Abstract
Extracellular pH variations are seen as the principal endogenous signal that triggers activation of Acid‐Sensing Ion Channels (ASICs), which are basically considered as proton sensors, and are involved in various processes associated with tissue acidification. Here, we show that human painful inflammatory exudates, displaying non‐acidic pH, induce a slow constitutive activation of human ASIC3 channels. This effect is largely driven by lipids, and we identify lysophosphatidylcholine (LPC) and arachidonic acid (AA) as endogenous activators of ASIC3 in the absence of any extracellular acidification. The combination of LPC and AA evokes robust depolarizing current in DRG neurons at physiological pH 7.4, increases nociceptive C‐fiber firing, and induces pain behavior in rats, effects that are all prevented by ASIC3 blockers. Lipid‐induced pain is also significantly reduced in ASIC3 knockout mice. These findings open new perspectives on the roles of ASIC3 in the absence of tissue pH variation, as well as on the contribution of those channels to lipid‐mediated signaling.
Keywords: acid‐sensing ion channels, arachidonic acid, lysophosphatidylcholine, pain, sodium channels
Subject Categories: Membrane & Intracellular Transport, Neuroscience
Introduction
Acid‐sensing ion channels (ASICs) are depolarizing sodium channels formed by the trimeric association of different subunits numbered from ASIC1 to ASIC3 (for review, see Deval et al, 2010). They are gated by extracellular protons (Waldmann et al, 1997) and are widely expressed throughout the nervous system where they have been involved in different physiological and pathophysiological processes (for reviews, see Noël et al, 2010; Wemmie et al, 2013). Among these processes, pain is particularly interesting since an increasing amount of data identifies ASICs as promising therapeutic targets for the development of new analgesic strategies (Mazzuca et al, 2007; Deval et al, 2008, 2011; Karczewski et al, 2010; Bohlen et al, 2011; Walder et al, 2011; Diochot et al, 2012, 2015; Izumi et al, 2012).
ASICs are basically considered as fine sensors of extracellular pH variations, and proton is actually the only endogenous ligand known to directly activate these channels. A non‐proton ligand‐sensing domain has also been described in ASIC3 (Yu et al, 2010), which contributes to the direct effect of the synthetic compound GMQ (2‐guanidine‐4‐methylquinazoline) and of the arginine metabolite agmatine (Li et al, 2010; Yu et al, 2010). However, agmatine has a very low affinity for the channel (EC50 ~10 mM), suggesting that it is probably not a direct endogenous activator of ASIC3, but rather a physiological modulator of the acid‐evoked current. Several other stimuli linked to ischemia and/or inflammation have also been reported to potentiate the acid‐induced ASIC3 current, including lactate (Immke & McCleskey, 2001), arachidonic acid (Allen & Attwell, 2002; Smith et al, 2007; Deval et al, 2008), nitric oxide (Cadiou et al, 2007), hypertonicity (Deval et al, 2008; Li et al, 2010), serotonin (Wang et al, 2013), and ATP (Birdsong et al, 2010).
We show here that human painful inflammatory exudates that exhibit a physiological non‐acidic pH are able to activate ASIC3 channels and that this effect is largely supported by lipids. Lysophosphatidylcholine (LPC) and arachidonic acid (AA), which are co‐released following hydrolysis of membrane phospholipids by phospholipase A2 (PLA2) enzymes, are present at high concentrations in these exudates and can directly activate ASIC3 channels at physiological pH 7.4, that is, without any extracellular acidification. LPC is also a strong potentiator of the acid‐induced ASIC3 current, similarly to what has been shown for AA (Deval et al, 2008). The combination of LPC and AA forms a potent endogenous signal that induces firing in nociceptive C‐fibers and triggers pain responses in animals through an ASIC3‐dependent mechanism. These results illustrate the capacity of endogenous lipids to effectively activate ASIC3 in vitro and in vivo, in the absence of any extracellular pH variations to generate pain in rodents and also probably in humans.
Results
Non‐acidic painful human inflammatory exudates activate ASIC3 channels
Several studies have reported a role for ASIC3 in mediating pain in animal models of joint inflammation (Ikeuchi et al, 2008; Izumi et al, 2012; Sugimura et al, 2015). We thus tested the effect of crude human inflammatory exudates, collected from patients with joint effusions, on human ASIC3 channels expressed in HEK293 cells (Fig 1). Interestingly, exudates were not acid and two had a pH close to 7.4 (exudates #2 and #13 with pH values of 7.45 ± 0.02 and 7.49 ± 0.01, respectively, see Fig EV1), although the visual analog scale (VAS) for pain was elevated in patients (8/10 and 9/10 for patients #2 and #13, respectively, see Fig EV1). Extracellular applications of these exudates on cells expressing human ASIC3 induced a slow sustained current, but not in non‐transfected cells nor in cells expressing human ASIC1a (Fig 1A–C). The exudate‐induced current reversed at +59 mV (Fig 1D) and was substantially inhibited by the ASIC3 blocker APETx2 (Diochot et al, 2004) (Fig 1E), confirming the direct activation of human ASIC3 channels in the absence of extracellular acidification. Chelating lipids in these exudates with bovine serum albumin (BSA, see Materials and Methods) significantly decreased their efficiency to activate human ASIC3 current (Fig 1F). Analysis of the total fatty acids (FAs) content of exudates from patients #2 and #13, by transmethylation and gas chromatography experiments (Fig 1G), revealed high levels of palmitic acid (16:0), oleic acid (18:1), stearic acid (18:0), and arachidonic acid (20:4/AA), with concentrations estimated to 441 ± 43, 914 ± 129, 200 ± 27 and 148 ± 42 μM, respectively. As these FAs could be either free or esterified into complex lipids such as phospholipids, we also performed mass spectrometry experiments (Fig 1G, inset) that notably revealed high levels of lysophosphatidylcholine (LPC) in exudates (average concentrations estimated to 113 ± 14, 26 ± 3, 47 ± 6 and 14 ± 1 μM for LPC16:0, LPC18:1, LPC18:0, and LPC20:4, respectively, data from 11 patients, see Fig EV1). High contents of LPC have been similarly reported in synovial fluids from patients with rheumatoid arthritis (Fuchs et al, 2005). Together, these data indicate that painful human inflammatory exudates with a physiological pH close to 7.4 are able to activate a constitutive current generated by human ASIC3 channels and that lipids are involved in this effect.
Figure 1. Non‐acidic human inflammatory exudates activate recombinant human ASIC3 channels.
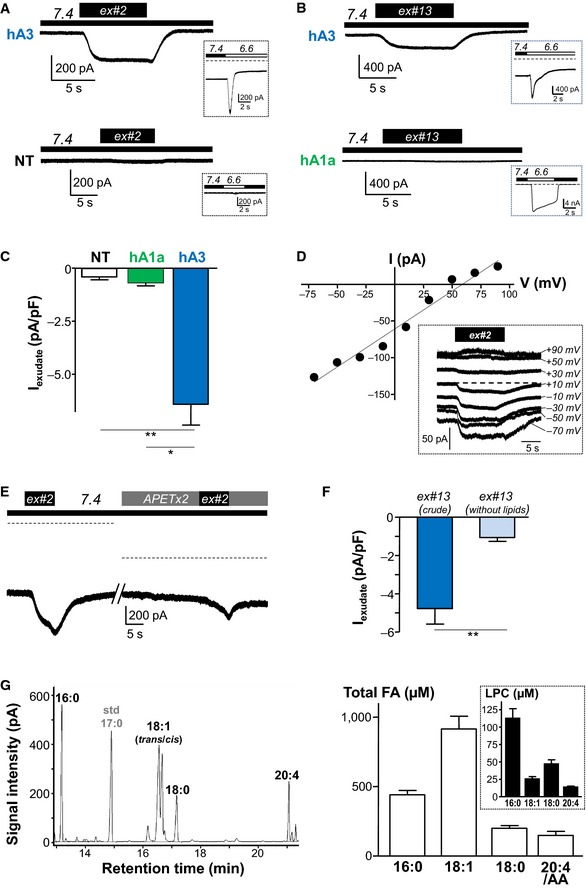
- Whole‐cell recording experiments performed at −80 mV showing that extracellular application of joint exudate from patient #2 (ex#2, pH = 7.45 ± 0.02) activates an inward sustained current in HEK293 cells transfected with human ASIC3 channels (hA3), but not in non‐transfected cells (NT). Functional expression of hA3 is shown by the presence of a typical current in response to extracellular acidification (from pH 7.4 to pH 6.6, insets).
- Effect of exudate from patient #13 (ex#13, pH = 7.49 ± 0.01) on HEK293 cells expressing either human ASIC3 or ASIC1a channels (insets: currents induced by extracellular acidification from pH 7.4 to pH 6.6).
- Histograms showing the mean amplitudes of exudate‐induced currents (ex#2 and ex#13) measured from hASIC3 (n = 9), hASIC1a (n = 5), or non‐transfected cells (n = 5) as illustrated in (A) and (B) (*P < 0.05 and **P < 0.01, Kruskal–Wallis test followed by a Dunn's post hoc test; error bars indicate ± SEM).
- Current–voltage relationship of the exudate‐induced current (ex#2) as shown in (A). The current reverses at +59 mV (inset: current traces recorded at different voltages and at pH 7.4).
- Effect of APETx2 on ex#2‐induced current. The dashed line indicates the zero current level (difference between recordings is due to inhibition by APETx2 of the human ASIC3 alkaline‐activated current already present at pH 7.4 (Delaunay et al, 2012).
- Mean amplitude of the hASIC3 currents induced by either the crude exudate from patient #13 or its delipidated fraction (n = 6 for each condition, **P < 0.01, Mann–Whitney U‐test; error bars indicate ± SEM).
- Left panel: Typical chromatography (GC) analysis of human inflammatory exudate from patient #13 showing the presence of different fatty acids. Right panel: Estimated mean concentrations of palmitic (16:0), oleic (18:1), stearic (18:0), and arachidonic (20:4) acids in exudates from patients #2 and #13 (n = 2 for each fatty acid). Inset shows the estimated mean concentrations of different LPC species (LPC16:0, LPC18:1, LPC18:0, and LPC 20:4) obtained following MS analysis of exudates from 11 patients (n = 11 for each LPC, see also Fig EV1). Error bars indicate ± SEM.
Figure EV1. Detailed analysis of exudates from 11 patients.
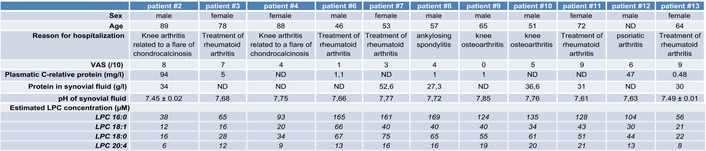
ND, not determined.
Arachidonic acid (AA) and lysophosphatidylcholine (LPC) activate ASIC3 at pH 7.4
We next tested the effects of the different lipids on ASIC3 channels. The fatty acid AA (20:4, ω6) was already known to strongly potentiate the acid‐induced ASIC3 currents (Smith et al, 2007; Deval et al, 2008; Delaunay et al, 2012). We show here that AA (10 μM) was also able to activate a slow sustained ASIC3 current at pH 7.4 (Fig 2A), that is, in the absence of extracellular acidification. In contrast, palmitic acid (16:0 at 10 μM), stearic acid (18:0, 10 μM), or oleic acid (18:1 at 10 μM) failed to activate a similar current when applied at pH 7.4 on cells transfected with human ASIC3 (Fig 2A). Because we also found high levels of LPC in exudates (Fig 1G, inset), we tested the effect of this lysophospholipid on human ASIC3 current at resting pH 7.4. We found that LPC16:0, LPC18:0, or LPC18:1 (10 μM each) had potent activating effects (Fig 2A), and the most efficient activation was obtained with LPC16:0 and LPC18:1. In good agreement with these results, LPC16:0 and LPC18:1 also activated a slow sustained current when applied at pH 7.4 on cells transfected with rat ASIC3, whereas palmitic acid, oleic acid, or phosphatidylcholine containing both palmitic and arachidonic acid (PC 16:0/20:4) had no effect (Fig 2B). Activation of rat ASIC3 by LPC16:0 and LPC18:1 was comparable, while LPC10:0 or LPC18:0 had a much smaller effect (see Fig EV2). We next tested the effect of crude LPC extracted from bovine brain, which mainly contains a mix of LPC16:0, LPC18:0, and LPC18:1 (Fig 2C–E). Crude brain LPC at 10 μM also activated a current at resting pH 7.4 in different cell lines transfected with rat ASIC3, but not in non‐transfected cells (F‐11, HEK293, and CHO cells, Figs 2C, 2D–E, and EV3, respectively). The activation of ASIC3 by LPC was observed for concentrations > 1 μM, with an estimated EC50 of 4.3 μM (Fig 2D). Hydrolysis of membrane phospholipids by PLA2 enzymes leads to the release of different lysophospholipids, including LPC, but also lysophosphatidylethanolamine (LPE), lysophosphatidylserine (LPS), lysophosphatidylinositol (LPI), and lysophosphatidic acid (LPA). LPC was the only lysophospholipid able to significantly activate an ASIC3 current at resting pH 7.4 (Fig 2E). Moreover, the activating effect of LPC was specific for ASIC3 channels since it failed to induce a similar current in cells expressing either ASIC1a, ASIC1b, or ASIC2a channels (Fig 2E, inset). Together, these data indicate that individual application of micromolar (> 1 μM) concentrations of LPC (and most probably also AA), in the extracellular medium and at resting pH 7.4, led to the activation of ASIC3 channels in a slow and sustained manner. The most potent activation was obtained with LPC16:0 and LPC18:1, showing the importance for the effect of the amphipathic properties of LPC molecules and of the length of their lipid chain.
Figure 2. Effect of fatty acids and lysophosphatidylcholine on recombinant ASIC3 channels.
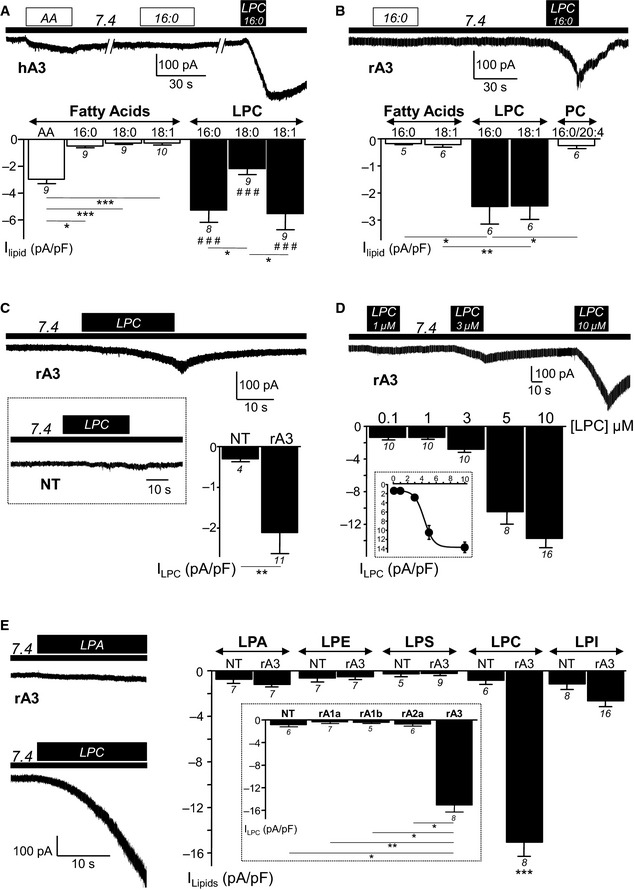
- Extracellular applications of different fatty acids (20:4/AA, 16:0, 18:0, and 18:1) and lysophosphatidylcholine (LPC16:0, LPC18:0, and LPC18:1) at 10 μM and at pH 7.4 on HEK293 cells expressing human ASIC3 channels (hA3). Histograms that represent the statistical analysis of data are shown in bottom panel, and current densities are measured following 30‐s applications of fatty acids or 10‐s applications of LPC (*P < 0.05 and ***P < 0.001, Kruskal–Wallis test followed by a Dunn's post hoc test; ### P < 0.001 as compared to effects of the respective fatty acids).
- Comparison of the effects of palmitic acid (16:0, 10 μM), oleic acid (18:1, 10 μM), LPC16:0 (10 μM), LPC18:1 (10 μM), and phosphatidylcholine (PC) 16:0/20:4 (10 μM) on HEK293 cells expressing rat ASIC3 channels. Current densities are measured following 30‐s applications of fatty acids (16:0, 18:1), or 10‐s applications of LPC, in the extracellular medium at resting pH 7.4. Typical current trace and statistical analysis of data are shown on the top and bottom, respectively (*P < 0.05 and **P < 0.01, Kruskal–Wallis test followed by a Dunn's post hoc test).
- Crude LPC extracted from bovine brain elicits a current on F‐11 cells expressing rat ASIC3 channels but not on non‐transfected cells. Current densities are measured following 30‐s applications of 10 μM LPC at resting pH 7.4 (**P < 0.01, Mann–Whitney U‐test).
- Dose‐dependent activation of rat ASIC3 channels by crude LPC in HEK293 cells. ASIC3 current densities are measured at resting pH 7.4, following extracellular applications (20 s) of different concentrations of LPC. Inset shows the dose–response curve of the activating effect of LPC fitted with a Boltzmann (IC50 estimated at 4.3 μM).
- Effects of different lysophospholipids (10 μM each) applied extracellularly for 20 s at resting pH 7.4 on HEK293 cells expressing or not rat ASIC3 channels (rA3 or NT, respectively). Left panel: Typical current traces recorded from ASIC3‐transfected cells upon application of lysophosphatidic acid (LPA, upper left) and lysophosphatidylcholine (LPC, lower left). Right panel: Bar graph representing the mean current densities measured following application of LPA, lysophosphatidylethanolamine (LPE), lysophosphatidylserine (LPS), LPC, or lysophosphatidylinositol (LPI) (***P < 0.001 as compared to NT, Mann–Whitney U‐test). Inset shows the effect of LPC 10 μM at resting pH 7.4 on cells transfected with either rat ASIC1a, ASIC1b, ASIC2a or ASIC3 channels, or on non‐transfected (NT) cells (*P < 0.05 and **P < 0.01, Kruskal–Wallis test followed by a Dunn's post hoc test).
Figure EV2. Effects of LPC molecules containing different fatty acid chains on ASIC3‐transfected cells at resting pH 7.4.
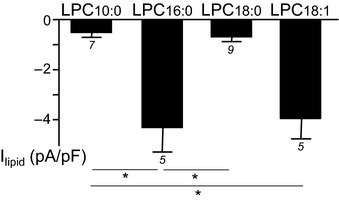
Current densities are measured at −80 mV from F‐11 cells following 30‐s applications of LPC10:0, LPC16:0, LPC18:0, or LPC18:1 at 10 μM (*P < 0.05 Kruskal–Wallis test followed by a Dunn's post hoc test). The number of experiments (n) is indicated below each bar graph, and error bars indicate ± SEM.
Figure EV3. Activating effect of LPC on ASIC3 channels expressed in CHO cells.
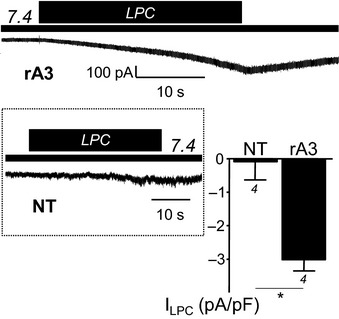
Rat ASIC3 (rA3) currents are generated by extracellular application of LPC (crude extract from bovine brain, 10 μM) at resting pH 7.4. Current densities are measured at −80 mV and are compared to the currents induced in non‐transfected (NT) cells (*P < 0.05, Mann–Whitney U‐test). The number of experiments (n) is indicated below each bar graph, and error bars indicate ± SEM.
LPC also potentiates the acid‐induced ASIC3 current and it acts by shifting the pH dependence of the channel
To further explore the mechanism of action of lipids on ASIC3, we tested the effect of LPC on its acid‐induced current. In addition to the activating effect at resting pH 7.4 (Fig 3A, I basal), LPC (10 μM) also strongly potentiated the pH 7.0‐induced ASIC3 current (Fig 3A). The effect started immediately after its application on the extracellular side of the cells and reached a maximum after 3 min (Fig 3A, bottom panel). Although submicromolar concentrations of LPC did not activate ASIC3 channels at pH 7.4 (Fig 2D), they potentiated the acid‐induced ASIC3 current (Fig 3B, inset). The potentiating effect was reversible upon washout (Fig 3B, inset) and was clearly dependent on LPC extracellular concentration. However, the EC50 was not calculated because it was not possible to reach the maximal effect. The efficacy of different lysophospholipids to modulate the pH 7.0‐induced ASIC3 current was also tested on transfected cells (see Fig EV4). LPC had the strongest potentiating effect with a ~fivefold increase of the pH 7.0‐activated ASIC3 peak current while LPA and LPE had no significant effects. LPS and LPI also displayed a significant but much smaller potentiating effect, with ~1.6‐fold increases for both lysophospholipids. LPC acted on the channel by shifting its pH‐dependent activation curve toward more alkaline pH values, with no effect on inactivation (Fig 3C), leading to an increase of the ASIC3 window current (Fig 3D), similarly to the effect we previously described for AA (Deval et al, 2008). As a result, LPC is not only a strong potentiator of the acid‐induced ASIC3 current, but it also generates a small but constitutive ASIC3 current at resting physiological pH 7.4, that is, in the absence of any extracellular acidification.
Figure 3. LPC activates ASIC3 channels at pH 7.4 and potentiates its acid‐induced activity.
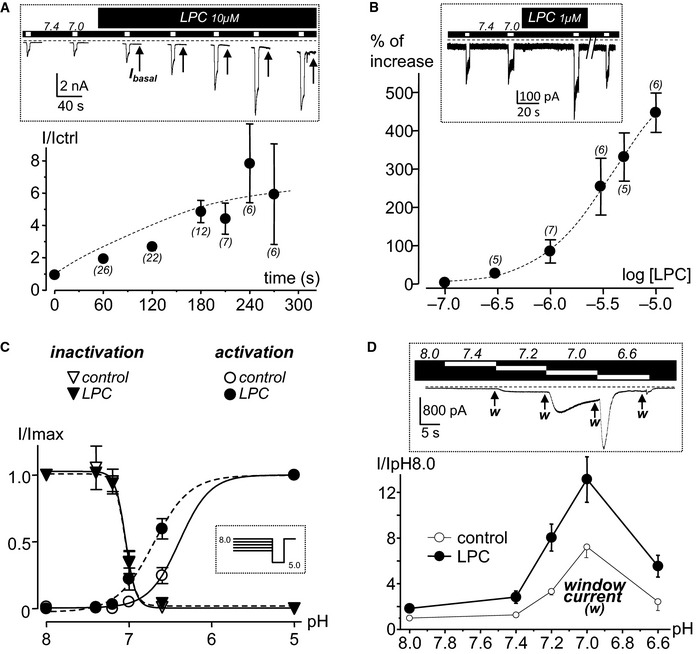
- Effect of LPC (10 μM) on pH 7.0‐induced ASIC3 current represented as a function of time. Inset: typical recording illustrating the potentiating effect of LPC on the pH 7.0‐induced current recorded in F‐11 cells. The curve on the bottom represents current amplitudes measured after LPC application and normalized (I/Ictrl) to those measured in control conditions, that is, before the application of lipid. Note the basal constitutive current induced by LPC at resting pH 7.4 (arrows, Ibasal).
- Dose–response effect of the potentiation by LPC of the pH 7.0‐induced ASIC3 current obtained from HEK293‐transfected cells. The percentage of effect is calculated by comparing current amplitudes before and after application of LPC. Inset: typical potentiating effect of LPC (1 μM) on a pH 7.0‐induced ASIC3 current showing reversibility upon washout. Note that this concentration is not sufficient to induce the basal constitutive current at pH 7.4 shown in (A).
- Effect of LPC (10 μM) on the pH‐dependent activation and inactivation curves of ASIC3 channels expressed in F‐11 cells. Acid‐induced ASIC3 currents are normalized to their maximal peak amplitude measured at pH 5.0, and the protocol used to obtain the curves is shown in the inset (data from three different cells).
- Effect of LPC (10 μM) on the ASIC3 window current measured between pH 8.0 and pH 6.6 from HEK293 cells. Inset: typical current trace obtained in control condition with the arrows (w) indicating the different points at which the window current is measured. Amplitudes of the ASIC3 window current are measured in control condition (without LPC, data from 8 different cells) and after 30‐s applications of LPC (data from 6 different cells). Current amplitudes, measured at different external pH in both control and LPC condition, are normalized to the basal current level measured at pH 8.0 without LPC (bottom graph).
Figure EV4. Effects of different lysophospholipids on acid‐induced ASIC3 current.

Comparison of the effects of LPA, LPE, LPS, LPC, and LPI at 10 μM on the pH 7.0‐induced current recorded from F‐11 cells (each compound is applied for 3 min, *P < 0.05, and **P < 0.01 and ***P < 0.001, Wilcoxon test; ## P < 0.01 and ### P < 0.001, Kruskal–Wallis test followed by a Dunn's post hoc test). The number of experiments (n) is indicated above each bar graph, and error bars indicate ± SEM.
These data demonstrate that LPC is the most efficient lysophospholipid acting on ASIC3 channels. It induces an alkaline shift of the pH‐dependent activation curve, leading to (i) a strong potentiating effect on the acid (pH 7.0)‐induced current and (ii) activation of a constitutive current at physiological pH 7.4.
Interaction between lipids and ASIC3 channels
The effects of LPC on ASIC3 channels could be either direct or indirect, because of lysophospholipid insertion into the external leaflet of the plasma membrane and subsequent non‐symmetrical membrane deformation. We therefore tested the effects of another amphipathic molecule known to modify the cell shape (Sheetz & Singer, 1974), that is, the crenator trinitrophenol (TNP), which preferentially inserts into the external leaflet of the bilayer. As expected, TNP‐induced strong modifications of cell surfaces as shown by experiments of scanning ion conductance microscopy (Fig 4A; left panel, average roughness of membranes of 0.21 ± 0.10 μm vs. 0.12 ± 0.04 μm in control conditions, n = 30 cells, P < 0.01). On the other hand, TNP was not able to activate a significant ASIC3 current at resting pH 7.4 (Fig 4A, right panel). The TNP‐induced membrane modifications observed here were typical “crenations”, with sizes similar to those previously observed by electron microscopy (Sheetz et al, 1976). Conversely, extracellular application of LPC or AA, which actually both activated significant ASIC3 currents at resting pH 7.4 (Fig 4A, right panel), did not induce any membrane deformation (Fig 4A, left panel; average roughness of membranes of 0.10 ± 0.02 μm for LPC and 0.12 ± 0.04 μm for AA as compared to 0.12 ± 0.04 μm in control conditions, n = 30 cells, P > 0.05). Figure 4A (right panel) also shows that the activation of ASIC3 was more important with LPC than with AA. Furthermore, we also show that the activation of ASIC3 by LPC at pH 7.4 occurred in the outside‐out patch‐clamp configuration, when the lipid was applied to the extracellular side of the membrane (Fig 4B, left panel). In contrast, no inward ASIC3 current was recorded in the inside‐out patch‐clamp configuration (Fig 4B, right panel), when the lipid was applied to the intracellular side of the membrane. Smith and colleagues reported a potentiating effect of AA on the acid‐induced ASIC2a current from both sides of the membrane (Smith et al, 2007). Difference between these results and our data could suggest differential mechanisms of action of AA and LPC on ASICs. However, both works cannot be readily compared because (i) the channel subtypes tested in excised patch‐clamp experiments are different (ASIC3 vs. ASIC2a) and (ii) the activating effect of LPC at resting pH 7.4 was not tested in the work of Smith and colleagues. Consistently, we did not observe any activation of rat ASIC2a channel following application at physiological pH 7.4 of LPC (see Fig 2E, inset) or co‐application of LPC and AA (see Fig 5B).
Figure 4. Effect of trinitrophenol (TNP), LPC, and AA on the cell membrane shape and ASIC3 channel activity at pH 7.4.
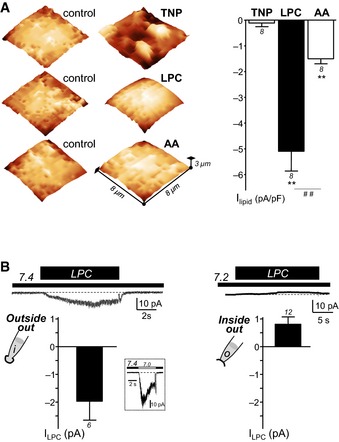
- Representative SICM experiments (left panel) performed on HEK293 cells showing the effects of the crenator trinitrophenol (TNP), of LPC (bovine brain extract) and of AA on the membrane shape (TNP at 5 mM, LPC at 30 μM and AA at 10 μM). SICM images are obtained before (control) and after extracellular application of TNP, LPC, or AA. Whole‐cell recording experiments (right panel), performed at −80 mV, showing the corresponding effect of TNP (5 mM, 30‐s applications), LPC (10 μM, 10‐s applications), and AA (10 μM, 30–s applications) on rat ASIC3 channels at resting pH 7.4 (**P < 0.01, significant current induced at pH 7.4, and ## P < 0.01, Wilcoxon tests).
- Effect of LPC applied either intracellularly or extracellularly in excised patch‐clamp experiments recorded from rat ASIC3‐transfected F‐11 cells. Outside‐out (left panel) and inside‐out (right panel) currents were recorded at −50 mV and +50 mV, respectively. Histograms show the mean amplitudes of the currents induced by LPC (10 μM) applied either at pH 7.4 (extracellular side, outside‐out) or at pH 7.2 (intracellular side, inside‐out). These two different pH values mimic the resting physiological pH of extracellular and intracellular media, respectively. For outside‐out experiments, LPC‐induced current amplitude is measured from patches that also displayed a typical ASIC3 inward current following extracellular acidification from pH 7.4 to pH 7.0 (inset). The dotted lines represent the basal current level.
Figure 5. LPC and AA act together to specifically activate ASIC3 at physiological pH 7.4.
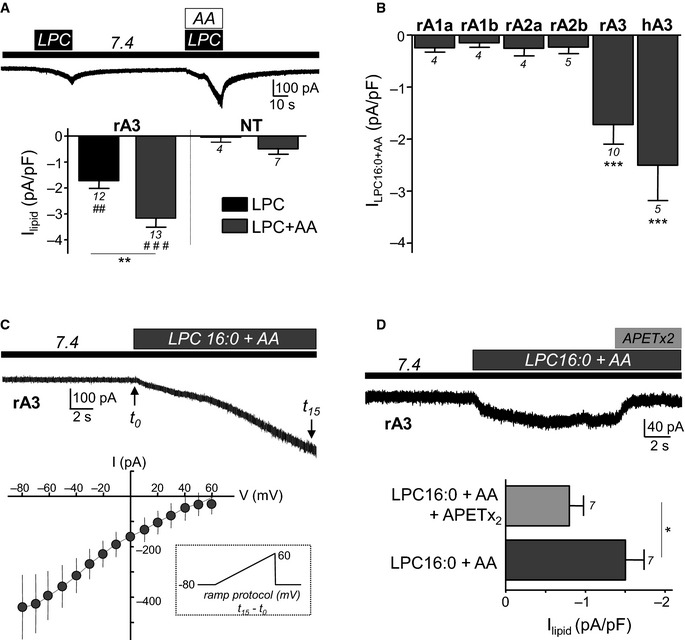
- Whole‐cell recording experiments performed at −80 mV and showing that co‐application of LPC (bovine brain extract) and AA (10 μM each) at pH 7.4 results in a stronger activation of the rat ASIC3 current in transfected F‐11 cells, as compared to the effect of LPC alone (**P < 0.05, Mann–Whitney U‐test; ## P < 0.01 and ### P < 0.001, significantly different from non‐transfected (NT) cells, Mann–Whitney U‐tests).
- Effect of a co‐application of LPC and AA on different ASIC channel subtypes expressed in transfected F‐11 cells. Extracellular application of both lipids during 20 s (LPC16:0 + AA, 10 μM each) at resting pH significantly activates rat and human ASIC3 channels but not rat ASIC1a, ASIC1b, ASIC2a, or ASIC2b channels (***P < 0.001, significantly different as compared to the effect of AA + LPC on non‐transfected cells shown in A, Kruskal–Wallis test followed by a Dunn's post hoc test).
- Current–voltage relationship of the rat ASIC3 current induced by extracellular application of LPC16:0 + AA (10 μM each, data from 9 different cells). Voltage ramp protocols (inset) were imposed to the cells for 10 ms before (t 0) and after (t 15) a 15‐s application of both lipids (see current trace at the top). The lipid‐induced ASIC3 current is obtained as the difference between t 15 and t 0 currents, and the estimated reversal potential is near +60 mV.
- Effect of the ASIC3 inhibitory peptide APETx2 (1 μM) on the current induced by co‐application of LPC16:0 and AA (10 μM each) at pH 7.4 on ASIC3‐transfected cells (*P < 0.05, Wilcoxon test).
Altogether, these results suggest that the effects of LPC and AA on ASIC3 channels are probably not due to an indirect mechanical deformation of the plasma membrane, but rather to a more direct effect of the lipid on the extracellular side of the channels.
The combination of LPC and AA strongly activates ASIC3 channels without any extracellular acidification
Lysophosphatidylcholine and arachidonic acid are co‐released following hydrolysis of cell plasma membrane by PLA2 enzymes. We therefore tested the co‐application of both lipids on the ASIC3 channels activity at pH 7.4 (Fig 5). LPC and AA activated a stronger ASIC3 current than LPC alone (Fig 5A). This effect was specific of ASIC3 channels, since we did not observe any significant current on cells transfected with ASIC1a, ASIC1b, ASIC2a, or ASIC2b subunits following co‐applications of LPC16:0 + AA at pH 7.4 (Fig 5B). A voltage ramp protocol was then used before and during application of lipids to measure the reversal potential of the current activated by LPC16:0 + AA in ASIC3‐transfected cells (Fig 5C). The lipid‐activated current had a reversal potential estimated to be close to +60 mV, indicating that it is mainly carried by Na+ ions, and it was significantly inhibited by the ASIC3‐inhibitory peptide APETx2 (Fig 5D). These data demonstrate that ASIC3 can specifically integrate endogenous lipid signals produced by hydrolysis of the membrane phospholipids in the absence of extracellular pH variations.
LPC and AA constitute a lipid signal that activates native ASIC3 channels and produces pain in rodents
To test the relevance of the activation of ASIC3 by lipids at resting pH 7.4 in a context of native ASIC3 channels, LPC and AA were co‐applied at pH 7.4 to rat dorsal root ganglia (DRG) neurons. Co‐application of both lipids induced a constitutive current, which was largely prevented or inhibited by the non‐selective ASIC blocker amiloride (Fig 6A). The native lipid‐induced current was observed in 11 out of 25 neurons (44%, see Fig 6B, inset), corresponding to small and medium diameter neurons (8 and 3 cells, respectively) that all displayed an acid (pH 6.6)‐induced ASIC current (ASIC+ neurons). Lipid‐induced current was also sensitive to the ASIC3 inhibitory peptide APETx2 (Fig 6B, lower panel). Similarly to what we observed on primary cultures of DRG neurons, nerve‐skin experiments showed that co‐application of lipids (LPC16:0 + AA) to the peripheral endings of rat C‐fibers provoked a significant increase of action potential (AP) firing, which was prevented when lipids were co‐applied together with amiloride (Fig 6C). Furthermore, the in vivo injections of LPC16:0 + AA to the dorsal face of rat hindpaws produced a significant pain behavior (flinches) as compared to injections of vehicle (Fig 6D). The lipid‐induced pain was significantly reduced when the pharmacological inhibitors APETx2 or amiloride were co‐injected together with LPC16:0 and AA in rats, strongly suggesting an involvement of ASIC3‐containing channels in the observed effect. Finally, injections of LPC16:0 + AA into mouse hindpaws induced a pain behavior in wild‐type animals that was significantly reduced in ASIC3 knockout mice (Fig 6E), further supporting the specificity of the effects with regard to ASIC3 channels.
Figure 6. LPC and AA activate native ASIC channels in DRG neurons and increase C‐fibers firing and pain behavior through an ASIC3‐dependent mechanism.
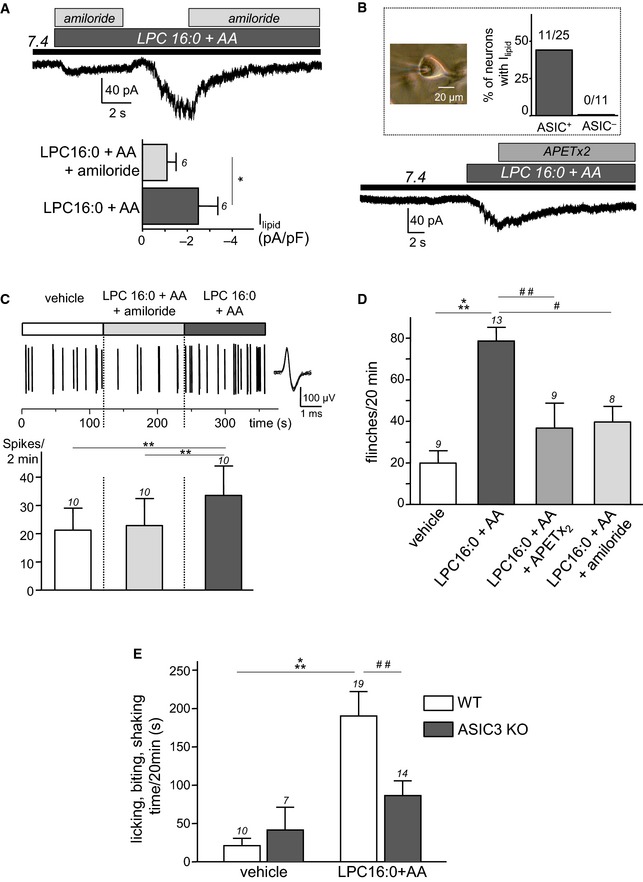
- Effect of amiloride (1 mM) on native whole‐cell currents recorded at −80 mV and induced by the co‐application of LPC and AA (10 μM each) at pH 7.4 on rat DRG neurons (*P < 0.05, Wilcoxon test).
- Typical inhibitory effect of APETx2 (1 μM) on the lipid‐induced (LPC16:0 + AA) current recorded at pH 7.4 from rat DRG neurons. Inset: percentage of DRG neurons (picture, scale bar = 20 μm) responding to lipids and that displayed (ASIC+) or do not displayed (ASIC−) a pH 6.6‐induced ASIC current.
- Nerve‐skin experiments showing the effect of lipids (LPC16:0 + AA, 48 nmole each), applied for 2 min onto the receptive fields of C‐fibers in the skin, either with amiloride (1 mM) or alone. Top trace shows a typical recording of C‐fiber firing with an enlarged action potential (AP) on the right, while the average spike frequency is represented at the bottom. Lipids provoke an increase of AP firing, which is blocked when co‐applied with amiloride (**P < 0.01 for LPC16:0 + AA vs. vehicle and LPC16:0 + AA vs. LPC16:0 + AA + amiloride, Friedman test followed by a Dunn's post hoc test).
- Pain behavioral experiments showing the number of rat flinches induced by a hindpaw injection of LPC16:0 + AA (2.4 nmole each) or vehicle (0.24% ethanol). Lipids are injected either alone or in the presence of APETx2 (0.2 nmole) or amiloride (20 nmole) (# P < 0.05, ## P < 0.01 and ***P < 0.001, Kruskal–Wallis test followed by Dunn's post hoc tests).
- Lipid‐induced pain in wild‐type (WT) and ASIC3 knockout mice (ASIC3 KO). Lipids (LPC16:0 + AA, 4.8 nmole each) or vehicles (0.96% ethanol) are injected into a hindpaw, and the pain behavior is represented as the time spent by mice licking, biting, and shaking their injected paw for 20 min (## P < 0.01 and ***P < 0.001, two‐way ANOVA tests followed by Bonferroni's post hoc tests).
Combination of these in vitro, ex vivo, and in vivo results demonstrate that native ASIC3 currents in DRG neurons are also activated by lipids at physiological pH 7.4, increasing the firing rate of C‐fibers and producing pain in rodents.
Discussion
ASIC channels are gated by extracellular protons and are considered as pH sensors. However, in addition to protons, several endogenous modulators of these channels have also been described. For instance, FMRFamide‐related peptides have been reported to directly interact with ASICs to potentiate their activity (Askwith et al, 2000; Deval et al, 2003). ATP, lactic acid, arachidonic acid, agmatine, serotonin, and hypertonicity also enhance the proton‐induced ASIC3 current (Immke & McCleskey, 2001; Smith et al, 2007; Deval et al, 2008; Birdsong et al, 2010; Li et al, 2010; Yu et al, 2010; Wang et al, 2013), supporting the idea that this channel behaves as a coincidence detector able to sense protons and several other endogenous stimuli. In addition, ASIC3 has been shown to be activated at physiological pH 7.4 by the synthetic compound GMQ (2‐guanidine‐4‐methylquinazoline), and this effect involves a site in the channel different from the one identified for the effect of protons (Yu et al, 2010). This non‐proton binding site has also been proposed to participate in the effect of the endogenous arginine metabolite agmatine (Li et al, 2010), and it is required for the paradoxical stimulation of ASIC3 by amiloride (Li et al, 2011). Finally, a toxin isolated from the Texas coral snake's venom, MitTx, is able to robustly activate ASIC1 channels (Bohlen et al, 2011), independently of protons. These data suggest the attractive possibility that endogenous stimuli can physiologically activate ASIC channels in the absence of any extracellular acidification.
Activation of ASIC channels by endogenous compounds in the absence of extracellular pH variations is further supported by our observation that non‐acidic exudates from patients with painful joint effusions can activate recombinant ASIC3 channels in vitro. The activating effect is largely supported by lipids, which are present at high levels in the human exudates. We further identify lysophosphatidylcholine and arachidonic acid as activators of ASIC3 at resting pH 7.4. Extracellular acidification is therefore not the unique signal able to generate an ASIC activity, and lipids can also induce a slowly activating sustained ASIC3 current. Individually, LPC and AA act on the channel with similar kinetics, both shifting their pH dependence for activation toward more alkaline values. This effect leads to a strong potentiation of the pH‐activated ASIC3 current (this work, but see also Deval et al, 2008) and, importantly, to a constitutive depolarizing ASIC3 current at resting physiological pH 7.4. LPC has a more potent activating effect than AA, most probably because of a stronger shift of the channel pH dependence. When applied together, LPC and AA have a cumulative effect on ASIC3 channels. As the two lipids are released simultaneously following hydrolysis of membrane phospholipids by PLA2 (Murakami et al, 2011), this finding supports the idea that they represent an endogenous lipid signal able to trigger constitutive ASIC3 activity in the absence of extracellular pH variations. Such activation by lipids significantly extends the contribution of ASIC3 in pain sensing. At resting pH 7.4 and in the presence of lipids, ASIC3 can indeed behave as a background depolarizing channel, which sensitizes neurons toward other stimuli and/or could trigger long‐lasting pain. On the other hand, lipids also strengthen the sensitivity of ASIC3 channels to extracellular pH acidifications, an effect that can greatly contribute to pain enhancement in conditions of tissue acidosis.
The constitutive ASIC3 current is activated by micromolar concentrations of AA and LPC (> 1 μM), which correlate well with the levels of lipids (> 20 μM) found in human exudates in the present study. LPC is particularly interesting because it has the strongest effect on ASIC3, which has never been described before, and because its role in pain remains poorly documented. Indeed, although LPC is known to indirectly induce peripheral neuropathy and pain through nerve demyelination (Wallace et al, 2003; Inoue et al, 2008), our data also support a more direct contribution to pain through an effect on the activity of ASIC3 channels. Moreover, high level of LPC (> 300 μM) has been reported in human plasma (Wiesner et al, 2009), and it is considered as a lipid second messenger involved in the pathogenesis of inflammatory diseases. For instance, both the plasma and the synovial fluids of patients with rheumatoid arthritis are enriched with LPC‐containing saturated fatty acid (Fuchs et al, 2005), in good agreement with our data showing a concentration of LPC16:0 greater than 50 μM in exudate of patient with a similar pathology. Effects of LPC on other pain‐related channels have also been reported in vitro, on the thermal sensitivity of the TRPM8 cold/menthol receptor (Andersson et al, 2007), and on the potassium channel TREK‐1 (Maingret et al, 2000). Interestingly, the tissue level of LPC rapidly increases following myocardial ischemia (Sedlis et al, 1993; Daleau, 1999), a pathological condition for which ASIC3 channels have been proposed to be the sensor of cardiac pain (Immke & McCleskey, 2001; Sutherland et al, 2001).
Lysophosphatidylcholine is the most effective lysophospholipid acting on ASIC3 (with a much lower effect of LPI and LPS, and no effect of LPA), showing the importance of the choline head, and suggesting some specificity in the effect. The fatty acid chain of LPC is also important since the activation of ASIC3 mainly occurs when the lysophospholipid contains palmitic acid (16:0) or oleic acid (18:1), which are the principal fatty acids that compose phosphatidylcholine in normal human brain (Svennerholm, 1968). On the other hand, the fatty acids 16:0, 18:0, or 18:1 are not able to activate ASIC3 channels, demonstrating that the amphipathic structure of LPC is necessary to produce the activation. Due to their abilities to insert into lipid bilayers, amphipathic molecules such as lysophospholipids or AA can be considered as compounds inducing membrane curvature changes according to the “bilayer couple theory” (Sheetz & Singer, 1974) and/or to their cone shapes (Pascher et al, 1992; Lundbaek & Andersen, 1994). The activating effects of LPA, LPC, and AA on the mechano‐sensitive TREK/TRAAK channels have been suggested to be the consequence of such membrane deformations (Maingret et al, 2000; Chemin et al, 2005; Brohawn et al, 2014). In the case of ASIC3, the activating mechanism appears to be different since trinitrophenol, which crenates cell membranes, failed to reproduce the effects of LPC or AA. Moreover, although ASIC3 has been involved in mechano‐sensory processes (Price et al, 2001; Chen et al, 2002; Page et al, 2005; Fromy et al, 2012), the channel does not appear to be directly gated by mechanical stimulation (Drew et al, 2004). Even if it is difficult to definitely rule out whether the effect of LPC or AA on ASIC3 is an indirect consequence of changes in the plasma membrane physicochemical properties, we favor more direct activating mechanisms associated with accumulation of the two lipids onto, or in the close vicinity of the channel. However, LPC can also act via G‐protein‐coupled receptors (GPCRs, for review, see Meyer zu Heringdorf & Jakobs, 2007), and we cannot completely exclude a possible indirect activation of ASIC3 via such membrane receptors.
In conclusion, the present work reveals that LPC and AA constitutively activate ASIC3 channels expressed in peripheral sensory neurons in the absence of pH variations, leading to pain under resting pH conditions. This activation may have broader consequences than only pain, especially in humans where ASIC3 is more largely expressed than in rodents (Delaunay et al, 2012). The discovery of such endogenous lipid activators of ASIC3 at physiological pH opens new perspectives on the role of ASIC channels in both physiological and pathophysiological processes that are not necessarily associated with extracellular pH variations.
Materials and Methods
F‐11, CHO, and HEK293 cell cultures and transfection
F‐11, CHO, and HEK293 cell lines were grown as described previously (Ettaiche et al, 2006). One day after plating, cells were transfected with pIRES2‐rASIC1a‐EGFP, pIRES2‐rASIC1b‐EGFP, pIRES2‐rASIC2a‐EGFP, pIRES2‐rASIC2b‐EGFP, pIRES2‐rASIC3‐EGFP, or pIRES2‐hASIC3a‐EGFP vectors using the JetPEI reagent according to the supplier's protocol (Polyplus transfection SA, Illkirch, France). Fluorescent cells were used for patch‐clamp recordings 2–4 days after transfection.
Primary cultures of DRG neurons
Lumbar DRG neurons were dissected from adult male Wistar rats, enzymatically dissociated and maintained in primary culture as already described (Deval et al, 2008). The diameter of DRG neurons recorded in patch‐clamp ranged between 12 and 32 μm, with an average of 24 ± 1 μm. Based on a classification where small, medium and large neurons have diameters ≤ 25 μm, between 25 and 40 μm, and larger than 40 μm, respectively (Scroggs & Fox, 1992; Scholz et al, 1998; Molliver et al, 2005), we recorded 61% (22/36) of small neurons, 33% (12/36) of medium neurons and 6% (2/36) of large neurons.
Patch‐clamp experiments
We used whole‐cell and excised configurations of the patch‐clamp technique to measure membrane currents (voltage clamp). Recordings were made at room temperature using an axopatch 200B amplifier (Axon Instruments) with a 2 kHz low‐pass filter. Data were sampled at 10 kHz, digitized by a Digidata 1440 A‐D/D‐A converter (Axon Instruments) and recorded on a hard disk using pClamp software (version 10; Axon Instruments). For whole‐cell and outside‐out experiments, the patch pipettes (2–8 MΩ) contained (in mM) the following: 135 KCl, 2.5 Na2‐ATP, 2 MgCl2, 2.1 CaCl2, 5 EGTA, and 10 HEPES (pH 7.25 with KOH). The control bath solution contained (in mM) the following: 145 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH 7.4 with N‐methyl‐D‐glucamine or NaOH). For inside‐out experiments, the pipette and bath solutions were reversed. MES was used instead of HEPES to buffer solution pH ranging from 6.0 to 5.0, and ASIC currents were induced by shifting one out of eight outlets of the microperfusion system from a holding control solution (i.e., pH 7.4 or pH 8.0) to an acidic test solution.
SICM experiments
Scanning ion conductance microscopy (SICM) is a technique in which the ion current flowing into a nanopipette is used to control the vertical (z‐axis) position of the pipette tip relative to the cell surface. Basic arrangement of the SICM for topographical imaging of living cells has previously been described (Korchev et al, 1997). To scan sample, nanopipette probe was driven in the x‐ and y‐directions by a piezo stage mounted on 25‐mm translation stage DC motors. Information on the lateral and vertical positions was recorded and used to generate a three‐dimensional (3D) topographic image (ScanIC Image software, Ionscope). In this study, we used the developed hopping mode of SICM (Novak et al, 2009). The present instrument is based on an inverted optical microscope (Olympus IX70). Ionscope Ltd provided SICM imaging software for DSP card. Nanopipettes were made from 1.00 mm outer diameter, 0.58 mm inner diameter glass microcapillaries (Intrafil) on a laser‐based Brown–Flaming puller (model P‐2000, Sutter Instrument Compagny). The measured nanopipette resistance was usually 80 MΩ. Experiments were performed in HEPES control bath solution for extracellular medium and for intrapipette solution. SICM images were made on HEK293 cells before and after 30‐min incubations with TNP (5 mM), LPC (30 μM), or AA (10 μM). The membrane roughness of cells, which is an arithmetical mean deviation of the surface, was measured from the different SICM images using Gwyddion software.
Nerve‐skin preparation and single fiber recordings
The isolated skin‐saphenous nerve preparation and single C‐fiber recording technique were used as described previously (Alloui et al, 2006). The skin of the hindpaw of 8‐ to 14‐week‐old male rat was dissected with the saphenous nerve. The skin was superfused with warm (~30°C) synthetic interstitial fluid (SIF), in mM : 120 NaCl, 3.48 KCl, 5 NaHCO3, 1.67 NaH2PO4, 2 CaCl2, 0.69 MgSO4, 9.64 Na‐gluconate, 5.5 glucose, 7.6 sucrose, and 10 HEPES, pH adjusted to 7.4 with NaOH, saturated with O2/CO2—95%/5%. The receptive field of an identified C‐fiber was searched by mechanical probing of the skin and further characterized for mechano‐sensitivity with calibrated von Frey filaments. This protocol implies that all C‐fibers were mechano‐sensitive. Conduction velocity was < 1.3 m/s. C‐fibers' receptive fields were isolated with a thick‐walled elrin ring inside which solutions were applied (internal volume of 400 μl) through local perfusion pipes of a CL‐100 bipolar temperature controller (Warner instrument). Recordings were band‐pass filtered between 60 Hz and 2 kHz and sampled at 10 kHz on computer with pClamp 9 software (Axon Instrument). Action potential were analyzed with Spike2 software (Cambridge Electronic Design). Spikes were discriminated off‐line and visualized individually.
Pain behavior experiments in rodents
Experiments were performed on adult male Wistar rats (Charles River) and on adult male C57Black6J wild‐type mice (Charles River) and ASIC3 knockout mice (Wultsch et al, 2008). The protocol was approved by the local ethical committee and the French government (agreement # 02233.01) and was in agreement with the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983). Animals were kept with a 12‐h light/dark cycle with ad libitum access to food and water and were acclimated to housing and husbandry conditions for at least 1 week before experiments. For nociceptive behavior experiments, rats or mice were placed in a transparent observation chamber where they were acclimated for at least 15 min. They were then gently restrained while saline solution (0.9% NaCl, 20 μl for rat and 10 μl for mouse) containing either vehicle (0.24% or 0.96% ethanol for rat and mouse, respectively), lipids (LPC and AA, 2.4 or 4.8 nmole each for rats and mice, respectively), lipids + APETx2 (0.2 nmole), or lipids + amiloride (20 nmole) was administered subcutaneously into the dorsal face of the hindpaw using a 26‐gauge needle connected to a 100‐ml Hamilton syringe. The amount of lipids injected into the rat hindpaws corresponds to the amount applied to a cell during a 30‐s super‐fusion of 10 μM LPC or AA in patch‐clamp experiments. Nociceptive behaviors (i.e., number of flinches of the injected paw for rats or time spent licking, biting, or shaking their injected paw for mice) were counted over a 20‐min period starting immediately after the injection.
Chemicals
APETx2 was synthesized by Synprosis/Provepep (France), and amiloride was purchased from Sigma (Saint Quentitn Fallavier, France). Lipids were purchase from Sigma and/or Avanti (Coger, France). All lipids were prepared as stock solutions in either saline, DMSO, or EtOH, stored at −20°C, and diluted to the final concentration into saline solutions just before the experiments. LPA was obtained from chicken egg; LPE and LPS were obtained from porcine brain; LPC and LPI were obtained from bovine brain and liver, respectively.
Human inflammatory exudates
Human samples of inflammatory exudates were collected from patients with painful acute knee‐joint effusions requiring joint aspiration for diagnosis or treatment. All patients were recruited from the Rheumatology Department of Nice University Hospital (CHU‐Nice, France) and provided informed consent before inclusion. The study was approved by the Nice University Institutional Review Board for Research on Human Subjects and has been conducted in accordance with the French national regulations regarding patient consent and ethical review. The study was registered in the ClinicalTrials.gov protocol registration system (NCT 01867840), and it includes patients with rheumatoid arthritis, psoriatic arthritis, osteoarthritis, ankylosing spondylitis, and chondrocalcinosis. After biological analysis for patients care, the remaining exudates were aliquoted, stored at −80°C, and further used for in vitro electrophysiological experiments on human ASIC3 channels. For experiments in which lipids were chelated, 1 ml aliquots of exudates were supplemented with BSA powder (2 μM final, 99% free fatty acids, Sigma), vortexed, and centrifugated 10 min at 18,000 g and 4°C.
Lipid extraction
Lipid extraction has been adapted from the Folch method (Folch et al, 1957). Fifty microliters of exudates was diluted in 950 μl of water and transferred into glass tubes with PTFE caps (Pyrex Labware) containing 500 μl of glass beads (Ø 0.3–0.4 mm, Sigma‐Aldrich). Mixtures were supplemented with 4 ml of 2/1 (v/v) chloroform (CHCl3)/methanol (CH3OH) solution and lipid standard (LPC17:0 or LPC13:0), and lipid extractions were carried out on an orbital shaker (IKA® VX® basic Vibrax®, Sigma‐Aldrich) at 1,500 rpm for 2 h at room temperature. From the resulting emulsions, aqueous phases were dissociated from the lipid‐containing organic phases with a swing‐out centrifuge for 5 min at 410 g. Aqueous phases were discarded, and organic ones were supplemented with 1 ml of 4/1 (v/v) 2N KCl/CH3OH solution. Samples were shaked for 10 min, and organic and aqueous phases were separated as described before. The resulting organic phases were complemented with 1 ml of 3/48/47 (v/v/v) CHCl3/CH3OH/H2O solution, and an additional shaking step was carried out. As previously described, the resulting organic phases were isolated and, then, transferred into new glass tubes. Those mixtures were supplemented with 1 ml of 3/48/47 (v/v/v) CHCl3/CH3OH/H2O solution and shaked for 10 min. The final organic phases containing the whole lipids were isolated and solvent was evaporated at 80°C under a nitrogen beam (1 bar) before storage at −80°C.
Phospholipids purification and mass spectrometry analysis
Dry samples of total lipids were resuspended in 1 ml dichloromethane, by shaking for 30 s on a vortex. These solutions were loaded onto silica columns (BOND ELUT‐SI, 100 mg 1 ml, Agilent Technologies) previously conditioned (cleared with 3 ml methanol and 2 ml dichloromethane successively). The bounded fractions were cleaned with 2 ml dichloromethane (for non‐polar lipids elution) and 3 ml acetone (for glycolipids elution) successively. Finally, 2 ml of 50/45/5 (v/v/v) CHCl3/CH3OH/H2O solution was loaded onto the columns and the eluted fractions containing the phospholipids were collected into glass tubes. Solvent was evaporated at 80°C under a nitrogen beam (1 bar), and samples were stored at −80°C prior to analysis. Dry purified phospholipids were resuspended in 100 μl of 2/1/1 (v/v/v) isopropanol/acetonitrile/water + 1% (v/v) formic acid, and the molecular species were analyzed by electrospray ionization–mass spectrometry (ESI–MS), in positive ion mode. Resulting spectrograms were notably used to analyze the acyl content of different phospholipid species by MS–MS.
Fatty acids transmethylation and gas chromatography analysis
Dry samples of total lipids were resuspended in 200 μl of cyclohexane, by shaking for 30 s on a vortex. Samples were supplemented with 2.5 ml of 2% (v/v) sulfuric acid (H2SO4)/CH3OH, and transmethylations of fatty acids (free or esterified in species such as triglycerides, sterylesters, phospholipids or lysophospholipids) were carried out at 80°C for 1.5 h (volumes were regularly adjusted with 2% (v/v) H2SO4/CH3OH). Mixtures were supplemented with 1.5 ml of water and 1.5 ml of cyclohexane, shaked on a vortex for 30 s, and then centrifuged at 410 g for 30 s. The resulting cyclo‐hexane phases containing the methylesters of fatty acids were collected into separated new glass tubes. An additional extraction step was carried out with 1.5 ml of cyclohexane as previously described, and the resulting cyclohexane phases were pooled with the formers. Solvent was evaporated at 80°C under a nitrogen beam (0.5 bar), and samples were stored at −80°C prior to analysis. Dry methylesters of fatty acids were resuspended in 100 μl of cyclohexane, and 2 μl of cyclohexane was injected for gas chromatography (GC) analysis on a DB5 column (Agilent Technologies, France). Program of column elution was as follows: 50°C held for 2 min, temperature increased by 30°C/min until 230°C and held for 12 min, and finally, temperature increased by 30°C/min until 300°C and held for 15 min. Retention time's areas under peaks of lipid standards were used to identify and quantify lipids from exudates.
Statistical analysis
Data analysis was performed using Microcal Origin 8.5 and GraphPad Prism 4.03 softwares. Data are presented as mean ± s.e.m. and statistical differences between sets of data were assessed using either parametric or nonparametric tests followed by post hoc tests, when appropriate.
Author contributions
SM, AD, and ED did electrophysiological and behavioral experiments and analyzed the data. VB, CR, and LE‐Z performed the clinical aspects of the study. MC and JN did the nerve‐skin experiments and analyzed the data. VF provided technical support. SS and CC did the SICM experiments and analyzed the data. RF‐C and TF did the lipid extractions and further analyses. ED and EL wrote the manuscript with input from all authors and supervised the project.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgements
We thank G. Lambeau, G. Drin, B. Antonny, H. Barelli, D. Debayle, A. Baron, S. Diochot, M. Salinas, M. Chafai, and T. Besson for helpful discussions, M. Lazdunski for his support, and C. Chevance for secretarial assistance. The authors thank the imaging plateform “ImageUP” (Université de Poitiers) for providing the SICM technical tools and F.J. Richard (Université de Poitiers) for providing GC technical tools. We thank the Fondation pour la Recherche Medicale (DEQ20110421309) and the Agence Nationale de la Recherche (ANR‐13‐BSV4‐0009) for financial support.
The EMBO Journal (2016) 35: 414–428
References
- Allen NJ, Attwell D (2002) Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischaemia‐related signals. J Physiol 543(Pt 2): 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat‐Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M (2006) TREK‐1, a K+ channel involved in polymodal pain perception. EMBO J 25: 2368–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Nash M, Bevan S (2007) Modulation of the cold‐activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci 27: 3347–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ (2000) Neuropeptide FF and FMRFamide potentiate acid‐evoked currents from sensory neurons and proton‐gated DEG/ENaC channels. Neuron 26: 133–141 [DOI] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh‐Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW (2010) Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68: 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Chesler AT, Sharif‐Naeini R, Medzihradszky KF, Zhou S, King D, Sanchez EE, Burlingame AL, Basbaum AI, Julius D (2011) A heteromeric Texas coral snake toxin targets acid‐sensing ion channels to produce pain. Nature 479: 410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Su Z, MacKinnon R (2014) Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci USA 111: 3614–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA (2007) Modulation of acid‐sensing ion channel activity by nitric oxide. J Neurosci 27: 13251–13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Patel A, Duprat F, Zanzouri M, Lazdunski M, Honore E (2005) Lysophosphatidic acid‐operated K+ channels. J Biol Chem 280: 4415–4421 [DOI] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ (2002) A role for ASIC3 in the modulation of high‐intensity pain stimuli. Proc Natl Acad Sci USA 99: 8992–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daleau P (1999) Lysophosphatidylcholine, a metabolite which accumulates early in myocardium during ischemia, reduces gap junctional coupling in cardiac cells. J Mol Cell Cardiol 31: 1391–1401 [DOI] [PubMed] [Google Scholar]
- Delaunay A, Gasull X, Salinas M, Noel J, Friend V, Lingueglia E, Deval E (2012) Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc Natl Acad Sci USA 109: 13124–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Baron A, Lingueglia E, Mazarguil H, Zajac JM, Lazdunski M (2003) Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology 44: 662–671 [DOI] [PubMed] [Google Scholar]
- Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E (2008) ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 27: 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, Lingueglia E (2010) Acid‐sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther 128: 549–558 [DOI] [PubMed] [Google Scholar]
- Deval E, Noel J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E (2011) Acid‐sensing ion channels in postoperative pain. J Neurosci 31: 6059–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M (2004) A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid‐sensitive channel in sensory neurons. EMBO J 23: 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Salinas M, Douguet D, Scarzello S, Dabert‐Gay AS, Debayle D, Friend V, Alloui A, Lazdunski M, Lingueglia E (2012) Black mamba venom peptides target acid‐sensing ion channels to abolish pain. Nature 490: 552–555 [DOI] [PubMed] [Google Scholar]
- Diochot S, Alloui A, Rodrigues P, Dauvois M, Friend V, Aissouni Y, Eschalier A, Lingueglia E, Baron A (2015) Analgesic effects of mambalgin peptide inhibitors of acid‐sensing ion channels in inflammatory and neuropathic pain. Pain doi:10.1097/j.pain.0000000000000397 [DOI] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN (2004) Acid‐sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556(Pt 3): 691–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N (2006) Silencing acid‐sensing ion channel 1a alters cone‐mediated retinal function. J Neurosci 26: 5800–5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509 [PubMed] [Google Scholar]
- Fromy B, Lingueglia E, Sigaudo‐Roussel D, Saumet JL, Lazdunski M (2012) Asic3 is a neuronal mechanosensor for pressure‐induced vasodilation that protects against pressure ulcers. Nat Med 18: 1205–1207 [DOI] [PubMed] [Google Scholar]
- Fuchs B, Schiller J, Wagner U, Hantzschel H, Arnold K (2005) The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31P NMR and MALDI‐TOF MS. Clin Biochem 38: 925–933 [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA (2008) Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain 137: 662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW (2001) Lactate enhances the acid‐sensing Na+ channel on ischemia‐sensing neurons. Nat Neurosci 4: 869–870 [DOI] [PubMed] [Google Scholar]
- Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H (2008) Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience 152: 296–298 [DOI] [PubMed] [Google Scholar]
- Izumi M, Ikeuchi M, Ji Q, Tani T (2012) Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci 19: 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO (2010) Reversal of acid‐induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol 161: 950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchev YE, Bashford CL, Milovanovic M, Vodyanoy I, Lab MJ (1997) Scanning ion conductance microscopy of living cells. Biophys J 73: 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WG, Yu Y, Zhang ZD, Cao H, Xu TL (2010) ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain 6: 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WG, Yu Y, Huang C, Cao H, Xu TL (2011) Nonproton ligand sensing domain is required for paradoxical stimulation of acid‐sensing ion channel 3 (ASIC3) channels by amiloride. J Biol Chem 286: 42635–42646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek JA, Andersen OS (1994) Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol 104: 645–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E (2000) Lysophospholipids open the two‐pore domain mechano‐gated K(+) channels TREK‐1 and TRAAK. J Biol Chem 275: 10128–10133 [DOI] [PubMed] [Google Scholar]
- Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M (2007) A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci 10: 943–945 [DOI] [PubMed] [Google Scholar]
- Meyer zu Heringdorf D, Jakobs KH (2007) Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta 1768: 923–940 [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW (2005) ASIC3, an acid‐sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K (2011) Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog Lipid Res 50: 152–192 [DOI] [PubMed] [Google Scholar]
- Noël J, Salinas M, Baron A, Diochot S, Deval E, Lingueglia E (2010) Current perspectives on acid‐sensing ion channels: new advances and therapeutic implications. Expert Rev Clin Pharmacol 3: 331–346. [DOI] [PubMed] [Google Scholar]
- Novak P, Li C, Shevchuk AI, Stepanyan R, Caldwell M, Hughes S, Smart TG, Gorelik J, Ostanin VP, Lab MJ, Moss GW, Frolenkov GI, Klenerman D, Korchev YE (2009) Nanoscale live‐cell imaging using hopping probe ion conductance microscopy. Nat Methods 6: 279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA (2005) Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54: 1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascher I, Lundmark M, Nyholm PG, Sundell S (1992) Crystal structures of membrane lipids. Biochim Biophys Acta 1113: 339–373 [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Scholz A, Appel N, Vogel W (1998) Two types of TTX‐resistant and one TTX‐sensitive Na+ channel in rat dorsal root ganglion neurons and their blockade by halothane. Eur J Neurosci 10: 2547–2556 [PubMed] [Google Scholar]
- Scroggs RS, Fox AP (1992) Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol 445: 639–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlis SP, Hom M, Sequeira JM, Esposito R (1993) Lysophosphatidylcholine accumulation in ischemic human myocardium. J Lab Clin Med 121: 111–117 [PubMed] [Google Scholar]
- Sheetz MP, Singer SJ (1974) Biological membranes as bilayer couples. A molecular mechanism of drug‐erythrocyte interactions. Proc Natl Acad Sci USA 71: 4457–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP, Painter RG, Singer SJ (1976) Biological membranes as bilayer couples. III. Compensatory shape changes induced in membranes. J Cell Biol 70: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Cadiou H, McNaughton PA (2007) Arachidonic acid potentiates acid‐sensing ion channels in rat sensory neurons by a direct action. Neuroscience 145: 686–698 [DOI] [PubMed] [Google Scholar]
- Sugimura N, Ikeuchi M, Izumi M, Kawano T, Aso K, Kato T, Ushida T, Yokoyama M, Tani T (2015) Repeated intra‐articular injections of acidic saline produce long‐lasting joint pain and widespread hyperalgesia. Eur J Pain 19: 629‐638 [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW (2001) Acid‐sensing ion channel 3 matches the acid‐gated current in cardiac ischemia‐sensing neurons. Proc Natl Acad Sci USA 98: 711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L (1968) Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res 9: 570–579 [PubMed] [Google Scholar]
- Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA (2011) Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain 152: 2348–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997) A proton‐gated cation channel involved in acid‐sensing. Nature 386: 173–177 [DOI] [PubMed] [Google Scholar]
- Wallace VC, Cottrell DF, Brophy PJ, Fleetwood‐Walker SM (2003) Focal lysolecithin‐induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci 23: 3221–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li WG, Yu Y, Xiao X, Cheng J, Zeng WZ, Peng Z, Xi Zhu M, Xu TL (2013) Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci 33: 4265–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ (2013) Acid‐sensing ion channels in pain and disease. Nat Rev Neurosci 14: 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G (2009) Lipid profiling of FPLC‐separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res 50: 574–585 [DOI] [PubMed] [Google Scholar]
- Wultsch T, Painsipp E, Shahbazian A, Mitrovic M, Edelsbrunner M, Lazdunski M, Waldmann R, Holzer P (2008) Deletion of the acid‐sensing ion channel ASIC3 prevents gastritis‐induced acid hyperresponsiveness of the stomach‐brainstem axis. Pain 134: 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL (2010) A nonproton ligand sensor in the acid‐sensing ion channel. Neuron 68: 61–72 [DOI] [PubMed] [Google Scholar]
- Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File


