Summary
Notwithstanding different meningococcal serogroups have changed their distribution and their impact in different age classes over time, N. meningitidis' invasive diseases are a major public health issue worldwide, due to the related complications and severe sequelae. Nowadays, the highest rates of invasive disease are registered in children younger than 1 year of age, with a second lesser peak in adolescents and young adults (15-25 years of age). On the contrary, the prevalence of carriage is low in newborns and in school-age children, and increases during adolescence and young-adult age; then it decreases again in older age. N. meningitidis' infection prevalence has greatly decreased in Europe and North America thanks to the use of conjugate vaccines (MenC and MenACWY) as well as the incidence of invasive disease due to serogroup A in sub-saharian Africa after the introduction of MenAfriVac conjugate vaccine.
The great success of conjugate vaccines is related not only to the direct protection from disease but also to the impact on carriage; this latter allows an indirect protection of unimmunized subjects. For these reasons, the implementation of immunization with the new generation vaccines in the age classes most impacted by disease and carriage (first year of life, adolescence and young adulthood) could permit to achieve an extraordinary decrease of the incidence of meningococcal disease.
Key words: Neisseria meningitidis, Age classes, Carriage
Introduction
Neisseria meningitidis (N. meningitidis) is an aerobic, Gram-negative diplococcus, exclusively hosted by man; it usually lives as a temporal commensal in the upper respiratory tract without causing any disease. Reasons for the transition from asymptomatic carriage to invasive disease have still not completely understood; anyway, some factors, such as genetic and capsular structure of pathogenic strains, are believed to play a relevant role [1, 2]. N. meningitidis is classified in 12 serogroups (accordingly to capsular polysaccharide structure) and in serotypes and sub-serotypes (accordingly to outer membrane proteins). The role played by each most epidemiological relevant serogroup (A, B, C, W-135, and Y) greatly changes in relation to time period and geographical area considered; anyway, notwithstanding the ample underestimate of its global epidemiological impact, meningococcus is a relevant public health issue worldwide [3, 4].
N. meningitidis is transmitted through respiratory droplets of infected subjects or, more often, of asymptomatic carriers. Usually humoral immune response is enough to prevent the spreading of the pathogen and the occurrence of invasive disease. Anyway, if the humoral response is not adequate, bacteriaemia occurs due to not yet completely understood mechanisms [5]. Once in the bloodstream, meningococci circumvent immunological response by several virulence factors (capsule, IgA protease, surface "blebs" containing LPS, that act as an endotoxin). Endotoxin induces a cascade of pro-inflammatory citokines with a subsequent endotelial damage, increase of vascular permeability, protrombotic condition with subsequent development of microthrombosis. Meningococcal disease is a quite rare event and meningitis is its most common feature (about 50% of cases) [6], followed by bacteriaemia (40% of cases). Fulminant disease occurs in 10-20% of cases and it is characterized by organ failure and disseminated intravascular clotting (e.g. Waterhouse-Friderichsen syndrome); in these cases, mortality could be equal to 50% [7]. Lethality of meningococcal infections could reach 10%, while permanent sequaelae occur in up to 20% of survivors. Permanent sequelae involve neurological damage, psychological disturbances, hearing loss, visual loss, cutaneous scarring and/or limb amputations [8].
Pathogenesis and Epidemiology
The global incidence of meningococcal disease greatly changes in relation to considered geographical areas; worldwide, 500,000-1,200,000 invasive meningococcal diseases occur each year, with 50,000-135,000 deaths [9,10].
Nowadays, in Europe, North America and Australia incidence ranges between 0.3 and 3 cases per 100,000 inhabitants [11], while the same could reach 10-1,000 cases/100,000 in Africa during epidemics (in particular in the so-called sub-saharian "meningitis belt").
The epidemiology of meningococcal infections has significantly changed over the years in many regions of the world. Serogroup A has been the principal agent of invasive meningococcal disease in Europe before and during I and II World Wars, serogroup B has been prevalent since 1970 in Europe and since 1980 in South America; epidemic outbreaks due to W-135 and Y serogroups have emerged more recently during the XXIst century. Besides, a change in the age classes affected by invasive disease has occurred, with an increase of incidence of serogroup Y in elderly and a decrease of serogroup C in adolescents. The epidemiological trend of invasive disease has almost remained unchanged in Africa, where serogroup A is most prevalent; very recently, serogroups X and W-135 have had a relevant impact in terms of morbidity and mortality [12].
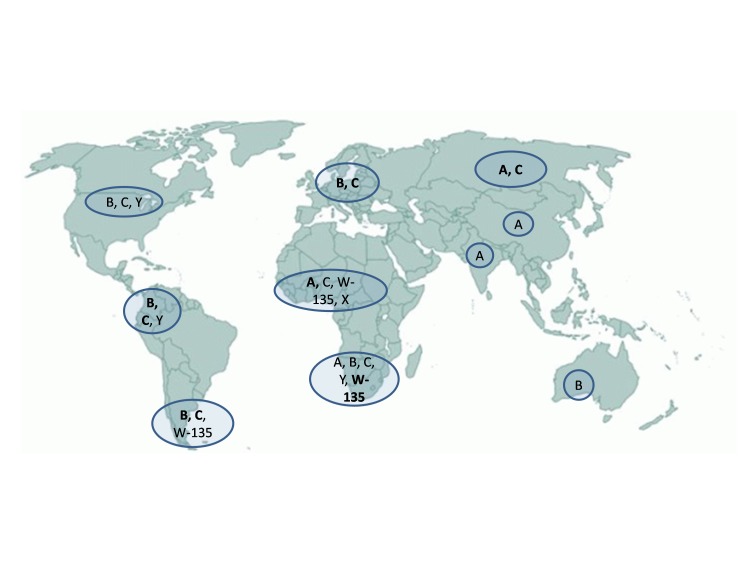
Disease caused by serogroup A in Africa has an annual incidence equal to 10-20 cases per 100,000 inhabitants; epidemic outbreaks, occurring during dry season, imply an attack rate greater than 1,000 cases per 100,000. Data from Latin America and Asia are limited. In Latin America, incidence ranges between 0.1/100,000 in Mexico to 2 cases/100,000 in Brasil, with a predominance of serogroups B and C [13]. In Asia, the epidemiological burden of meningococcal disease is not well defined. Serogroup A has been considered prevalent; anyway, all five serogroups (A, B, C, Y and W-135) have been reported, even if with a regional variation [14]. In Australia, meningococcal incidence is greater than 3 case/100,000. In most American and European countries a low level of endemicity is registered. In 2011 [15], 29 European countries (27 UE countries plus Norway and Iceland) have reported 3,808 confirmed cases of invasive disease; the global notification rate has been equal to 0.77/100,000 (range 0.09-1.99), serogroup B has been the most relevant (73.6% of cases), followed by serogroup C (14.4%) and Y (8.2%). Invasive disease incidence sustained by serogroup B in Europe accounted for 0.5 cases per 100,000 inhabitants. Italy reports the lowest incidence rate, equal to 0.25 case per 100,000 [16]. Figure 1 shows the distribution of main N. meningitidis serogroups in different geographical areas.
Fig. 1.
Meningococcal serogroups in the world (reference 9, modified).
Age classes and categories at risk of developing meningococcal disease
There is an ample consensus that the categories at highest risk of developing meningococcal disease are newborns and children in the first year of life (as natural immunity against N. meningitids is particularly low), adolescents (due to their habits and behaviours that facilitate strict interpersonal contacts; besides, they have the highest carriage rate), travellers that stay for long time in endemic areas (Sub-saharian Africa, ect.), immunosuppressed subjects (functional or anatomic asplenia, thalassemia, sickle cell anemia, persistent complement deficiencies, organ transplant, cancer or high dosage corticosteroid therapy, diabetes, HIV infection, congenital immunodeficiencies), and elder subjects [17, 11].
The highest rate of disease is registered in children younger than 1 year of age, with a second lower peak in adolescents and young adults (15-25 years of age) [18].
Since 2008, European incidence rate has decreased form 0.95/100,000 to 0.68/100,000; higher rates have been registered in Lituania and UK (1.77 and 1.36, respectively). Newborns, 1-4year-old children and adolescents (15-25 years of age) are the most affected subjects in all countries, irrespective of ongoing or not immunization programs against MenC.
The most relevant rate of invasive disease, in particular in children younger than 5 years of age, is related to serogroup B, followed by serogroup C. In 2012, the notification rate of MenB infection in children <1 year of age has been three-fold higher than the one registered in the age group 1-4 years (12.3 and 4.1 per 100,000, respectively). The highest rate of MenC cases has been registered among young adults and adults (25-44 years of age); serogroup Y has been mostly identified in >65 year-old subjects [19].
In Italy, Azzari e co-workers have confirmed, in a study conducted in the period 2006-2012 [20], that as in other European countries, meningococcal disease caused by serogroup B has a greater incidence during the first 5 years of life and that 70% of cases are registered during the first year of age, with a peak between 4 and 8 months.
As far as immunization with MenC conjugated vaccine has been implemented in Italian regions, the incidence of the disease related to this serogroup has progressively decreased. Nowadays, serogroup B is the most common serogroup causing invasive meningococcal disease, being involved in more than 80% of cases in patients <24 years of age. Figure 2 shows the distribution of MenB invasive disease in Italy in the period 2007-2012, and its peak of incidence in the first year of life [16].
Fig. 2.
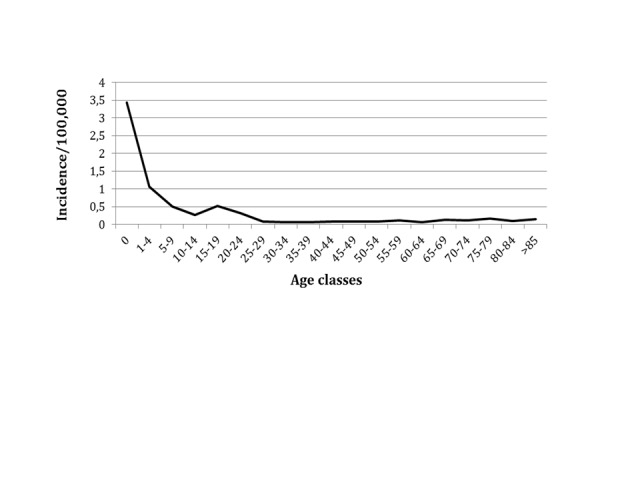
Estimated incidence of invasive MenB disease in Italy, 2007-2012 (data from reference 16).
In USA, the rate of invasive meningococcal disease has been equal to 0.14/100,000 in 2013; incidence mainly involved children younger than 5 years and subjects aged 18-35 years (in the two groups the incidence was almost equal: 1.7/100,000). Serogroup B impacted mostly in <1year-old babies (0.68/100,000), while serogroup C showed an higher incidence in the age class 1-4 years (0.41/100,000) [21].
Carriage
N. meningitidis is a human infective agent usually residing in the nasopharynx. Human upper respiratory tract is a stable ecological niche; anyway, meningococci can be habitual components of the microbial flora in buccal mucosa, anus, urethra, urogenital mucosa, and dental plaque. Carriage at pharingeal level involves 8-25% of subjects; this means hundreds of millions people in the world, adolescents being the most relevant group [22].
The relationship between asymptomatic carriage and development of invasive disease in not completely known, nor the timeframe necessary for the transition from one status to the other. Concerning this point, humoral immunity certainly plays a crucial role. In most cases the microorganism persists in the nasopharynx for days or weeks, and even months. Carriage is crucial not only in the transmission dynamics but even in the onset of invasive disease. As a matter of fact, the lack of bactericidal antibodies is a relevant risk factor for the transition to invasive disease. The repeated occurrence of carrier status, not only of N. meningitidis but also of N. lactamica, even not protective against subsequent new carriage, can elicit a cross-protection against invasive disease [23].
Strains in carriers are genetically and antigenically different from the ones isolated in subjects affected by invasive disease. Age is one of the most relevant factor related to meningococcal carriage.
Carrier status has been studied and even more in depth understood during last years, in particular after the implementation of immunization with MenC conjugate vaccine and the achievement of relevant results in terms of epidemiological and immunological impact. Differently from natural infection, carriage has a low prevalence in the first years of life and in older age classes, and reaches its peak in adolescents and young adults. In the "meningitis belt" meningococci are, in respect to other geographical areas, more uniformly distributed irrespective from age [24].
Besides, more than age, other factors influence carriage such as male gender, concomitant viral or bacterial respiratory infections, active and passive smoke, low socioeconomic status. One of the most relevant risk factor is the number and the mixing pattern of social interactions; seasonality does not seem relevant [25].
Both in Europe and in North America, the highest rate of nasopharingeal carriage is registered in adolescents and in young adults; they act as the most relevant source of infection. Carriage prevalence increases from 4.5% in infancy to a peak of 23.7% in the age class 19-20 years, and then decreases to about 10% in adults [26, 27]. These data have been recently confirmed in a study performed in Italy evaluating the molecular and serological diversity of N. meningitidis carrier strains isolated from students aged 14 to 22 years [28].
Vaccines and herd immunity
Knowledge on the epidemiology of N. meningitidis, the role of carriers and the invasive disease, has allowed to better understand both the relevance and the impact of immunization. There is an ample consensus on the point that immunization is the best and most efficacious preventive approach against meningococcal disease.
Since '70-'80s polysaccharide vaccines against serogroups A, C, Y and W-135 have been available. Later, conjugate vaccines has been developed; these vaccines, differently from polysaccharide ones, elicit a T-dependent immune response with the production of high affinity antibodies, immune memory and responsivity to subsequent doses. Conjugate vaccines are efficacious also in newborns, have an impact on carriage and induce herd immunity.
For all these reasons, the availability of conjugate vaccines and the implementation of immunization programs has allowed to achieve a great impact on the epidemiology of meningococcal disease. Since 2005, the evaluation of the results obtained after the immunization program adopted in UK has demonstrated that MenC conjugate vaccine directly protects immunized subjects, decreases carriage and blocks the spreading of the agent [24]. All these effects have amplified the impact of vaccination against serogroup C even in age classes not directly involved in the immunization program [29]. Another study performed in Africa in 2012 has showed a persistent decrease of carriage following the use of a MenA conjugate vaccine (MenAfriVac); carriage prevalence decreased from 0.39% in the pre-immunization period to 0,02% two years after the implementation of vaccination [30].
In addition to conjugate vaccines against serogroups A, C, Y and W-135, since January 2013 a new 4-components MenB vaccine (4cMenB) has been licensed in Europe. Read and co-workers in UK have recently evaluated the impact on carriage after immunization with Men ACWYCRM conjugate (1 dose) and 4CMenB (2 doses; time interval between doses: 1 month) vaccines in university students aged 18-24 years [31]. The impact on carriage of both vaccines has been evaluated 1 month and during the 12 months following immunization. Concerning 4cMenB, since the 3rd month after administration a significant decrease of carriage of any meningococcal strain (18.2%), capsular groups BCWY (26.6%), capsular groups and serogroups CWY (29.6% and 28.5%, respectively) has been registered. A significant decrease of carriage rate has been registered also in subjects immunized with MenACWYCRM compared to controls; the decrease was equal to 39% and 36.2% for serogroup Y and CWY, respectively. This study confirms that 4cMenB vaccine could impact on carriage not only for meningococcus B but also for other serogroups, as it does not contain capsular antigens but proteins shared with other nonB serogroups. Anyway, even if this study shows a first evidence of the impact on carriage of 4cMenB vaccine (as well as of MenACWYCRM vaccine), its results should be considered with caution; the impact on carriage at individual level cannot be considered predictive of herd immunity. As a matter of fact, several other factors play a relevant role in the determinism of herd immunity, not only the ability of the vaccine to block or decrease the acquisition of the carriage status.
Conclusions
Notwithstanding the results achieved in the fight against disease caused by N. meningitidis, this etiological agent continues to be a relevant worldwide treat for health. Knowledge about age classes at highest risk and about relationship between nasopharingeal carriage and disease is fundamental in order to understand epidemiology and pathogenesis of meningococcal disease and to identify adequate immunization strategies.
Newborns and children <1year of age are at highest risk for the disease as their immune system is not completely developed and the maternal passive immunity tends to progressively fade out. The highest prevalence rate of carriage is registered in adolescents and young adults; in these age groups the efficacy of conjugate vaccines against carriage is equal to 75% [32].
The incidence of meningococcal disease has decreased during the last decade thanks to the immunization programs with conjugate vaccines against serogroups A, C, Y, W-135. More recently the new 4CMenB vaccine has been introduced with the aim to decrease the incidence of the disease sustained by serogroup B.
All these vaccines are safe, well tolerated and highly efficacious against the most relevant invasive serogroups; they elicit a long-lasting immune response in all age groups and induce herd immunity.
For all these reasons, the implementation of immunization programs against meningococcal disease should be a public health priority.
References
- 1.Vernikos G, Medini D. Bexsero® chronicle. Pathog Glob Health. 2014;108:305–316. doi: 10.1179/2047773214Y.0000000162. doi: 10.1179/2047773214Y.0000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasparini R, Amicizia D, Lai PL, et al. Neisseria meningitidis: pathogenetic mechanisms to overcome the human immune defences. J Prev Med Hyg. 2012;53:50–55. [PubMed] [Google Scholar]
- 3.Halperin SA, Bettinger JA, Greenwood BG, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30:B26–B36. doi: 10.1016/j.vaccine.2011.12.032. doi: 10.1016/j.vaccine.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) , author. Meningococcal vaccines: WHO position paper. November 2011. Wkly Epidemiol Rec. 2011;86:521–539. [PubMed] [Google Scholar]
- 5.Stephens DS, Hoffman LH, McGee ZA. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J Infect Dis. 1983;148:369–376. doi: 10.1093/infdis/148.3.369. [DOI] [PubMed] [Google Scholar]
- 6.Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20. doi: 10.1007/978-1-61779-346-2_1. doi: 10.1007/978-1-61779-346-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Agati VC, Marangoni BA. The Waterhouse-Friderichsen Syndrome. N Engl J Med. 1945;232:1–7. [Google Scholar]
- 8.Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30:B3–B9. doi: 10.1016/j.vaccine.2011.12.062. doi: 10.1016/j.vaccine. 2011.12.062. [DOI] [PubMed] [Google Scholar]
- 9.Jafri RZ, Ali A, Messonnier NE, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11:17–17. doi: 10.1186/1478-7954-11-17. doi: 10.1186/1478-7954-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Q, Tzeng YL, Stephens DS. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 2012;4:237–245. doi: 10.2147/CLEP.S28410. doi: 10.2147/CLEP.S28410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwilow R, Fanella S. Invasive Meningococcal Disease in the 21st Century-An Update for the Clinician 2015. Curr Neurol Neurosci Rep. 2015;15:2–2. doi: 10.1007/s11910-015-0524-6. doi: 10.1007/s11910-015-0524-6. [DOI] [PubMed] [Google Scholar]
- 12.Abio A, Neal KR, Beck CR. An epidemiological review of changes in meningococcal biology during the last 100 years. Pathog Glob Health. 2013;107:373–380. doi: 10.1179/2047773213Y.0000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Tawfiq JA, Clark TA, Memish ZA. Meningococcal disease: the organism, clinical presentation, and worldwide epidemiology. J Travel Med. 2010;17:3–8. doi: 10.1111/j.1708-8305.2010.00448.x. doi: 10.1111/j.1708- 8305.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- 14.Bermal N, Huang LM, Dubey AP, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Human Vaccines. 2011;7:239–247. doi: 10.4161/hv.7.2.14068. doi: 10.4161/hv.7.2.14068. [DOI] [PubMed] [Google Scholar]
- 15. European Centre for Disease Prevention and Control (ECDC). Surveillance of invasive bacterial diseases in Europe. ECDC 2011 [Accessed 2014 May 28]; URL: http://www.ecdc.europa.eu/en/publications/Publications/invasive-bacterial-diseasessurveillance-2011.pdf.
- 16. Istituto Superiore di Sanità (ISS). Gruppo di Lavoro del centro nazionale di epidemiologia, sorveglianza e promozione della salute (CNEPS) Dati e evidenze disponibili per l'introduzione della vaccinazione antimeningococco B nei nuovi nati e negli adolescenti. June 2014. Available at: http://www.epicentro.iss.it/temi/vaccinazioni/pdf/Istruttoria%20MENINGOCOCCO%20B.Pdf.
- 17.Panatto D, Amicizia D, Lai PL, et al. New versus old meningococcal group B vaccines: how the new ones may benefit infants & toddlers. Indian J Med Res. 2013;138:835–846. [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis Lisa A, Sanjay Ram. Meningococcal disease and the complement system. Virulence. 2014;5:98–126. doi: 10.4161/viru.26515. doi: 10.4161/ viru.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Centre for Disease Prevention and Control (ECDC) , author. Annual epidemiological report 2014 – Vaccine-preventable diseases – invasive bacterial diseases.
- 20.Azzari C, Canessa C, Lippi F, et al. Distribution of invasive meningococcal B disease in Italian pediatric population: Implications for vaccination timing. Vaccine. 2014;32:1187–1191. doi: 10.1016/j.vaccine.2013.09.055. doi: 10.1016/j.vaccine.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention (CDC) , author. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Neisseria meningitidis, 2011. 2012. Available via the Internet: http://www.cdc.gov/abcs/reports-findings/survreports/mening11.pdf.
- 22.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitides. Vaccine. 2009;27:B71–B77. doi: 10.1016/j.vaccine.2009.04.070. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzzetta G, Manfredi P, Gasparini R, et al. On the relationship between meningococcal transmission dynamics and disease: remarks on humoral immunity. Vaccine. 2009;27:3429–3434. doi: 10.1016/j.vaccine.2009.01.092. doi: 10.1016/j.vaccine.2009.01.092. [DOI] [PubMed] [Google Scholar]
- 24.Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8:851–861. doi: 10.1586/erv.09.48. doi: 10.1586/erv.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caugant DA, Maiden MC. Meningococcal carriage and disease- Population biology and evolution. Vaccine. 2009;27:B64–B70. doi: 10.1016/j.vaccine.2009.04.061. doi: 10.1016/j.vaccine.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–861. doi: 10.1016/S1473-3099(10)70251-6. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 27.Maiden MC, Ibarz-Pavòn AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–743. doi: 10.1086/527401. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasparini R, Comanducci M, Amicizia D, et al. Molecular and serological diversity of Neisseria meningitidis carrier strains isolated from Italian students aged 14 to 22 years. JCM. 2015;52:1901–1910. doi: 10.1128/JCM.03584-13. doi: 10.1128/JCM.03584-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitides. Vaccine. 2009;27:B71–B77. doi: 10.1016/j.vaccine.2009.04.070. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristiansen PA, Ba AK, Ouédraogo AS, et al. Persistent low carriage of serogroup A Neisseria meningitidis two years after mass vaccination with the meningococcal conjugate vaccine, MenAfriVac. BMC Infect Dis. 2014;14:663–663. doi: 10.1186/s12879-014-0663-4. doi: 10.1186/ s12879-014-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–2131. doi: 10.1016/S0140-6736(14)60842-4. doi: 10.1016/S0140-6736(14)60842-4. [DOI] [PubMed] [Google Scholar]
- 32.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nature reviews. Immunology. 2009;9:213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]