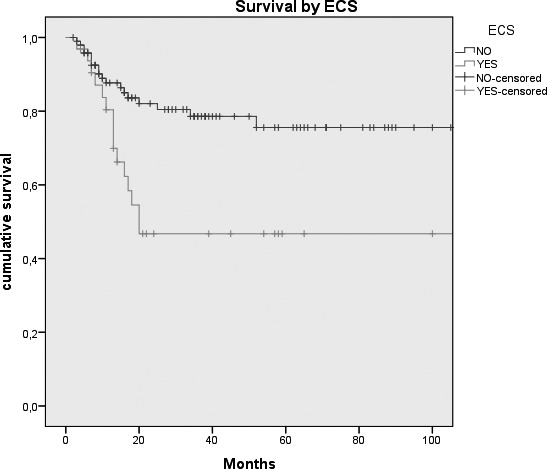
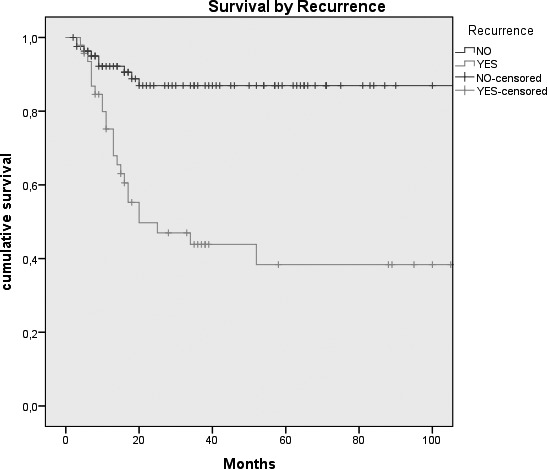
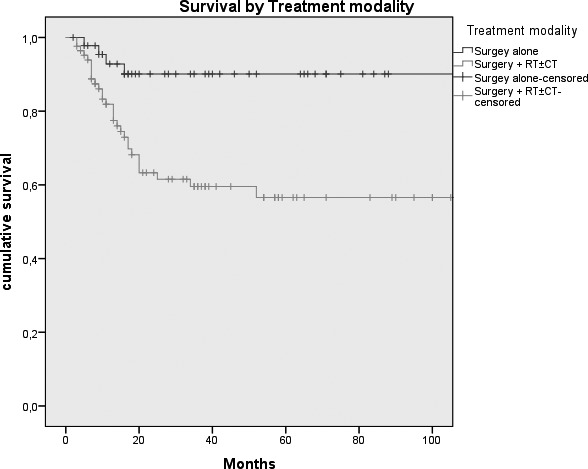
Kaplan-Meier survival curves by: A, Tumour size (p = 0.026); B, N status (p = < 0.0005); C, Clinical Stage (p = 0.007); D, Margins of resection (p = 0.001); E, Extracapsular spread (p = 0.005); F, Recurrence (p = < 0.0005); G, Treatment modality (p = 0.004).
On multivariate Cox regression analysis, the same variables showed a significant relationship with DSS, and in particular N stage (HR 2.2; p = 0.0001), margins of resection (HR 2; p = 0.0001) and recurrence of disease (HR 5.3; p = 0.00001). Results are summarised in Table III.
Table III.
Cox regression analysis.
| Variables | χ2 | SE | Exp(B) | 95% confidence interval | p value | |
|---|---|---|---|---|---|---|
| Inferior | Superior | |||||
| Gender | 1.8 | 0.40 | 0.58 | 0.26 | 1.28 | 0.18 |
| Age | 0.1 | 0.37 | 1.15 | 0.55 | 2.38 | 0.7 |
| Primary site | 1.1 | 0.08 | 1.09 | 0.92 | 1.29 | 0.28 |
| Histology | 1.3 | 0.58 | 0.51 | 0.16 | 1.64 | 0.26 |
| T status | 11.6 | 0.21 | 1.98 | 1.31 | 3.01 | 0.001 |
| N status | 15.5 | 0.21 | 2.24 | 1.46 | 3.43 | 0.0001 |
| Clinical stage* | 11.4 | 0.35 | 3 | 1.49 | 6.03 | 0.002 |
| Type of flap | 4.8 | 0.09 | 1.24 | 1.02 | 1.50 | 0.03 |
| Margins | 15.7 | 0.19 | 2.04 | 1.40 | 2.97 | 0.0001 |
| Extracapsular spread | 9.2 | 0.35 | 2.77 | 1.39 | 5.52 | 0.004 |
| Perineural invasion | 1.1 | 0.53 | 0.56 | 0.19 | 1.60 | 0.28 |
| Mandible/Maxilla involvement | 0.4 | 0.35 | 1.26 | 0.63 | 2.51 | 0.5 |
| Recurrence | 23 | 0.39 | 5.35 | 2.48 | 11.54 | 0.00001 |
| Treatment modality | 9.5 | 0.53 | 4.50 | 1.58 | 12.82 | 0.005 |
| Lymph node dissection | 0.4 | 0.53 | 0.68 | 0.24 | 1.96 | 0.48 |
According to UICC TNM Sixth Edition.
Flap complications
Nine patients experienced major complications after surgery (9/130; 6.9%). Five patients had total flap failure for venous thrombosis (5/130; 3.8%); one of these was saved after vascular re-exploration, and the other four required a second flap. Three patients had exposure of osteoplastic plates and one patient had salivary fistula. The majority of these complications occurred when a fibula flap was used for reconstruction (6 cases) and 2 of 8 complications occurred when using other types of flaps.
Discussion
Oral carcinoma still remains a neoplasm with poor prognosis, especially for advanced stage tumours. The local control rate extends from 95% of T1-T2 lip carcinoma to 20% of T4 tongue and retromolar trigone cancer 11 12.
In the past 20 years, the contribution of plastic and reconstructive surgery has resulted in a breakthrough in the treatment of these tumours, and in particular for reconstruction of large and complex tissue defects 13-25. As demonstrated by De Vicente et al. 26, free flap surgery leads to a trend toward better survival than loco-regional flap, primary closure or skin grafts; this is because the chance to re-establish anatomical and functional continuity guarantees genuine oncological radicality, improving, on the other hand, quality of life. The study by Marchetti et al. 27 showed an overall 5-year survival rate of 41.9% in a cohort of 42 oral cancer patients treated by microvascular flap reconstruction, while De Vicente et al26 reported a 5-year survival rate of 58.6% in the "free flap group" (49 patients) 28. The best results were achieved by Rogers et al. 9; with an overall 5-year survival of 51% (2% SE), they obtained a 5-year DSS of 70% (3% SE). In our experience, we obtained a DSS of 67.8% (4.9% S.E.). Results from statistical analysis highlight the role that some of the variables we considered play in influencing prognosis. Some of these, such as tumour size (expressed by pT stage), presence of lymph node metastasis, extracapsular spread and recurrence of disease are well known prognostic indicators for survival of oral cancer patients 29-31, and our results are in agreement with the findings in the literature. Other variables which, in our series, were shown to influence the survival are treatment modality and involved margins of resection. Moreover, the relationship between these prognostic indicators and DSS was statistically significant. These findings are not surprising: in fact, patients needing adjuvant radio/chemotherapy are usually affected by advanced stage disease (T4 stage, lymph node metastasis > N1, presence of extracapsular spread or involved margins of resection) or a particularly aggressive neoplasm (in fact, only 10% of patients suffering a relapse of disease underwent surgery alone), and in our opinion this is the reason for the poor prognosis. Our study sample included a large percentage of patients affected by stage IIIIV disease; 14.6% and 10.8%, respectively, had involved and close margins of resection. Analysing the corresponding Kaplan-Meier curve, it is possible to appreciate that patients with negative and close margins showed approximately the same trend of survival, in contrast to those with positive margins, which are characterised by a much more unfavourable prognosis.
Microvascular flap surgery could ideally lead to better control of disease, because the possibility of bridging extended tissue defects can push surgeons to perform more aggressive resections to achieve a truly oncological radical result, especially in light of the close correlation between prognosis and disease-free resection margins.
This hypothesis has already been advanced by Hanasono 32 and reinforced by the data of De Vicente 26. As previously noted, in our series 19/130 patients (14.6%) had involved margins and 47/130 patients (36.1%) suffered recurrence of disease. Multivariate Cox backward logistic regression model demonstrated an influence of these two variables on patient survival (HR = 1.75 for margins of resection and HR = 4.63 for recurrence of disease; p = 0.003 and 0.0001 respectively). This result unfortunately deviates from those obtained by the authors mentioned above whom found positive margins in 7% and 8.2% of microvascular group patients, respectively 32 26.
To explain this finding, we have to underline that the majority of patients with positive resection margins underwent microvascular flap surgery during the first years after the introduction of this technique in our surgical practice. Over the following years, the trend has shown a marked improvement, and in our opinion this result could be explained by looking on one hand at the enhancement of our reconstructive technique, and on the other at the increased confidence of highly aggressive surgery dismissing the mentality of a resection "cut on reconstruction", in favour of an ablation which aims only to achieve oncological radicality 33 34.
Furthermore, during tumour resection not only of the tongue, but also in other sites of the oral cavity, we adopted the principles of compartmental surgery which advocate removal of compartments (anatomo-functional units) containing the primary tumour, eliminating the disease and potential muscular, vascular, glandular and lymphatic pathways of spread and recurrence 35. This could also explain the improvement of DSS observed in our series even if we had a large number of patients with advanced stages of disease.
Finally, the progressively increasing use of the anterolateral thigh perforator flap and the DIEAP-polygonal flap, which have become workhorses for head and neck soft tissue reconstruction, are associated with better results in terms of disease-free margins of resection, also because their use as an "on-site tailoring" flap instead of a "premarked" flap allows to tailor the flap at the end of the oncologic resection 36.
Conclusions
In conclusion, in our experience, reconstruction of oral cavity defects with microvascular flaps combined with compartmental surgery confirms that it can play an important role in increasing survival in oral cancer patients.
Acknowledgements
The authors would like to thank Dr Stefano Granieri for his important contribution.
References
- 1. Fast stats-world in Globocan 2008. International Agency for research on Cancer-WHO.
- 2. Fast stats-Italy in Globocan 2008. International Agency for research on Cancer-WHO.
- 3.Tarsitano A, Vietti MV, Cipriani R, et al. Functional results of microvascular reconstruction after hemiglossectomy: free anterolateral thigh flap versus free forearm flap. Acta Otorhinolaryngol Ital. 2013;33:374–379. [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers SN, Humphris G, Lowe D, et al. The impact of surgery for oral cancer on quality of life as measured by the Medical Outcomes Short Form 36. Oral Oncol. 1998;34:171–179. doi: 10.1016/s1368-8375(97)00069-9. [DOI] [PubMed] [Google Scholar]
- 5.Graeff A, Leeuw JRJ, Ros WJG, et al. A prospective study on quality of life of patients with cancer of the oral cavity or oropharynx treated with surgery with or without radiotherapy. Oral Oncol. 1999;35:27–32. doi: 10.1016/s1368-8375(98)00049-9. [DOI] [PubMed] [Google Scholar]
- 6.Schliephake H, Jamil MU. Prospective evaluation of quality of life after oncologic surgery for oral cancer. Int J Oral Maxillofac Surg. 2002;31:427–433. doi: 10.1054/ijom.2001.0194. [DOI] [PubMed] [Google Scholar]
- 7.Sharp HM, List M, MacCracken E, et al. Patients' priorities among treatment effects in head and neck cancer: evaluation of a new assessment tool. Head Neck. 1999;21:538–546. doi: 10.1002/(sici)1097-0347(199909)21:6<538::aid-hed7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.List MA, Stracks J, Colangelo L, et al. How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol. 2000;18:877–884. doi: 10.1200/JCO.2000.18.4.877. [DOI] [PubMed] [Google Scholar]
- 9.Rogers SN, Brown JS, Woolgar JA, et al. Survival following primary surgery for oral cancer. Oral Oncol. 2009;45:201–211. doi: 10.1016/j.oraloncology.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Spiro RH, Huvos AG, Wong JY, et al. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986;152:345–350. doi: 10.1016/0002-9610(86)90302-8. [DOI] [PubMed] [Google Scholar]
- 11.Sessions DG, Spector GJ, Lenox J, et al. Analysis of treatment results for oral tongue cancer. Laryngoscope. 2002;112:616–625. doi: 10.1097/00005537-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Gorsky M, Epstein JB, Oakley C, et al. Carcinoma of the tongue: a case series analysis of clinical presentation, risk factors, staging, and outcome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:546–552. doi: 10.1016/j.tripleo.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Rinaldo A, Shaha AR, Wei WI, et al. Microvascular free flaps: a major advance in head and neck reconstruction. Acta Otolaryngol. 2002;122:779–784. [PubMed] [Google Scholar]
- 14.Eckardt A, Fokas K. Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. J Craniomaxillofac Surg. 2003;31:197–201. doi: 10.1016/s1010-5182(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 15.Berrone M, Crosetti E, Succo G. Repositioning template for mandibular reconstruction with fibular free flaps: an alternative technique to pre-plating and virtual surgical planning. Acta Otorhinolaryngol Ital. 2014;34:278–282. [PMC free article] [PubMed] [Google Scholar]
- 16.Deganello A, Gitti G, Parrinello G, et al. Cost analysis in oral cavity and oropharyngeal reconstructions with microvascular and pedicled flaps. Acta Otorhinolaryngol Ital. 2013;33:380–387. [PMC free article] [PubMed] [Google Scholar]
- 17.Giordano L, Bondi S, Toma S, et al. Versatility of the supraclavicular pedicle flap in head and neck reconstruction. Acta Otorhinolaryngol Ital. 2014;34:394–398. [PMC free article] [PubMed] [Google Scholar]
- 18.Leite AK, Matos LL, Belli M, et al. Pectoralis major myocutaneous flap for head and neck reconstruction: risk factors for fistula formation. Acta Otorhinolaryngol Ital. 2014;34:389–393. [PMC free article] [PubMed] [Google Scholar]
- 19.Baj A, Bolzoni A, Torretta S, et al. Arterial microanastomoses on the reverse flow of the internal carotid artery reverse flow: an extreme solution in free-flap revascularisation. How we do it. Acta Otorhinolaryngol Ital. 2014;34:368–371. [PMC free article] [PubMed] [Google Scholar]
- 20.Colletti G, Autelitano L, Rabbiosi D, et al. Technical refinements in mandibular reconstruction with free fibula flaps: outcome-oriented retrospective review of 99 cases. Acta Otorhinolaryngol Ital. 2014;34:342–348. [PMC free article] [PubMed] [Google Scholar]
- 21.Bussu F, Gallus R, Navach V, et al. Contemporary role of pectoralis major regional flaps in head and neck surgery. Acta Otorhinolaryngol Ital. 2014;34:327–341. [PMC free article] [PubMed] [Google Scholar]
- 22.Baj A, Beltramini GA, Demarchi M, et al. Extended-pedicle peroneal artery perforator flap in intraoral reconstruction. Acta Otorhinolaryngol Ital. 2013;33:282–285. [PMC free article] [PubMed] [Google Scholar]
- 23.Baj A, Capparé P, Autelitano L, et al. The park-bench position in cervico-facial reconstructive surgery: a technical note. Acta Otorhinolaryngol Ital. 2013;33:129–132. [PMC free article] [PubMed] [Google Scholar]
- 24.Bussu F, Salgarello M, Adesi LB, et al. Oral cavity defect reconstruction using anterolateral thigh free flaps. B-ENT. 2011;7:19–25. [PubMed] [Google Scholar]
- 25.Salgarello M, Snider F, Finocchi V, et al. The Pruitt-Inahara carotid shunt as an assisting tool to anastomose the arterial free flap pedicle to the internal carotid artery in the vesseldepleted neck. Microsurgery. 2011;31:234–236. doi: 10.1002/micr.20849. [DOI] [PubMed] [Google Scholar]
- 26.Vicente JC, Rodriguez-Santamarta T, Rosado P, et al. Survival after free flap reconstruction in patients with advanced oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70:453–459. doi: 10.1016/j.joms.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti C, Pizzigallo A, Cipriani R, et al. Does microvascular free flap reconstruction in oral squamous cell carcinoma improve patient survival? Otolaryngol Head Neck Surg. 2008;139:775–780. doi: 10.1016/j.otohns.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Salvatori P, Paradisi S, Calabrese L, et al. Patients' survival after free flap reconstructive surgery of head and neck squamous cell carcinoma: a retrospective multicentre study. Acta Otorhinolaryngol Ital. 2014;34:99–104. [PMC free article] [PubMed] [Google Scholar]
- 29.Massano J, Regateiro FS, Januário G, et al. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 30.Grandi C, Allossio M, Moglia DEA. Prognostic significance of lymphatic spread in head and neck carcinomas: Therapeutic implications. Head Neck Surg. 1985;8:67–73. doi: 10.1002/hed.2890080202. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson RJ, Ironside JAD. Squamous cell carcinomas of the head and neck. BMJ. 2002;325:822–827. doi: 10.1136/bmj.325.7368.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanasono MM, Friel MT, Klem C, et al. Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck. 2009;31:1289–1289. doi: 10.1002/hed.21100. [DOI] [PubMed] [Google Scholar]
- 33.Durmus K, Apuhan T, Ozer E. Transoral robotic surgery for retromolar trigone tumours. Acta Otorhinolaryngol Ital. 2013;33:425–427. [PMC free article] [PubMed] [Google Scholar]
- 34.Basaran B, Ulusan M, Orhan KS, et al. Is it necessary to remove submandibular glands in squamous cell carcinomas of the oral cavity? Acta Otorhinolaryngol Ital. 2013;33:88–92. [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrese L, Bruschini R, Giugliano G, et al. Compartmental tongue surgery: Long term oncologic results in the treatment of tongue cancer. Oral Oncol. 2011;47:174–179. doi: 10.1016/j.oraloncology.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Tarsitano A, Sgarzani R, Betti E, et al. Vascular pedicle ossification of free fibular flap: is it a rare phenomenon? Is it possible to avoid this risk? Acta Otorhinolaryngol Ital. 2013;33:307–310. [PMC free article] [PubMed] [Google Scholar]


