Abstract
Background:
Women with polycystic ovary syndrome have lower pregnancy rates, possibly due to the decreased uterine receptivity. Successful implantation depends on protein networks that are essential for cross-talk between the embryo and endometrium. Apolipoprotein A1 has been proposed as a putative anti-implantation factor. In this study, we evaluated apolipoprotein A1 expression in human endometrial tissues.
Materials and Methods:
Endometrial apolipoprotein A1 messenger RNA (mRNA) and protein expression were investigated using quantitative real-time polymerase chain reaction (PCR) and Western blot. The distribution of apolipoprotein A1 was also detected by immunostaining. Samples were obtained from 10 patients with polycystic ovary syndrome and 15 healthy fertile women in the proliferative (on day 2 or day 3 before ovulation, n = 7) and secretory (on days 3-5 after ovulation, n = 8) phases.
Results:
Endometrial apolipoprotein A1 expression was upregulated in patients with polycystic ovary syndrome compared to normal subjects. However, apolipoprotein A1 expression in the proliferative phase was significantly higher than in the luteal phase (P value < 0.05).
Conclusion:
It seems that differentially expressed apolipoprotein A1 negatively affects endometrial receptivity in patients with polycystic ovary syndrome. The results showed that apolipoprotein A1 level significantly changes in the human endometrium during the menstrual cycle with minimum expression in the secretory phase, coincident with the receptive phase (window of implantation). Further studies are required to clarify the clinical application of this protein.
Keywords: Apolipoprotein A1, endometrium, polycystic ovary syndrome, proliferative phase, secretory phase
INTRODUCTION
Polycystic ovary syndrome is one of the most common endocrine disorders in premenopausal women and the most frequent cause of ovulatory infertility.[1,2,3] Prevalence of this condition in women of the reproductive age is approximately between 5% and 10%.[1,4,5] Polycystic ovary syndrome as a complex disorder is associated with several health complications including obesity, hyperandrogenism, metabolic syndrome, hirsutism, acne, ovarian dysfunction, and infertility.[4,6,7] Although anovulation is an obvious cause of infertility in polycystic ovary syndrome, growing evidence suggests that endometrial receptivity also contributes to infertility of these patients.[1,7,8]
Endometrial receptivity means the ability of the uterine lining to accept an implanting embryo, resulting in a successful pregnancy. It seems that a shortened or absent receptive endometrium is the main cause of conception delay and lack of pregnancy.[9,10] Successful implantation depends on the regulation of protein networks that are essential for communication between the nascent embryo and endometrium. Several studies have been performed to provide potential genes and protein candidates for endometrial receptivity. However, none of them have been verified as a clinical biomarker.[1,2] Apolipoprotein A1 is the predominant protein for high density lipoproteins.[2,11] Apolipoprotein A1 is a primary acceptor for cholesterol in extrahepatic tissues.[2,11] Apolipoprotein A1 is dysregulated in diverse tissues and body fluids in a variety of diseases.[12,13] Recent works showed that apolipoprotein A1 may also play a role in endometrial receptivity and it has been identified as a putative anti-implantation factor secreted by the differentiating endometrium.[2,11,13] A proteomic analysis of the endometrium from women with repeated implantation failure and normal fertile women showed a significantly higher apolipoprotein A1 expression in patients with repeated implantation failure.[14] Further studies confirmed that upregulation of apolipoprotein A1 is upregulated in the endometrium of women with unexplained infertility.[2] The abovementioned evidences provide a potential role for apolipoprotein A1 in female infertility associated with endometrium disturbances. Therefore, in the present study the possible in vivo role of apolipoprotein A1 in endometrial receptivity of patients with polycystic ovary syndrome was investigated.
MATERIALS AND METHODS
Patients and tissue selection
Human endometrial samples were obtained from 10 patients with polycystic ovary syndrome and 15 healthy fertile women in the proliferative (on day 2 or day 3 before ovulation, n = 7) and secretory (on days 3-5 after ovulation, n = 8) phases. Daily urinary luteinizing hormone levels were monitored using an ovulation prediction kit. The mean age of the participants was 26 (range: 18-35) years. The participants did not receive any form of hormonal therapy and none of them used an intrauterine contraceptive device during the previous 3 months. Patients with chronic anovulation, clinical and/or biochemical signs of hyperandrogenism, and those who were polycystic in the ultrasound scanning of ovaries were diagnosed as polycystic ovary syndrome (Rotterdam ESHRE/ASRM-sponsored polycystic ovary syndrome Consensus Workshop Group, 2004). Endometrial biopsies were taken using Pipelle catheters under sterile conditions. Each biopsy was divided into three pieces. The first portion was transferred to a cryotube containing 1 mL RNAlater (Ambion, Austin, TX, USA), for RNA extraction. Another portion was fixed in formalin for histological dating. The last part was dry-frozen at –80°C for protein extraction. Approval for the use of tissues was prepared by the local ethics committee and written informed consent was obtained prior to the sample collection (IUMS, No. 19950).
RNA extraction, cDNA synthesis, and reverse transcription polymerase chain reaction
Total RNA was extracted from endometrial samples by homogenization in trizol reagent (Sigma Pool, UK), according to the manufacturer's instructions. RNA samples were treated with DNase I (Fermentas, Sankt Leon-Rot, Germany) to remove genomic DNA contamination. First-strand cDNA synthesis was performed using oligo dT primers (Metabion, Martinsried, Bavaria, Germany) and was reverse-transcribed using SuperScript II (200 U/μl, fermentase). Negative controls were prepared without an enzyme (nonreverse transcribed controls, RT controls). The reverse transcription polymerase chain reaction (RT-PCR) was performed with the prepared cDNA, Platinum Blue PCR SuperMix (Invitrogen, Paisley, Scotland, UK) and the forward and reverse primers for apolipoprotein A1 (Metabion, Martinsried, Bavaria, Germany). The following primer pairs were used: β-actin forward 5’-TGA CCC AGA TCA TGT TTG AGA CC-3’ and β-actin reverse 5’-GGA GGA GCA ATG ATC TTG ATC TTC-3,’ apolipoprotein A1 forward 5’-CCCAGTTGTCAAGGAGCTTT-3,’ and apolipoprotein A1 reverse 5’-TGGATGTGCTCAAAGACAGC-3.’ The amplification was performed for 40 cycles under the following setting: 95° for 30 s, 60° for 1 min, and 72° for 1 min. All experiments included RT controls as negative controls. To separate PCR products, 10 μl of each sample was resolved on a 1.2% agarose gel and electrophoresis was performed with 1x tris-acetate-EDTA (TAE) buffer and a voltage of 95 V for 30-40 min. The bands were visualized by using an ultraviolet transillumination. The RT-PCR products were sequenced to confirm the identity of the amplified product. β-actin was used as a housekeeping control and its expression was checked between the three groups.[15]
Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed using cDNA prepared from endometrial samples. qPCR reactions were performed in triplicates using an ABI Prism 7300 Sequence Detector (Applied Biosystems, Foster City, California, USA) in a total volume of 20 μl containing 250 ng cDNA, 5 pmol gene-specific primers, and SYBR Green reagent (Applied Biosystems) with ROX dye as a passive control for signal intensity. The thermal cycle profile followed 50 cycles at 95° for 30 s, 60° for 30 s, and 72° for 30 s. Melting curves of PCR reactions were monitored to ensure that there is one single PCR product and no primer dimmer. Standard curves were used to assess primers efficiency.
The qPCR data were analyzed using the comparative CT method. In brief, the difference in cycle time (ΔCT) was determined as the difference between the number of cycles required for amplification of the test gene and the reference housekeeping gene, human β-actin. Then, ΔΔCT was assessed as the difference between the groups. The fold change (FC) was calculated as 2-ΔΔ.[15]
Protein extraction
Briefly, endometrial tissue samples were homogenized in 1 mL of TRI reagent (Sigma Pool, UK) (VWR) for ∼1 min. The total protein was extracted using TRI reagent (Invitrogen 15596-026) and quantified by Bradford's assay with a standard curve generated using a human serum albumin/gamma globulin (Fluka SP8119).[16]
Western blotting
Normal endometrial tissue in proliferative and secretory phases as well as endometrial tissues of women with polycystic ovary syndrome was used for apolipoprotein A1 expression analysis. Samples, containing 40 μg of total protein, were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 100V and transferred to polyvinylidene fluoride (PVDF) membrane (Bio-Rad, USA) by a wet transfer system (Bio-Rad, USA) at 20V overnight. Transferred blots were blocked with 5% BSA and 0.1% Tween-20 (Sigma-Aldrich, St. Louis, MO, USA). Afterward, blots were incubated overnight at 4°C with primary antibody against apolipoprotein A1 (1:5000; Abcam ab17278, Cambridge, Cambridgeshire, UK) and β-actin (1:10000; Sigma-Aldrich, St. Louis, MO, USA). The blots were then washed three times, each time for 20 min with TBST and were incubated with the secondary antibody, HRP-conjugated anti-mouse antibody (Abcam, ab195891) for 1 h at room temperature. After three washes, each time for 20 min with TBST, the blots were visualized by the chemiluminescent peroxidase substrate (ECL Advance, GE Healthcare, UK) in a dark room and were exposed to x-ray films (GE, 28906835). Subsequently, the films were scanned with densitometer (GS-800, Bio-Rad, USA). The values were normalized to β-actin. For a negative control, the blots were incubated with nonimmunized serum as primary antibody.[16]
Immunohistochemistry
The antibody used in the experiment was obtained from Abcam (Cambridge, Cambridgeshire, UK). It was monoclonal antibody specific for apolipoprotein A1 (catalogue no. ab17278). Tissue samples were fixed in 10% neutral buffered formalin for 18-24 h, embedded in paraffin, and cut in 4 μm. Formal in-fixed sections were deparaffinized with xylosine twice for 5 min followed by rehydration in graded ethanol. Endogenous peroxidase activity was blocked with 3% v/v hydrogen peroxidase in methanol for 20 min. Antigen retrieval on these sections was performed by microwave irradiation for 10 min in 10 mmol/L sodium citrate pH = 6.0. Sections were allowed to cool for 20 min and then washed in phosphate buffer saline (PBS), then stained using a Vectastain Elite ABC peroxidase kit (Vector Laboratories Ltd., UK).
In addition, to avoid nonspecific binding, an avidin/biotin blocking kit was used. Briefly, the slides were blocked for 1 h at room temperature in PBS containing 0.2% appropriate serum and 25% avidin supplied in the blocking kit. The block was removed and the slides were incubated overnight at 4°C in primary antibody at an appropriate dilution using antibody diluent media (Dakocytomation Ltd., Ely, UK) and 250 ml biotin per mL of diluted antibody. Binding was visualized by incubation with peroxidase substrate 3-amino-9-ethylcarbazole (AEC) for 10 min, washed in distilled water for 3 min, and counterstained in 10% hematoxylin for 10 min. Slides were washed in tap water for 2 min and mounted with Aquamount (VWR). Control sections were obtained by the blocking of primary antibody with the corresponding specific peptide using a 20-fold excess of blocking peptide. Immunostained sections were examined using an Olympus BH2 microscope at ×250 magnification (Olympus, London, UK).
Statistical analysis
Western-blot images in each group were analyzed by Image J software. Also, relative apolipoprotein A1 gene expression quantities were compared between the groups. The threshold cycle values were normalized against the threshold value of human β-actin. Differences in normalized gene and protein expression values between the samples were tested for the significance using analysis of variance (ANOVA) statistical test. The results were expressed as mean ± standard error of the mean (SEM). P values <0.05 were considered to be statistically significant.
RESULTS
Apolipoprotein A1 immunoblotting analysis
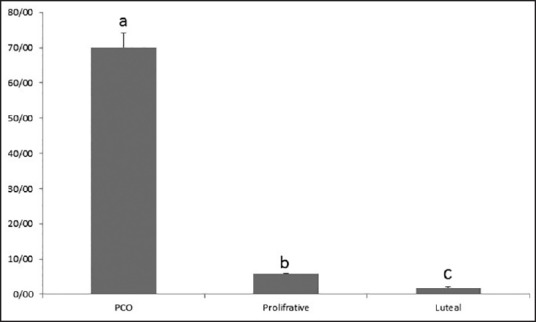
Demographic characteristics, hormonal and clinical parameters of polycystic ovary syndrome, and the control subjects are summarized in Table 1. A representative western blotting analysis of apolipoprotein A1.
Table 1.
Demographic characteristics, hormonal, and clinical parameters of polycystic ovary syndrome and control subjects
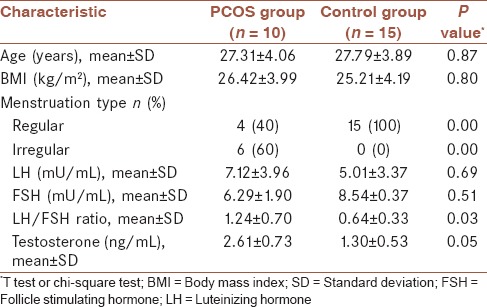
Protein expression in endometrial specimens is shown in Figure 1. Western blot analysis showed a significantly higher apolipoprotein A1 protein expression in the endometrium of patients with polycystic ovary syndrome compared to normal ones. However, expression of this protein in the proliferative phase was significantly higher than in the luteal phase.
Figure 1.
Western blot analysis of apolipoprotein A1 expression in proliferative and secretory endometrial samples obtained from healthy fertile women and patients with polycystic ovary syndrome. A clear reduction trend was observed in apolipoprotein A1 expression of normal samples. The results also showed lower expression of apolipoprotein A1 in luteal samples compared to proliferative samples. (a) Different letters denote significant differences (b) 1, polycystic ovary syndrome; 2, proliferative; 3, luteal. (P value < 0.05)
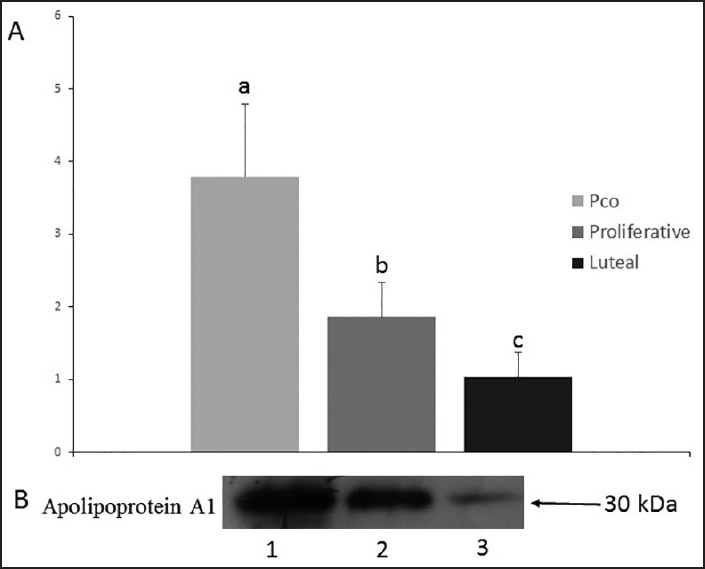
Reverse transcription polymerase chain reaction
Figure 2 shows the result of RT-PCR for mRNA expression of apolipoprotein A1 and human β actin genes in the human endometrium of normal women and patients with polycystic ovary syndrome. All amplified products were of the expected size for that particular gene. There was no amplified product in the control samples, which indicate the lack of genomic DNA contamination. Human β actin as the housekeeping gene showed a stable gene expression between the different groups.
Figure 2.
Expression of apolipoprotein A1 gene in human endometrial tissue. Forward and reverse primers produced a specific product with the specific predicted size. (P value < 0.05)
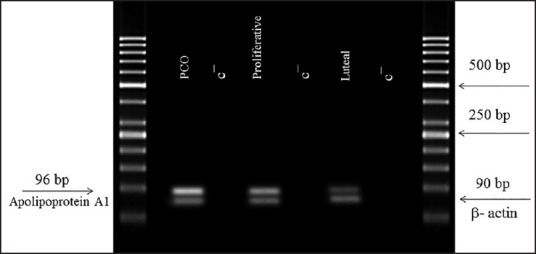
Quantitative real-time polymerase chain reaction
Apolipoprotein A1 gene expression in endometrial biopsies was evaluated using quantitative real-time PCR [Figure 3]. There was a significantly higher expression of apolipoprotein A1 in patients with polycystic ovary syndrome than in normal women. The qPCR results also revealed a higher expression of apolipoprotein A1 in proliferative samples compared to luteal.
Figure 3.
qPCR was used to quantify apolipoprotein A1 mRNA expression in endometrial biopsies of 10 healthy fertile women and 10 polycystic ovary syndrome women obtained in the proliferative and luteal phases. The figure shows mean ± SEM of normalized apolipoprotein A1 expression. Apolipoprotein A1 level was significantly increased in the endometrial samples of polycystic ovary syndrome women compared to normal females. There was also a significantly higher apolipoprotein A1 expression in proliferative samples compared to luteal samples. Different letters denote significant differences. (P value < 0.05)
Immunohistochemical localization of apolipoprotein A1
Positive immunostaining for apolipoprotein A1 was observed in the human endometrial tissues [Figure 4]. Apolipoprotein A1 was localized in glandular as well as stromal compartments of the endometrium. Moderate apolipoprotein A1 staining was observed in proliferative phase endometria with downregulation of staining in the secretory phase of normal women.
Figure 4.
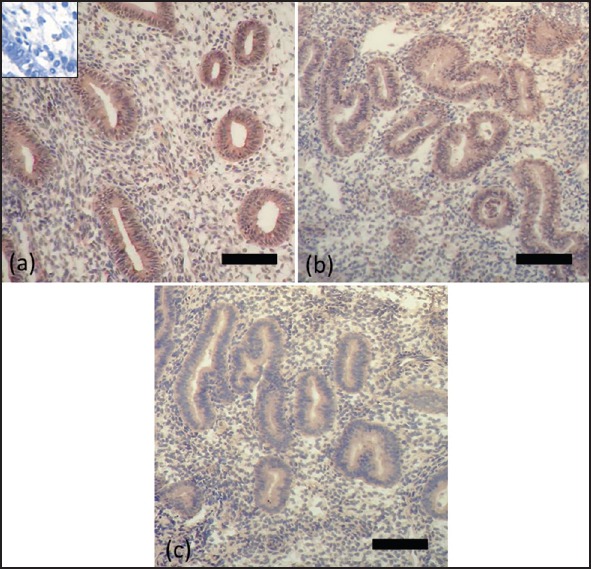
Immunohistochemistry for apolipoprotein A1 using endometrial samples from PCOS patients (a) and normal women during proliferative phase (b) and secretory phase (c). Positive staining is brown and negative staining is blue. Insets show blocking of the antiapolipoprotein A1 antibody with its specific peptides. Bar = 50 μm
It is interesting that glandular epithelium and stroma in the endometrium of patients with polycystic ovary syndrome showed a significantly higher expression of apolipoprotein A1 when compared with their respective counterparts in the endometrium of normal women.
In the endometrium of women with polycystic ovary syndrome, strong staining for apolipoprotein A1 was observed in the glandular epithelium with weaker staining in the stroma.
DISCUSSION
Polycystic ovary syndrome is associated with infertility not only to anovulation but also to endometrial dysfunction.[9,17] Despite many recent advances in assisted reproduction techniques even with the selection of good quality embryos, the rate of success is mainly limited because of implantation failures.[7,8,18] Endometrial factors at the molecular level have been suggested to explain implantation failure and poor reproductive potential of patients with polycystic ovary syndrome.[1,7]
In women with polycystic ovary syndrome, dyslipidemia and lipoprotein abnormalities are common metabolic disorders.[19,20] Apolipoprotein A1 is the main protein component of high-density lipoprotein (HDL).[11,21] Dysregulation of apolipoprotein A1 levels have been reported in a variety of diseases such as preeclampsia, endometriosis, and repeated implantation failure.[2,12] There are no reports describing apolipoprotein A1 expression and function in the endometria of women with polycystic ovary syndrome. In this study, we assessed the endometrial expression of apolipoprotein A1 as a potential biomarker of nonreceptive endometria in patients with polycystic ovary syndrome without pharmacological treatment. The results showed a higher endometrial apolipoprotein A1 mRNA and protein expression in women with polycystic ovary syndrome compared to fertile women. We also confirmed the presence and determined the localization of apolipoprotein A1 in the endometrial tissue using immunohistochemistry. Our work revealed that endometrial apolipoprotein A1 expression specifically in the glandular epithelium was upregulated in patients with polycystic ovary syndrome compared to normal subjects. However, immunohistochemical staining is a qualitative technique and as such is not ideal for quantitative analysis; so the results were verified by western blot as a semi-quantitative technique.
Recent studies performed on human endometrial samples have revealed that elevated apolipoprotein A1 levels are associated with unexplained infertility and also repeated implantation failure.[2,14] Nyalwidhe et al.[13] demonstrated that apolipoprotein A1 is consumed by human preimplantation embryos suggesting a possible role in implantation. Indeed, embryos with the highest likelihood of implantation consume or metabolize apolipoprotein A1, which prepares a suitable microenvironment with low apolipoprotein A1 levels for successful implantation.[13] In an in vitro experiment, apolipoprotein A1 was also found to be have a higher expression in nondecidualized cells compared to decidualized stromal cells.[14] Thus, important negative effects can be predicted for apolipoprotein A1 in the initiation and maintenance of the window of implantation.
Apolipoprotein A1 also has anti-inflammatory properties including inhibition of interleukin 1 (IL-1) and tumor necrosis factor alpha (TNFα) production, and suppression of neutrophil degranulation.[22,23] Inflammatory processes are implicated in the pathophysiology of endometriosis. Upregulation of endometrial apolipoprotein A1 is observed in women with endometriosis.[22]
Moreover, at the time of implantation, the maternal endometrium shows characteristics of an acute aseptic inflammatory response.[24,25] Inflammatory cytokines and adhesion molecules that are expressed by the endometrium and embryo are essential for the appropriate interaction between the embryo and the endometrium for successful pregnancy.[24,25] Apolipoprotein A1 has the ability to inhibit the synthesis of inflammatory cytokines and adhesion molecules, such as selectins, that have essential roles in the embryo implantation.[23,26,27,28,29] Another potential mechanism through which apolipoprotein A1 contributes to the implantation failure may be via inhibition of angiogenesis. Tissue remodeling and angiogenesis are two crucial events during implantation and decidualization.[24] Apolipoprotein A1 has inhibitory effects on angiogenesis and tissue remodeling by downregulation of matrix metalloproteinase (MMP)-9, vascular endothelial growth factor (VEGF)/basic fibroblast growth factor (bFGF) production, and dendritic cell function.[30,31,32]
Our study demonstrates that apolipoprotein A1 expression in human endometria significantly changes during the menstrual cycle with minimum level in the secretory phase, coincident with the receptive phase (window of implantation). It appears that sex hormones regulate the expression of apolipoprotein A1 in a reverse manner. There is evidence that apolipoprotein A1 is upregulated by estradiol and is downregulated by progesterone.[33,34] Progesterone may protect the endometrium against the detrimental effects of apolipoprotein A1 during the critical period of the receptivity window. Endometrial apolipoprotein A1 expression is strongly inhibited by human chorionic gonadotropin (hCG), which is a key requirement for the promotion of implantation.[2] Thus, hCG treatment may improve uterine receptivity and pregnancy rate in in vitro fertilization (IVF) patients with polycystic ovary syndrome by decreasing apolipoprotein A1 levels.
In summary, the present study provides a novel insight into the mechanism of implantation failure and subfertility associated with polycystic ovary syndrome condition. Elevated apolipoprotein A1 levels can be considered as a biomarker for nonreceptive endometrium in patients with polycystic ovary syndrome. Further investigation should be performed to clarify the major clinical applications of this protein.
Financial support and sponsorship
This study was financed by the Cooperative of Iran University of Medical Sciences and Royan Institute (Grant no. 19950).
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTIONS
FSA contributed in conception of the work and drafting, approval of the final version of the manuscript, and agreed for all aspects of the work. RA contributed in the design of the work, conducting the study, approval of the final version of the manuscript, and agreed for all aspects of the work. SHHJ contributed in conception of the work, approval of the final version of the manuscript, and agreed for all aspects of the work. BS contributed in conception of the work, approval of the final version of the manuscript, and agreed for all aspects of the work. MA contributed in conception of the work, approval of the final version of the manuscript, and agreed for all aspects of the work. MM contributed in the design of the work, conducting the study, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgements
We wish to thank Miss Sara Taleahmad for her skillful technical assistance.
REFERENCES
- 1.Qiao J, Wang L, Li R, Zhang X. Microarray evaluation of endometrial receptivity in Chinese women with polycystic ovary syndrome. Reprod Biomed Online. 2008;17:425–35. doi: 10.1016/s1472-6483(10)60228-3. [DOI] [PubMed] [Google Scholar]
- 2.Brosens JJ, Hodgetts A, Feroze-Zaidi F, Sherwin JR, Fusi L, Salker MS, et al. Proteomic analysis of endometrium from fertile and infertile patients suggests a role for apolipoprotein AI in embryo implantation failure and endometriosis. Mol Hum Reprod. 2010;16:273–85. doi: 10.1093/molehr/gap108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoaei T, Heidari-Beni M, Tehrani HG, Feizi A, Esmaillzadeh A, Askari G. Effects of probiotic supplementation on pancreatic β-cell function and c-reactive protein in women with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Int J Prev Med. 2015;6:27. doi: 10.4103/2008-7802.153866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart R, Norman R. Polycystic ovarian syndrome — prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol. 2006;20:751–78. doi: 10.1016/j.bpobgyn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Raja-Khan N, Urbanek M, Rodgers RJ, Legro RS. The role of TGF-β in polycystic ovary syndrome. Reprod Sci. 2014;21:20–31. doi: 10.1177/1933719113485294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insenser M, Martinez-Garcia MA, Montes R, San-Millan JL, Escobar-Morreale HF. Proteomic analysis of plasma in the polycystic ovary syndrome identifies novel markers involved in iron metabolism, acute-phase response, and inflammation. J Clin Endocrinol Metab. 2010;95:3863–70. doi: 10.1210/jc.2010-0220. [DOI] [PubMed] [Google Scholar]
- 7.Lopes IM, Baracat MC, Simões Mde J, Simões RS, Baracat EC, Soares JM., Jr Endometrium in women with polycystic ovary syndrome during the window of implantation. Rev Assoc Med Bras. 2011;57:702–9. doi: 10.1590/s0104-42302011000600020. [DOI] [PubMed] [Google Scholar]
- 8.Cakmak H, Taylor HS. Implantation failure: Molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–53. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20:235–44. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Lessey BA. Assessment of endometrial receptivity. Fertil Steril. 2011;96:522–9. doi: 10.1016/j.fertnstert.2011.07.1095. [DOI] [PubMed] [Google Scholar]
- 11.Mains LM, Christenson L, Yang B, Sparks AE, Mathur S, Van Voorhis BJ. Identification of apolipoprotein A1 in the human embryonic secretome. Fertil Steril. 2011;96:422–7 e2. doi: 10.1016/j.fertnstert.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 12.Corton M, Botella-Carretero JI, Lopez JA, Camafeita E, San Millan JL, Escobar-Morreale HF, et al. Proteomic analysis of human omental adipose tissue in the polycystic ovary syndrome using two-dimensional difference gel electrophoresis and mass spectrometry. Hum Reprod. 2008;23:651–61. doi: 10.1093/humrep/dem380. [DOI] [PubMed] [Google Scholar]
- 13.Nyalwidhe J, Burch T, Bocca S, Cazares L, Green-Mitchell S, Cooke M, et al. The search for biomarkers of human embryo developmental potential in IVF: A comprehensive proteomic approach. Mol Hum Reprod. 2013;19:250–63. doi: 10.1093/molehr/gas063. [DOI] [PubMed] [Google Scholar]
- 14.Manohar M, Shukla V, Das V, Agarwal A, Pandey A, Siddiqui WA, et al. Proteomic identification and analysis of human endometrial proteins associated with unexplained infertility. J Proteomics Bioinform. 2014;7:359–66. [Google Scholar]
- 15.Taghavi SA, Ashrafi M, Mehdizadeh M, Karimian L, Joghataie MT, Aflatoonian R. Toll-like receptors expression in follicular cells of patients with poor ovarian response. Int J Fertil Steril. 2014;8:183–92. [PMC free article] [PubMed] [Google Scholar]
- 16.Fazeli AS, Nasrabadi D, Pouya A, Mirshavaladi S, Sanati MH, Baharvand H, et al. Proteome analysis of post-transplantation recovery mechanisms of an EAE model of multiple sclerosis treated with embryonic stem cell-derived neural precursors. J Proteomics. 2013;94:437–50. doi: 10.1016/j.jprot.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Donaghay M, Lessey BA. Uterine receptivity: Alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25:461–75. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- 18.Shang K, Jia X, Qiao J, Kang J, Guan Y. Endometrial abnormality in women with polycystic ovary syndrome. Reprod Sci. 2012;19:674–83. doi: 10.1177/1933719111430993. [DOI] [PubMed] [Google Scholar]
- 19.Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci. 2013;56:137–42. doi: 10.5468/ogs.2013.56.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: Relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf) 1994;41:463–71. doi: 10.1111/j.1365-2265.1994.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto VY, Kane JP, Ishida BY, Bloom MS, Browne RW. High-density lipoprotein metabolism and the human embryo. Hum Reprod Update. 2010;16:20–38. doi: 10.1093/humupd/dmp029. [DOI] [PubMed] [Google Scholar]
- 22.Ferrero S, Gillott DJ, Remorgida V, Anserini P, Leung KY, Ragni N, et al. Proteomic analysis of peritoneal fluid in women with endometriosis. J Proteome Res. 2007;6:3402–11. doi: 10.1021/pr060680q. [DOI] [PubMed] [Google Scholar]
- 23.Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, 3rd, Roux-Lombard P, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–9. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 24.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–73. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 25.Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: Roles in implantation and early placentation. Semin Reprod Med. 2007;25:437–44. doi: 10.1055/s-2007-991041. [DOI] [PubMed] [Google Scholar]
- 26.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Navab M, Reddy ST, Buga GM, et al. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–7. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi N, Wu MP. Apolipoprotein A-I attenuates renal ischemia/reperfusion injury in rats. J Biomed Sci. 2008;15:577–83. doi: 10.1007/s11373-008-9258-7. [DOI] [PubMed] [Google Scholar]
- 28.Liao XL, Lou B, Ma J, Wu MP. Neutrophils activation can be diminished by apolipoprotein A-I. Life Sci. 2005;77:325–35. doi: 10.1016/j.lfs.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 29.Chenaud C, Merlani PG, Roux-Lombard P, Burger D, Harbarth S, Luyasu S, et al. Low apolipoprotein A-I level at intensive care unit admission and systemic inflammatory response syndrome exacerbation. Crit Care Med. 2004;32:632–7. doi: 10.1097/01.ccm.0000114820.47460.0a. [DOI] [PubMed] [Google Scholar]
- 30.Kim KD, Lim HY, Lee HG, Yoon DY, Choe YK, Choi I, et al. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun. 2005;338:1126–36. doi: 10.1016/j.bbrc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 31.Zamanian-Daryoush M, Lindner D, Tallant TC, Wang Z, Buffa J, Klipfell E, et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J Biol Chem. 2013;288:21237–52. doi: 10.1074/jbc.M113.468967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao F, Vasquez SX, Su F, Roberts S, Shah N, Grijalva V, et al. L-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathways. Integr Biol (Camb) 2011;3:479–89. doi: 10.1039/c0ib00147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima K, Abe-Dohmae S, Arakawa R, Murakami I, Suzumori K, Yokoyama S. Progesterone inhibits apolipoprotein-mediated cellular lipid release: A putative mechanism for the decrease of high-density lipoprotein. Biochim Biophys Acta. 2001;1532:173–84. doi: 10.1016/s1388-1981(01)00124-x. [DOI] [PubMed] [Google Scholar]
- 34.Lamon-Fava S, Postfai B, Diffenderfer M, DeLuca C, O’Connor J, Jr, Welty FK, et al. Role of the estrogen and progestin in hormonal replacement therapy on apolipoprotein A-I kinetics in postmenopausal women. Arterioscler Thromb Vasc Biol. 2006;26:385–91. doi: 10.1161/01.ATV.0000199248.53590.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]


