Abstract
Benzofuran as an important heterocyclic compound is extensively found in natural products as well as synthetic materials. Since benzofuran drivatives display a diverse array of pharmacological activities, an interest in developing new biologically active agents from benzofuran is still under consideration. This review highlights recent findings on biological activities of benzofuran derivatives as antimicrobial and antibreast cancer agents and lays emphasis on the importance of benzofurans as a major source for drug design and development.
Keywords: Benzofuran, antibacterial, anti breast cancer
INTRODUCTION
Benzofuran is a fundamental structural unit in a variety of biologically active natural products as well as synthetic materials.[1] Benzofuran derivatives possess several biological properties such as anti-inflammatory, antimicrobial, antifungal, antihyperglycemic, analgesic, antiparasitic, and antitumor activities.[2,3,4,5,6,7] Such a wide range of biological properties inherent in benzofuran scaffold justifies the extensive interest in using benzofuran as building blocks of pharmacological agents. Many of the clinically approved drugs are synthetic or naturally occurring substituted benzofuran derivatives, some of which are fused with other heterocyclic moieties.[8] Existence of several new publications on the biological importance of these products has necessitated a new review on their biological activities.[9,10,11,12] However, due to the great biological importance of this scaffold, investigation of various methods for synthesis and structural modification of benzofuran derivatives have now become an important goal of several research groups.[13,14,15] Although benzofuran derivatives display a diverse array of pharmacological activities, in this review we only provide a literature overview on the antimicrobial and antibreast cancer activities of this scaffold and offer a summary of structure activity relationship (SAR) in some areas.
BENZOFURAN AS ANTIBACTERIAL AND ANTIFUNGAL AGENT
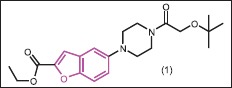
Due to the emerging and increasing bacterial resistance, development of new antibiotics is very important for public health globally.[16] Several benzofuran derivatives have been prepared and introduced as antibacterial agents.[17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] Renuka et al.[17] designed and synthesized a series of substituted benzofurans as DNA gyrase B inhibitors of Mycobacterium tuberculosis (MTB). DNA gyrase of MTB is a type II topoisomerase and is a well-established and validated target for the development of novel therapeutics. The compounds were tested for their biological activity; compound 1 emerged as the most active potent lead with an IC50 of 3.2 ± 0.15 μM against Mycobacterium smegmatis DNA gyraseB enzyme and 0.81 ± 0.24 μM in MTB supercoiling activity. Subsequently, the binding of compound 1 to the DNA gyraseB enzyme by the docking study indicated good interaction with the enzyme. This ligand with a glide score of −8.66 kcal/mol showed a H-bond with Arg141 and a well-fitted pose in the hydrophobic pocket within the vicinity of Ile84, Val128, Ile171, Val49, Ala53, Leu135, Val128, Val123, and Val99, and a few polar amino acid residues Glu 48, Ser 126, Glu 56, Gln 102. The binding pattern within the active site pocket of the crystal ligand and reference ligand was quite similar and additionally the van der Waals and columbic forces between Thr170, Asn52, Ala53, Ile84, and Glu48 and the ligand were observed.
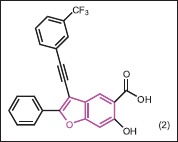
He et al.[18] synthesized a series of inhibitors of Mycobacterium protein tyrosine phosphatase B (mPTPB) from 6-hydroxy-benzofuran-5-carboxylic acid scaffold. mPTPB is a virulence factor secreted by the pathogen and mediates mycobacterial survival in macrophages by targeting host cell immune responses. Consequently, mPTPB represents an exciting new target to combat tuberculosis (TB) infection.[19] He et al.[18] described a medicinal chemistry-oriented approach that transforms a benzofuran salicylic acid scaffold into a highly potent (IC50 = 38 nM) and selective mPTPB inhibitor [>50-fold against a large panel of protein tyrosine phosphatase (PTPs)] (2). Importantly, the inhibitor is capable of reversing the altered host immune responses induced by the bacterial phosphatase and restoring the macrophage's full capacity to secrete interleukin-6 and undergo apoptosis in response to interferon-γ stimulation, validating the concept that chemical inhibition of mPTPB may be therapeutically useful for novel TB treatment. The study further demonstrates that bicyclic salicylic acid pharmacophores can be used to deliver PTP inhibitors with high potency, selectivity, and cellular efficacy.
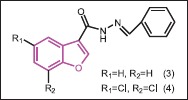
Telveka et al.[20] synthesized a series of benzofuran-3-carbohydrazide derivatives and evaluated them for in vitro inhibitory activity against M. tuberculosis H37Rv strains. The synthesized compounds showed promising antimycobacterial and antifungal activities. Compounds 3 and 4 were found to be the most active compounds with minimum inhibitory concentration (MIC) of 8 μg/mL and 2 μg/mL, respectively. For antitubercular activity, ortho-hydroxyl and protected hydroxyl groups’ substitution on the benzylidene group have showed good antitubercular activity while for antifungal activity, the unsubstituted benzofuran ring and highly substituted side chain attached to hydrazide appeared to be more effective.
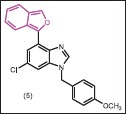
In another study, 6-benzofuryl purines were synthesized and their in vitro activities against M. tuberculosis H37Rv and mammalian cells (Vero cells) were determined.[21] The results indicated that several compounds displayed profound antimycobacterial activity in combination with low toxicity toward mammalian cells. 6-Benzofurylpurine (5) where the benzofuran substituent is connected directly to C-6 in the purine was found to be highly potent inhibitors of MTB (IC90 < 0.60 μM).
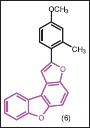
Yempala et al. designed and synthesized a series of 2-substituted-3H-benzofurobenzofurans through molecular hybridization[22] and screened them for in vitro antimycobacterial activity against M. tuberculosis H37Rv. Among them, 2-(4-methoxy-2-methyl phenyl)-3H-benzofuro [3,2-e] benzofuran (6) was found to be most active with MIC 3.12 μg/mL and exhibited lower cytotoxicity with good therapeutic index.
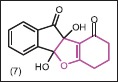
In order to investigate antimicrobial activity, Mehdi et al.[23] synthesized 4b,9b-dihydroxy-7,8-dihydro-4bH-indeno[1,2-b]benzofuran-9,10 (6H,9bH)-dione (7) and evaluated their biological activity, using microdilution method, against gram-positive (B. subtilis, B. cereus, S. pneumoniae, S. aureus) and gram-negative (K. pneumoniae, S. flexneri, P. aeruginosa, E. aerogenes, E. coli) bacterial and fungal (C. albicans) strains. Generally, MIC values of these compounds against all tested microorganisms were between 0.5 mg/mL and 1 mg/mL, which were comparable to that of clinically used antimicrobial agents against the selected microorganisms.
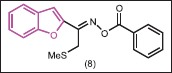
Kirilmis et al.[24] reported the synthesis of some 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. When the synthesized compounds were tested for antimicrobial activity, (E)-1-(1-benzofuran-2-yl)-2-mesitylethanone-O-benzoyloxime (8) was found to be the most active derivative against S. aureus ATCC 6538 and E. coli ATCC 25922. The other compounds exhibited moderate activity against the tested microorganisms.
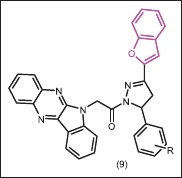
Manna and Agrawal[25] demonstrated that some indophenazine 1,3,5-trisubstituted pyrazoline derivatives of benzofuran (9), which were synthesized by microwave irradiation, exhibited good antibacterial activity with MICs lower than 10 μg/mL against E. coli, P. aeruginosa and S. aureus, which was comparable with sparfloxacin and norfloxacin.
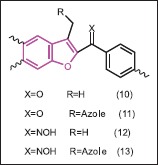
Gundogdu-Karaburun et al.[26] synthesized some aryl [3-(imidazol-1-yl/triazol-1-ylmethyl/methyl) benzofuran-2-yl] ketones (10, 11), aryl (3-methyl-benzofuran-2-yl) ketoximes and aryl [3-(imidazol-1-yl/triazol-1-ylmethyl)benzofuran-2-yl] ketoximes (12, 13). Antifungal activities of these compounds were examined and moderate activity was obtained. The activities of the aimed oxime and azole residue bearing compounds ranged between 5-12.5 μg/mL and 5-25 μg/mL against C. glabrata and C. albicans, respectively. It was seen that furnishing the oxime residue to the ketones increases the activity almost up to twofold to fourfold in most of the compounds. However, addition of the azole residue slightly increased some of the compound's activity. Uncooperatively, oxime azole combination was not worked and the most active compounds appeared to be ketoximes 12.
Jiang et al. synthesized a series of new benzofuran derivatives bearing aryl substituents at its C-3 position through methanone linker.[27] All compounds were screened for their antibacterial and antifungal activities against four bacteria: E. coli, S. aureus, methicillin-resistant Staphylococcus aureus (MRSA), B. subtilis, and a fungus C. albicans. All the tested compounds were found to be inactive against C. albicans. SAR studies revealed that hydroxyl substituents at C-3 and C-4 position resulted in good antibacterial activities while compounds possessing hydroxyl group at C-2 position impart no increasing activity against the bacteria. Compounds with the methylation of the hydroxyl group would reduce the solubility and consequently decrease their antimicrobial abilities. In this series, some compounds with halogen substituents showed no antibacterial activity, which is different from what was observed in benzufuran-containing natural products. Compound (14), with a hydroxyl group at C-4, displayed the excellent antibacterial activity against S. aureus and MRSA with MIC80 values of 0.39 μg/mL and 0.78 μg/mL, respectively.
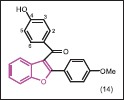
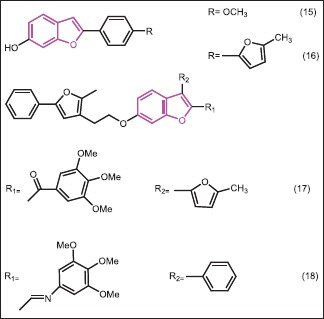
Liu et al.[28] reported the synthesis and antibacterial evaluation of a new series of 3-methanone-6-substituted-benzofuran derivatives with hydroxyl or (5-methyl-2-phenyloxazol-4-yl)ethyloxy at C-6 position of benzofuran. Antibacterial activities were evaluated against E. coli, S. aureus, MRSA, B. subtilis, and P. aeruginosa. The result indicated that substitutions at C-6 and C-3 positions of these derivatives were found to greatly impact the antibacterial activity and strains’ specificity, respectively. Compounds bearing a hydroxyl group at C-6 (15, 16) offered excellent antibacterial activities against all the strains (MIC80 = 0.78-3.12 μg/mL). Compounds with phenyl, 5- methylfuran-2-yl, and 4-methoxyphenyl groups at C-2 position of benzofuran showed good antibacterial activity with MIC80 values between 0.78 μg/mL and 6.25 μg/mL, comparable to those of control drugs. In contrast, compounds in which the hydroxyl group was blocked at the C-6 position of benzofuran, exhibited no antibacterial activity to any of the tested strains, indicating that the hydroxyl group at C-6 position of benzofuran was requisite for the activity. The strain specificity was also observed in compounds 17 and 18. C-6 position was fixed with a 5-methyl-2-phenyloxazole-4-ethyloxy group and the C-3 position was occupied by either a 3,4,5-trimethoxybenzoyl group (17) or an imine group (18). These two compounds displayed antibacterial activities only against S. aureus with MIC80 values of 12.5 μg/mL and 3.12 μg/mL, respectively. It was speculated that the strain-specificity may be owing to the methanone group or imine group between the 3,4,5-trimethoxyphenyl and benzofuran nucleus, which may play a specific role with the biological target of S. aureus. Moreover, the strain specificity lost when the double bond was introduced between the 3,4,5-trimethoxyphenyl and methanone or when the imine was reduced to amine.
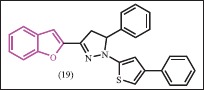
A series of benzofurans were synthesized and tested for antimicrobial evaluation by Abdel-Wahab et al.[29] In vitro antimicrobial activity was performed against the gram-positive (S. aureus, B. subtilis), gram-negative bacteria (E. coli) and fungi (C. albicans and A. niger). The results revealed that the inhibitory activity of the synthesized compounds against the gram-negative bacteria was higher than that of the gram-positive bacteria. The 1-(thiazol-2-yl)pyrazoline (19) showed excellent activity against gram-negative bacteria (inhibitory zone 25 mm), good activity against gram-positive bacteria (inhibitory zone 20 mm). The compounds showed remarkable activity against C. albicans. Most of the tested compounds showed none or weak antifungal activity against A. niger. According to the SAR studies, it can be concluded that benzofuran, pyrazoline, and thiazole moieties are essential for their antimicrobial activity.
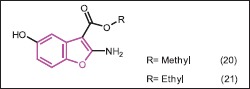
Ryu et al.[30] synthesized benzofuran-5-ol derivatives as potent antifungal agents. The antifungal activity of compounds 20 and 21 was superior or comparable to 5-fluorocytosine. These compounds completely inhibited the growth of all tested fungal species at the MIC level of 1.6-12.5 μg/mL. Many of 2-amino-4-arylthio-5-hydroxybenzofurans also showed potent antifungal activity against C. krusei, C. neoformans, and A. niger. The results suggested that benzofuran-5-ol scaffolds would be promising leads for the development of antifungal agents.
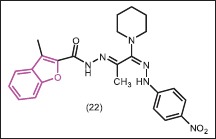
Benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones were synthesized by Abdel-Aziz and Mekawey[31] and evaluated for antifungal and antibacterial activities. The antifungal potencies were superior to the antibacterial activities in these series. Compound 22 was found to be a promising antifungal agent. From the results of the morphological features of A. fumigatus and C. albicans, it can be concluded that these derivatives can be considered very promising in the perspective of new drugs discovery.
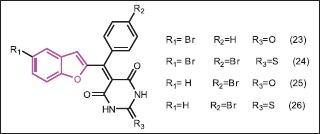
Kenchappa et al.[32] synthesized a novel series of benzofuran derivatives, containing barbitone and thiobarbitone moiety. The investigation of antimicrobial screening of this compounds revealed that all compounds exhibited a varied degree (MIC, 11.38-199.10 mmol/L) of antibacterial activity against all tested bacterial strains. SAR studies revealed that electron withdrawing groups in the ortho position of benzofuran ring and in the para position of aryl ring have a tendency to increase the potency while compounds containing electron-donating groups were found to weaken the antimicrobial activity. Compounds 23 and 24 having two bromo substituents on C-5 of benzofuran and C-4 of phenyl ring, respectively, were found to exhibit excellent antibacterial activity against all tested bacterial strains with MIC value of 29.76-31.96 mmol/L. Compounds bearing -Br substituent on C-4 position of the aryl ring showed good ability to inhibit S. tyhphi at MIC 36.61-37.92 mmol/L; the same compounds showed good activity against P. syringae with MIC 37.20-38.50 mmol/L while compounds having hydroxyl and bromo substituent exhibited moderate to good activity against S. tyhphi with MIC value 36.08-36.73 mmol/L. The MIC of antifungal activity of the compounds indicated that compounds 25 and 26 exhibited remarkable activity against the tested organisms with MIC value 14.90-29.92 mmol/L. Further, the synthesized compounds were studied for docking on the enzyme, glucosamine-6-phosphate synthase, and the results showed that compounds 23 and 24 emerged as an active antimicrobial agents with lowest binding energy (−5.27 kJ mol/L and −4.85 kJ mol/L, respectively).
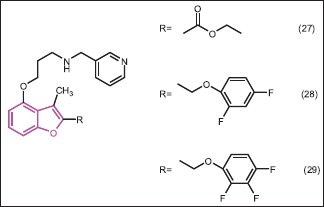
Hirosato et al.[33] performed the synthesis of several pyridyl-benzofuran derivatives, which are obtained by structural modification at C-2 of a known antifungal agent 27 (RO-09-4609) and evaluated their activity for inhibition of N-myristoyltransferase enzyme, an enzyme involved in fungal infection. Compounds 28 (enzyme inhibition IC50 = 0.0075 μM; antifungal activity IC50 = 0.03 μM) and 29 (enzyme inhibition IC50 = 0.0057 μM; antifungal activity IC50 = 0.035 μM) were found to be the most active among synthesized compounds. Both these compounds showed activity in the rat systematic candidiasis model.
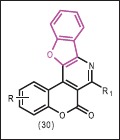
Fused benzofuran derivatives containing coumarin and pyridine rings (30) were synthesized by Khan et al.[34] and their antibacterial and antifungal activities were evaluated. In antibacterial screening, all compounds showed MIC of 25 μg/mL against P. chinchori but they were ineffective against M. aureus. In antifungal screening, the compounds showed MIC at 25 μg/mL and 100 μg/mL against A. fumigatus and P. wortmanni, respectively.
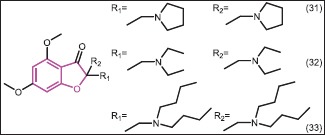
Antibacterial and antifungal activity of 2-bisaminomethylatedaurone benzofuran derivatives were evaluated by Bandgar et al.[35] against B. subtilis (NCIM 2546), E. coli (NCIM 2065), S. aureus (NCIM 2120), K. pneumonia (NCIM 5082), and P. vulgaris (NCIM 2813) bacterial strains by the disc diffusion method. Interestingly, all compounds have shown good antimicrobial activity. Compounds 31, 32, and 33 with MIC = 25 μg/mL exhibited promising activity.
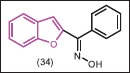
Some aryl (benzofuran-2-yl) ketoximes and their ethers as well as their esters were synthesized by Demirayak et al.[36] Antifungal activities of the compounds were examined and it is concluded that unsubstituted or small alkyl group substitution on the oxime residue resulted in more effective compounds. The most significant compound is appeared to be 34 with a MIC value lower than the oxiconazole, clotrimazole, and fluconazole as control.
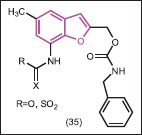
Venkateshwarlu et al.[37] synthesized some novel benzo[b]furan derivatives possessing sulfonamide and phenyl carbamate moieties (35) and performed antibacterial screening against four species of bacteria, i.e., S. aureus, S. pyogenes, E. coli and P. aeruginosa. It was observed that among all these compounds, sulphonyl derivatives showed good to excellent activity, while the carbonyl derivatives showed poor activity against all the tested bacterial strains.
Two series of 8-bromo-3-{[phenylmethylidene]amino}[1]benzofuro[3,2-d]pyrimidin-4(3H)-one derivatives (36) have been synthesized by Yamuna et al.[38] The results of antibacterial and antifungal activities showed that several derivatives of these analogs exhibit considerable activity against all the four bacteria and two fungal species.
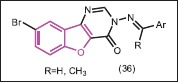
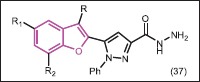
Two bacterial species E. coli, S. aureus, and one fungal species A. niger were used for antibacterial and antifungal evaluating of pyrazolyl-benzofuran derivatives (37), which were synthesized by Siddiqui et al.[39] The results demonstrated that all the tested compounds are biologically active due to the presence of different heterocycles and functional groups but their activities toward the fungus and bacteria are variable at different concentrations. All these compounds were found to be active at a high concentration against bacterial species while they were active at a low concentration against fungal species.
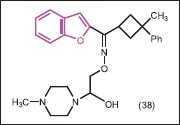
Koca et al.[40] synthesized a series derivative of benzofuran ketoxime containing cyclobutyl group and tested them for antimicrobial activity against S. aureus, S. epidermidis, E. coli, K. pneumonia, P. aeruginosa, S. typhi, S. flexneri, P. mirabilis, and C. albicans. Among tested compounds, benzofuran ketoxime 38 was most active against S. aureus (MIC = 0.039 μg/mL) while other benzofuran ketoxime derivatives showed good activity against C. albicans (MIC = 0.625-2.5 μg/mL).
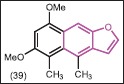
1,3-Dimethoxy-4,6-dimethylnaphthofuran (39), which is obtained from the root of Ligularia veitchiana (food supplement in China) was evaluated for antimicrobial activity by Liu et al.[41] It was found to be active against S. aureus (MIC = 62.5 μg/mL), which may be due to the existence of a modified eremophilane (metabolite of biological active Ligularia Cass) skeleton.
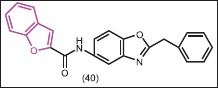
In vitro antimicrobial activity of a series of synthesized 2-(substitutedphenyl/benzyl)-5-[(2- benzofuryl)carboxamido]benzoxazole derivatives was determined by Alper-Hayta et al.[42] The results indicated that the synthesized compounds possessed a broad spectrum of activity. In the series, the most active compound against C. krusei and C. albicans was 40 with MIC value 31.25 μg/mL. SAR analysis of synthesized compound using three-dimensional (3D) common features pharmacophore hypotheses suggested that “N” was more important than “O” of benzoxazole for increasing the potency and also that the “O” of either benzofuran or benzoxazole placed at the same side played a very important role for increasing the activity against drug-resistant P. aeruginosa.
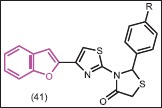
1-(1-Benzofuran-2-yl)-2-bromoethanone was utilized as a key intermediate by Venkatesh et al.[26] for synthesis of a series of 3-[4-(1-benzofuran-2-yl)-1,3-thiazol-2-yl]-2-(4-aryl)-1,3-thiazolidin-4-one derivatives (41). The title compounds showed good antimicrobial activity againest four bacteria and four fungal tested organisms.
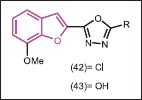
A series of benzofuran drivatives containing oxadiazoles and pyrazoles were synthesized and tested against bacterial (E. coli, K. pneumonia, P. aeruginosa, S. aureus, S. faecalis) and fungal strains (A. flavus, A. fumigatus, C. albicans, P. notatum, Rhizopus).[43] All the tested compounds showed moderate to good microbial inhibition. In the series, the compounds bearing chlorine 42 and hydroxyl group 43 exhibited potent activities compared to others. Compound 42 was more potent antibacterial than 43, whereas vice versa is true for antifungal activity.
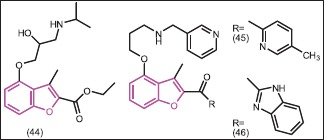
The C-4 side chain modification of lead compound 44 by Masubuchi et al.[44] resulted in the identification of potent and selective Candida albicans N-myristoyltransferase (CaNmt) inhibitors. Among the new inhibitors, pyridine derivative 45 and benzimidazole derivative 46 showed clear antifungal activity in a murine systemic candidiasis model.
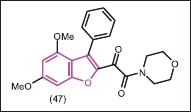
Mielczarek et al.[45] synthesized several classes of mono-indole and mono-benzofuran drivatives that targeted the essential protein-protein interaction between RNA polymerase core and σ70/σA factors in bacteria. The inhibitor behavior of the novel molecules was measured by enzyme-linked immunosorbent assay (ELISA) test. Synthesized derivatives inhibited the growth of both gram-positive and gram-negative bacteria in culture. Among mono-benzofuran derivatives, compound 47 was the most potent inhibitor of B. subtilis growth, with growth inhibition values of 70% at 200 μM. However, since this compound exhibited relatively low or no activity in the ELISA, its mechanism of antibacterial activity might not involve the inhibition of the B´-CH-σ70/σA2.2 interaction. SAR studies of the mono-benzofuran inhibitors suggested that the hydrophilic-hydrophobic balance was an important determinant of biological activity of these compounds.
BENZOFURAN AS ANTI BREAST CANCER AGENT
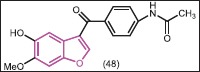
Some benzofuran derivatives have shown potential as therapeutic agents for breast cancer.[46,47,48,49,50,51,52,53] Li et al.[46] synthesized 3-acyl-5- hydroxybenzofuran derivatives by using a microwave-assisted method, which exhibited different antiproliferation against human breast cancer MCF-7 cells. Compound 48 showed the best activity with IC50 = 43.08 μM. To investigate the binding interactions between compounds and estrogen receptor alpha (ERα), a Quantum Mechanics Polarized Ligand Docking (QPLD) study was conducted. A detailed analysis indicated that compound 48 possesses the highest van der Waals and hydrogen bond interactions and can be used as attractive scaffold for designing anticancer agents.
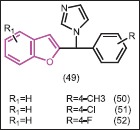
Aromatase inhibitors are also a good choice for the treatment of breast cancer in postmenopausal women.[47] Whomsley et al.[48] identified substituted l-[(benzofuran-2-yl)-phenylmethyl]-imidazoles (49) as a class of potent aromatase inhibitor with in vitro IC50 values <10 nM which is 80-1000 times of the inhibitory activity of aminoglutethimide. At a dose of 2 mg/kg in pregnant mares serum gonadotropin (PMSG)-stimulated rats, these compounds effectively reduce the estradiol levels by 82-98%. Subsequently, Khodarahmi et al.[49] studied their enantioselectivity as aromatase inhibitors. The enantioselectivity ratio ((+):(-)-forms) of three substituted l-[(benzofuran-2-yl) phenylmethyl] imidazoles (50, 51, 52) were 2.16, 12.3, and 1.0 for the 4-methyl-, 4-fluoro-, and 4-chloro-substituted compounds, respectively.
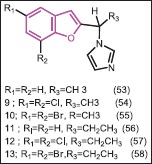
Additionally, Khodarahmi et al.[50] studied replacement of the phenyl group by methyl and ethyl functions within the related 1-(benzofuran-2-y1)-1-(1-H-imidazol-1-yl) alkan. They reported that achiral substituted 1-(benzofuran-2-yl alkan) imidazoles are much weaker inhibitors of the enzyme than the phenyl analogues. The (±)-methyl-substituted compounds (53, 54, 55) were 2.36-12.0 times more potent than (±)-aminoglutethimide and as expected, with an increase in hydrophobicity the (±)-ethyl substituted compounds were 5.4-33 times more potent (56). The 5,7-dichloro compounds (54 and 57) were the most potent in each series. The IC50 of the (−) form/IC50 of the (+) form of 4.8 (54) and 12.6 (55) for the the methyl-substituted indicate increased potency of (+) isomer compared with (±)-aminoglutethimide. In the ethyl-substituted series the stereoselective ratios were 8.3 and 5.2 for (57) and (58), both having about 39-fold greater potency than (±)-aminoglutethimide.
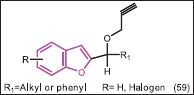
Khodarahmi et al.[51] synthesized some 2-(1-phenyl- 1 -(prop-2’-ynyloxy)methyl) benzofurans (59) and studied their behavior as inhibitors of aromatase. Unlike l-[benzofuran-2-y1)phenylmethyll imidazole, these series of compounds were weak reversible inhibitors of the enzyme (4-15%) while it would seem that prolonged exposure to aromatase leads to metabolism of the propargyl function and irreversible inhibition of the enzyme.
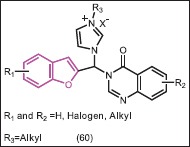
Considering the potent cytotoxic activities of hybrid benzofuran-imidazolium derivatives on the breast cancer cell line MCF-7, Khodarahmi et al.[52] also designed novel hybrid derivatives (60) incorporating benzofuran, imidazole, and quinazolinone pharmacophores using a molecular hybridization approach. The binding modes of these novel hybrid compounds to aromatase were investigated using a docking procedure applying a combined quantum mechanical/molecular mechanical (QM/MM) method. The results indicated that the hybrid compounds were adopted properly within the aromatase binding site, suggesting that they could be potential inhibitors of aromatase. These novel designed compounds engaged in hydrophobic and H-bond interactions with the aromatase binding site, which are in agreement with the basic physicochemical features of known aromatase inhibitors.
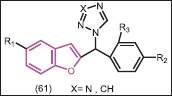
Vinh et al.[53] synthesized a series of 1-[(benzofuran-2-yl)phenyimethyl]-triazoles and -tetrazoles (61) and tested for human placental aromatase inhibition in vitro, using [1β-3H] -androstenedione as the substrate for the aromatase enzyme. In all cases, the triazoles exhibited greater inhibitory activity than the corresponding tetrazale analogues. Substitution in the phenyl ring, in particular substitution at R2, resulted in improved activity while substitution in the benzofuran ring had an adverse effect on in vitro activity. It should be mentioned that in vitro imidazole substituted benzofurans were more poten compared to triazole- and tetrazole-derivatives.
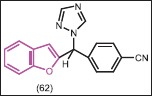
Palma et al.[54] reported that MEN-11066 (62) as a benzofuran containing aromatase inhibitor, which was 15-fold more potent (Ki = 0.098 nM) than the anastrozole (Ki = 1.2 nM). (+) enantiomer of 62 was again 10-fold more active than racemic form (Ki = 0.079 nM), suggesting that (+) enantiomer of 62 could be considered in estrogen responsive cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
GK contributed in the conception of the work, conducting the study, drafting and revising the draft, approval of the final version of the manuscript and agreed for all aspects of the work. PA contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript and agreed for all aspects of the work. FH contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript and agreed for all aspects of the work. EK contributed in revising the draft, approval of the final version of the manuscript and agreed for all aspects of the work.
Acknowledgments
This study was supported by the Research Council of Isfahan University of Medical Sciences, Isfahan (193071) and Iran National Science Foundation, Presidency of the Islamic Republic of Iran (93012407).
REFERENCES
- 1.Dawood KM. Benzofuran derivatives: A patent review. Expert Opin Ther Pat. 2013;23:1133–56. doi: 10.1517/13543776.2013.801455. [DOI] [PubMed] [Google Scholar]
- 2.Habtemariam S. Antiinflammatory activity of the antirheumatic herbal drug, gravel root (Eupatorium purpureum): Further biological activities and constituents. Phytother Res. 2001;15:687–90. doi: 10.1002/ptr.887. [DOI] [PubMed] [Google Scholar]
- 3.Pauletti PM, Araújo AR, Young MC, Giesbrecht AM, Bolzani VD. nor-Lignans from the leaves of Styrax ferrugineus (Styracaceae) with antibacterial and antifungal activity. Phytochemistry. 2000;55:597–601. doi: 10.1016/s0031-9422(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 4.Masubuchi M, Kawasaki K, Ebiike H, Ikeda Y, Tsujii S, Sogabe S, et al. Design and synthesis of novel benzofurans as a new class of antifungal agents targeting fungal N-myristoyltransferase. Part 1. Bioorg Med Chem Lett. 2001;11:1833–7. doi: 10.1016/s0960-894x(01)00319-5. [DOI] [PubMed] [Google Scholar]
- 5.Wrobel JE, Dietrich AJ, Antane MM. Benzothiophenes, Benzofurans, and Indoles Useful in the Treatment of Insulin Resistance and Hyperglycemia. US Patent 6,251,936. 2001:1–57. [Google Scholar]
- 6.Kayser O, Chen M, Kharazmi A, Kiderlen AF. Aurones Interfere with Leishmania major mitochondrial fumarate reductase. Z Naturforsch C. 2002;57:717–20. doi: 10.1515/znc-2002-7-828. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa I, Shioya R, Agatsuma T, Furukawa H, Naruto S, Sugano Y. 4-Hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as potent anti-tumor agents. Bioorg Med Chem Lett. 2004;14:455–8. doi: 10.1016/j.bmcl.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Proksch P, Rodriguez E. Chromenes and benzofurans of the asteraceae, their chemistry and biological significance. Phytochemistry. 1983;22:2335–48. [Google Scholar]
- 9.Khanam H, Shamsuzzaman Bioactive Benzofuran derivatives: A review. Eur J Med Chem. 2015;97:483–504. doi: 10.1016/j.ejmech.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Naik R, Harmalkar DS, Xu X, Jang K, Lee K. Bioactive benzofuran derivatives: Moracins A-Z in medicinal chemistry. Eur J Med Chem. 2015;90:379–93. doi: 10.1016/j.ejmech.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Radadiya A, Shah A. Bioactive benzofuran derivatives: An insight on lead developments, radioligands and advances of the last decade. Eur J Med Chem. 2015;97:356–76. doi: 10.1016/j.ejmech.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Nevagi RJ, Dighe SN, Dighe SN. Biological and medicinal significance of benzofuran. Eur J Med Chem. 2015;97:561–81. doi: 10.1016/j.ejmech.2014.10.085. [DOI] [PubMed] [Google Scholar]
- 13.Kadieva MG, Oganesyan ÉT. Methods for the synthesis of benzofuran derivatives (review) Chem Hetero Comp. 1997;33:1245–58. [Google Scholar]
- 14.Sidhu PS. Review: One-pot synthesis protocol for highly functionalized benzofuran scaffold. J Postdoc Res. 2014;1:31–6. [Google Scholar]
- 15.Rossy C, Fouquet E, Felpin FX. Practical synthesis of indoles and benzofurans in water using a heterogeneous bimetallic catalyst. Beilstein J Org Chem. 2013;9:1426–31. doi: 10.3762/bjoc.9.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien S. Meeting the societal need for new antibiotics: The challenges for the pharmaceutical industry. Br J Clin Pharmacol. 2015;79:168–72. doi: 10.1111/bcp.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renuka J, Reddy KI, Srihari K, Jeankumar VU, Shravan M, Sridevi JP, et al. Design, synthesis, biological evaluation of substituted benzofurans as DNA gyraseB inhibitors of Mycobacterium tuberculosis. Bioorg Med Chem. 2014;22:4924–34. doi: 10.1016/j.bmc.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Xu J, Yu ZH, Gunawan AM, Wu L, Wang L, et al. Discovery and evaluation of novel inhibitors of mycobacterium protein tyrosine phosphatase B from the 6-hydroxy-benzofuran-5-carboxylic acid Scaffold. J Med Chem. 2013;56:832–42. doi: 10.1021/jm301781p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B, He Y, Zhang X, Xu J, Luo Y, Wang Y, et al. Targeting mycobacterium protein tyrosine phosphatase B for antituberculosis agents. Proc Natl Acad Sci U S A. 2010;107:4573–8. doi: 10.1073/pnas.0909133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telvekar VN, Belubbi A, Bairwa VK, Satardekar K. Novel N´-benzylidene benzofuran-3-carbohydrazide derivatives as antitubercular and antifungal agents. Bioorg Med Chem Lett. 2012;22:2343–6. doi: 10.1016/j.bmcl.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 21.Brndvang M, Bakken V, Gundersen LL. Synthesis, structure, and antimycobacterial activity of 6-[1(3H)-isobenzofuranylidenemethyl]purines and analogs. Bioorg Med Chem. 2009;17:6512–6. doi: 10.1016/j.bmc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Yempala T, Sridevi JP, Yogeeswari P, Sriram D, Kantevari S. Design, synthesis and antitubercular evaluation of novel 2-substituted-3H-benzofuro benzofurans via palladium-copper catalysed Sonagashira coupling reaction. Bioorg Med Chem Lett. 2013;23:5393–6. doi: 10.1016/j.bmcl.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 23.Mehdi SH, Hashim R, Ghalib RM, Guedes da Silva MFC, Sulaiman O, Rahman SZ, et al. Synthesis, characterization, antimicrobial and enzymatic activity of 4b,9b-dihydroxy-7,8-dihydro -4bH-indeno [1,2-b] benzofuran-9,10 (6H,9bH) -dione. J Mol Struc. 2011;1006:318–23. [Google Scholar]
- 24.Kirilmis C, Ahmedzade M, Servi S, Koca M, Kizirgil A, Kazaz C. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur J Med Chem. 2008;43:300–8. doi: 10.1016/j.ejmech.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Manna K, Agrawal YK. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity. Bioorg Med Chem Lett. 2009;19:2688–92. doi: 10.1016/j.bmcl.2009.03.161. [DOI] [PubMed] [Google Scholar]
- 26.Gündoğdu-Karaburun N, Benkli K, Tunali Y, Uçucu U, Demirayak S. Synthesis and antifungal activities of some aryl [3-(imidazol-1-yl/triazol-1-ylmethyl) benzofuran-2-yl] ketoximes. Eur J Med Chem. 2006;41:651–6. doi: 10.1016/j.ejmech.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X, Liu W, Zhang W, Jiang F, Gao Z, Zhuang H, et al. Synthesis and antimicrobial evaluation of new benzofuran derivatives. Eur J Med Chem. 2011;46:3526–30. doi: 10.1016/j.ejmech.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Jiang F, Jiang X, Zhang W, Liu J, Liu W, et al. Synthesis and antimicrobial evaluation of 3-methanone-6-substituted-benzofuran derivatives. Eur J Med Chem. 2012;54:879–86. doi: 10.1016/j.ejmech.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Wahab BF, Abdel-Aziz HA, Ahmed EM. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur J Med Chem. 2009;44:2632–5. doi: 10.1016/j.ejmech.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Ryu CK, Song AL, Lee JY, Hong JA, Yoon JH, Kim A. Synthesis and antifungal activity of benzofuran-5-ols. Bioorg Med Chem Lett. 2010;20:6777–80. doi: 10.1016/j.bmcl.2010.08.129. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Aziz HA, Mekawey AA. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur J Med Chem. 2009;44:4985–97. doi: 10.1016/j.ejmech.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Kenchappa R, Bodke YD, Telkar BA, Sindhe MA. Synthesis, antimicrobial, and antioxidant activity of benzofuran barbitone and benzofuran thiobarbitone derivatives. Med Chem Res. 2014;23:3065–81. [Google Scholar]
- 33.Ebiike H, Masubuchi M, Liu P, Kawasaki K, Morikami K, Sogabe S, et al. Design and synthesis of novel benzofurans as a new class of antifungal agents targeting fungal N-Myristoyltransferase. Part 2. Bioorg Med Chem Lett. 2002;12:607–10. doi: 10.1016/s0960-894x(01)00808-3. [DOI] [PubMed] [Google Scholar]
- 34.Khan IA, Kulkarni MV, Gopal M, Shahabuddinb MS, Sun CM. Synthesis and biological evaluation of novel angularly fused polycyclic coumarins. Bioorg Med Chem Lett. 2005;15:3584–7. doi: 10.1016/j.bmcl.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 35.Bandgar BP, Patil SA, Korbad BL, Biradar SC, Nile SN, Khobragade CN. Synthesis and biological evaluation of a novel series of 2,2-bisaminomethylated aurone analogues as anti-inflammatory and antimicrobial agents. Eur J Med Chem. 2010;45:3223–7. doi: 10.1016/j.ejmech.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 36.Demirayak S, Uçucu U, Benkli K, Gündoğdu-Karaburun N, Karaburun AC, Akar D, et al. Synthesis and antifungal activities of some aryl(benzofuran-2-yl)ketoximes. Farmaco. 2002;57:609–12. doi: 10.1016/s0014-827x(02)01257-0. [DOI] [PubMed] [Google Scholar]
- 37.Oter O, Ertekin K, Kirilmis C, Koca M, Ahmedzade M. Characterization of a newly synthesized fluorescent benzofuran derivative and usage as a selective fiber optic sensor for Fe(III) Sens Actuators B: Chem. 2007;122:450–6. [Google Scholar]
- 38.Yamuna AJ, Vaidya VP, Shruthi E, Mahadevan KM. Synthesis, characterization and biological activities of some benzofuropyrimidine derivatives. Int J Pharm Pharm Sci. 2013;5(Suppl 2):450–5. [Google Scholar]
- 39.Siddiqui NJ, Idrees M, Khati NT, Dhonde MG. Use of transesterified 1,3-diketoesters in the synthesis of trisubstituted pyrazoles and their biological screening. Bull Chem Soc Ethiop. 2013;27:85–94. [Google Scholar]
- 40.Habermann J, Ley SV, Smits R. Three-step synthesis of an array of substituted benzofurans using polymer-supported reagents. J Chem Soc Perkin Trans. 1999;1:2421–3. [Google Scholar]
- 41.Liu Q, Shen L, Wanga TT, Chen CJ, Qi WY, Gao K. Novel modified furanoeremophilane-type sesquiterpenes and benzofuran derivatives from Ligularia veitchiana. Food Chem. 2010;122:55–9. [Google Scholar]
- 42.Alper-Hayta S, Arisoy M, Temiz-Arpaci O, Yildiz I, Aki E, Ozkan S, et al. Synthesis, antimicrobial activity, pharmacophore analysis of some new 2-(substitutedphenyl/benzyl)-5- [(2-benzofuryl)carboxamido]benzoxazoles. Eur J Med Chem. 2008;43:2568–78. doi: 10.1016/j.ejmech.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Hanumanagoud H, Basavaraja KM. Synthesis and biological evaluation of some new oxadiazole and pyrazole derivatives incorporating benzofuran moiety. Der Pharma Chemica. 2013;5:87–98. [Google Scholar]
- 44.Kamal M, Shakya AK, Jawaid T. Benzofurans: A new profile of biological activities. Int J Med Pharm Sci. 2011;1:1–15. [Google Scholar]
- 45.Mielczarek M, Thomas RV, Mac C, Kandemir H, Yang X, Bhadbhade M, et al. Synthesis and biological activity of novel mono-indole and mono-benzofuran inhibitors of bacterial transcription initiation complex formation. Bioorg Med Chem. 2015;23:1763–75. doi: 10.1016/j.bmc.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 46.Li XY, He BF, Luo HJ, Huang NY, Deng WQ. 3-Acyl-5-hydroxybenzofuran derivatives as potential anti-estrogen breast cancer agents: A combined experimental and theoretical investigation. Bioorg Med Chem Lett. 2013;23:4617–21. doi: 10.1016/j.bmcl.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Briest S, Davidson NE. Aromatase inhibitors for breast cancer. Rev Endocr Metab Disord. 2007;8:215–28. doi: 10.1007/s11154-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 48.Whomsley R, fernandez E, Nicholls PJ, Smith HJ, Lombardi P, Pestellin V. Substituted,1-[(benzofuran-2-Yl)-phenylmethyl]-imidazoles as potent inhibitors of aromatase in vitro and in female rats in vivo. J Steroid Biochem Mol Biol. 1993;44:675–6. doi: 10.1016/0960-0760(93)90279-6. [DOI] [PubMed] [Google Scholar]
- 49.Khodarahmi GA, Laughton CA, Smith HJ, Nicholls PJ. Enantioselectivity of some 1-[(Benzofuran-2-yl) phenylmethyl] imidazoles as aromatase (P450AROM) inhibitors. J Enzyme Inhib. 2001;16:401–16. doi: 10.1080/14756360109162389. [DOI] [PubMed] [Google Scholar]
- 50.Khodarahmi GA, Smith HJ, Nicholls PJ, Ahmadi M. Enantioselectivity of some 1-(benzofuran-2-yl)-1-(1-H-imidazol-1-yl) alkanes as inhibitors of P450Arom. J Pharm Pharmacol. 1998;50:1321–30. doi: 10.1111/j.2042-7158.1998.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 51.Khodarahmi GA, Saeed G, Smith HJ, Nicholls J. Some 2-(1 -phenyl- 1 -(prop-2’-nyloxy)methyl) benzofurans as irreversible inhibitors of aromatase (P450 AROM) J Pharm Pharmacol. 1998;50(Suppl):206. [Google Scholar]
- 52.Khodarahmi GA, Asadi P, Farrokhpour H, Hassanzadeh F, Dinari M. Design of novel potential aromatase inhibitors via hybrid pharmacophore approach: Docking improvement using the QM/MM method. RSC Adv. 2015;5:58055–64. [Google Scholar]
- 53.Vinh TK, Ahmadi M, Delgado PO, Perez SF, Waiters HM, Smith HJ, et al. 1-[(Benzofuran-2-yl)phenylmethyl]-triazoles and -tetrazoles- potent competitive inhibitors of aromatase. Bioorg Med Chem Lett. 1999;9:2105–8. doi: 10.1016/s0960-894x(99)00328-5. [DOI] [PubMed] [Google Scholar]
- 54.Palma C, Criscuoli M, Lippi A, Muratori M, Mauro S, Maggi CA. Effect of the aromatase inhibitor, MEN 11066, on growth of two different MCF-7 sublines. Eur J Pharmcol. 2000;409:93–101. doi: 10.1016/s0014-2999(00)00761-5. [DOI] [PubMed] [Google Scholar]


