Academics have a public duty to use their research to promote improvements in patient care and health. Here, we argue that there is an imperative to translate recent compelling evidence demonstrating the importance of the periconceptional period in determining the health of future generations, into improvements in pregnancy‐related care and perinatal health. Taking this action has the potential to interrupt cycles of deprivation and to reduce inequalities in health. These are among the biggest challenges in health care today.
It has been known for three decades that maternal exposures influence fetal growth and development by programming of the health of the newborn.1 These changes persist into later life, and also affect health in the next generation, one example being the ongoing effects of undernutrition in those born or conceived during the Dutch Hunger Winter.
It has become increasingly apparent that maternal characteristics not only affect fetal growth but also gametogenesis and embryonic development with lasting impact on health at birth and during childhood.2, 3 Periconceptional paternal influences on sex‐specific fetal growth and long‐term health of the offspring are also beginning to be seen.4 The periconceptional period is therefore one of the most critical periods in the life course, initiating epigenetic programming determining perinatal health and well‐being for generations to come.
Perinatal health outcomes differ widely between countries, but even within high‐income countries large differences in perinatal health outcomes exist. Perinatal mortality – as tip of the iceberg of perinatal morbidity – is an indicator of perinatal health. In cities like Rotterdam in the Netherlands, perinatal mortality in neighbourhoods ranges between 2 and 34 per 1000 births.5 Between 2006 and 2013 in Southampton in the UK, perinatal mortality across electoral wards ranged from 4‰ to 13‰,6 and an eightfold difference is observed in infant mortality rates across municipalities of Massachusetts, USA.7 Disparities in perinatal health outcomes are known to be related not only to differences in obstetric and medical risk factors but also to lifestyle, education, working conditions, experience of violence, geography and socio‐economic status of couples.8
Effects of poverty and deprivation on perinatal health are substantial and are seen across all immigrant and native European and US communities. Even after adjustment for determinants such as socio‐economic status, age, parity, race and ethnicity there remain increased risks associated with living in deprived neighbourhoods for perinatal mortality (20%), preterm delivery (16%) and fetal growth restriction (11%).9 Risk accumulation involving decreased literacy, lack of access to social facilities, health care, and support as well as exposure to urban environmental stressors including crime, noise, physical insecurity, inadequate housing, air pollution, and unemployment may also play a role.
These factors provide a compelling case for the provision of new, comprehensive pregnancy‐related care.2, 10 As long ago as 1963, the WHO was calling for attention to those aspects of personal and community life, which have an impact on reproductive, perinatal and child health. This shift requires general practitioners, obstetricians and community midwifes to include routine assessment of non‐medical risks such as those related to poverty as part of the process of risk analysis already carried out at the booking. To maximise benefits, there should be an equivalent mechanism for conducting such a risk assessment at some point prior to conception for couples. Analogous with the opportunity map of societal investment in health created by Fielding and Teutsch,11 an implementation map for preconception care should be drawn, in which health at conception is defined as a combination of an individual's biology with exposure to social and environmental exposures known to be determinants of health. In recognition of the importance of preconception care, the Centers for Disease Control and Prevention (CDC) offers preconception guidance, which emphasises the role of the male partner (http://www.cdc.gov/preconception).
Comprehensive, combined preconception and antenatal care requires coordination and delivery of services to address both medical and non‐medical issues particularly in at‐risk populations. Antenatal care pathways should be implemented in ways that provide support for this complex of inter‐related issues, including content geared towards nutritional and lifestyle improvements. Such a holistic approach to antenatal care will involve reorganisation and coordination of social and medical services so that they are coterminous across neighbourhoods and communities, allowing for care to be provided in an integrated chain combining the expertise not only of community midwifes and obstetricians but also of public health, social, and youth workers (Figure 1).
Figure 1.
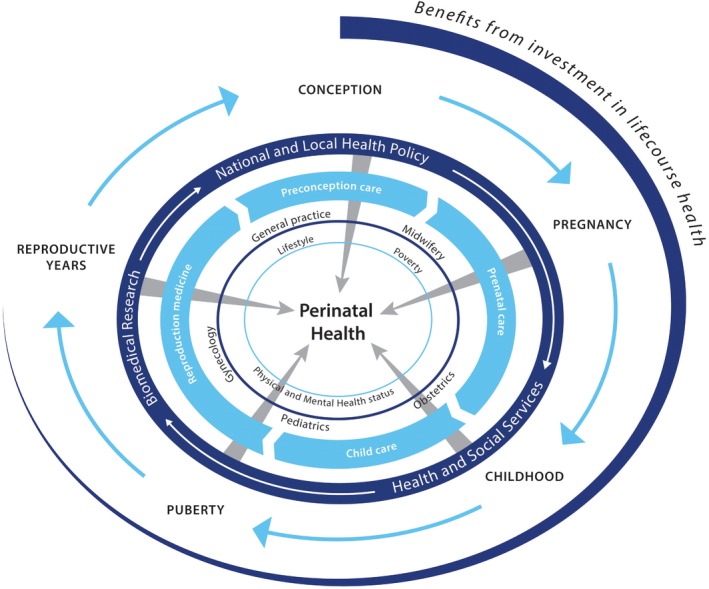
Holistic approach to pregnancy‐related care crossing medical and social domains.
One of the great challenges in generating evidence‐based public health is in translating interventions of proven effectiveness into health care practice. Failing to do so, however, may cost lives. The complex nature of population‐level interventions may make translation from evidence to practice more difficult than in some other areas of medicine, and the realities of health and social care systems have in the past led to the failure to implement effective interventions in community settings. The challenge for academic perinatal health researchers is to find ways to communicate the importance of preconception and antenatal health care to the general population and to specific communities. In the past, universities have been seen as ivory towers but medical faculties increasingly feel a responsibility for the health of the general population and have invested substantial capital in supporting major national initiatives to leverage existing academic health centre infrastructure through clinical and translational science awards and through strategic support for interdisciplinary programmes including the Life‐Course Research Network, designed to accelerate the translation of the life course theory to Maternal and Child Health (MCH) practice and policy to improve MCH outcomes.
Local and national governmental bodies can also direct valorisation processes by determining the content and by subsidising them. In the Netherlands, the municipality of Rotterdam finances the local programme ‘Ready for a Baby’ and the Ministry of Health, Welfare, and Sport funded the national programme ‘Healthy Pregnancy for All’ in 14 other Dutch cities.12 Analysis of public health data by academics had illuminated large differences in perinatal health between neighbourhoods in the cities involved. Sharing this new information with policy makers in a number of different ways, using city maps showing the distribution of perinatal health outcomes, was sufficient to convince them that action was needed. Essential components of these Dutch programmes are enhanced pre‐conceptional and inter‐conceptional care, careful risk assessment at pregnancy booking also addressing non‐medical risks tailored to the individual – and early involvement of youth care during pregnancy in the case of vulnerable families.
The US national ‘Healthy Start Program’ was designed to eliminate disparities in infant mortality and other adverse birth outcomes through the implementation of required programme components within the context of the community. Programme components included outreach, case management, inter‐conceptional care, local health system action plan, and sustainability planning. Collectively, the interventions were intended to help improve access to care and birth outcomes by enhancing health literacy, promoting healthy behaviours and mobilising the community to improve perinatal health by ensuring the delivery of social and medical services to support pregnant and inter‐conceptional women and their infants.13 In the Omaha Healthy Start Program, early analyses of the social and economic impact of community‐based prenatal care designed to reduce perinatal health disparities documented a 31% cost savings in average hospital expenditure for participants, as compared with non‐participants.14 Care targeted at vulnerable populations has produced clear, long‐term benefit. For example, the Special Supplemental Nutrition Program for Women, Infants and Children (WIC), which provides disadvantaged families with regular supplies of food from the food groups essential for physical and cognitive development, has produced demonstrable improvements in maternal and child nutrition quality, and the physical and cognitive development of children.15
Transfer of knowledge is essential, not only from within the university to outside, but also within and across fields of science, curative care, and public health and between different societal organisations, multiple stakeholders, and governmental bodies. In doing so, there should be mutual respect of differences in vision, strategies, and approaches to how challenges are addressed. Genuine partnership and communication are essential if health improvements are going to result from increases in scientific knowledge. This has not been a strength in academic medicine but gives impetus to the importance of the emerging area of implementation science. In the US, programmes as the annual CityMatCH Maternal and Child Health Urban Leadership Conference serve as a platform for promoting leadership activities, workforce development, and dissemination of innovations in epidemiologic, policy and health services research to stakeholders in scientific and non‐scientific arenas.
Supporting the most vulnerable families in society is one of the most challenging tasks facing health care systems but also one with the greatest possible impact. Women in these families also have high rates of unplanned and undesired pregnancies, helping to perpetuate the negative cycle of events associated with disparities in economic and health outcomes. We conclude that there is convincing evidence that improving perinatal health can reduce inequalities in health, and to make this happen societal valorisation programmes should be initiated and supported by both universities and governmental bodies.
Acknowledgements
This work was made possible by the department of Obstetrics and Gynecology of the Erasmus Medical Center, Erasmus University Rotterdam, the Netherlands, the MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom, and the Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, USA. All authors declare that they have no conflicts of interest.
References
- 1. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 8489:1077–1081. [DOI] [PubMed] [Google Scholar]
- 2. Steegers‐Theunissen RPM, Steegers EAP. Embryonic health: new insights, mHealth and personalised patient care. Reproduction, Fertility, and Development 2015; 27:712–715. [DOI] [PubMed] [Google Scholar]
- 3. Jaddoe VWV, de Jonge LL, Hofman A, Steegers EAP, Gaillard R. First‐trimester growth restriction and cardiovascular risk factors in childhood. British Medical Journal 2014; 348:g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen YP, Xiaom XM, Li J, Reichetzeder C, Wang ZN, Hocher B. Paternal body mass index (BMI) is associated with offspring intrauterine growth in a gender dependent manner. PLoS ONE 2012; 7:e36329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poeran J, Denktaş S, Birnie E, Bonsel GJ, Steegers EA. Urban perinatal health inequalities. Journal of Maternal‐Fetal & Neonatal Medicine 2011; 24:643–646. [DOI] [PubMed] [Google Scholar]
- 6. Personal communication to Mary E. Barker . Statistics provided by Public Health Southampton, Southampton City Council, 14th November 2014.
- 7. Massachusetts Department of Public Health . MassCHIP, Massachusetts community health information profile. 2013. http://www.mass.gov/eohhs/docs/dph/masschip/health-indicators/hsicountyessex.rtf [last accessed December 2014].
- 8. de Graaf JP, Steegers EA, Bonsel GJ. Inequalities in perinatal and maternal health. Current Opinion in Obstetrics and Gynecology 2013; 25:98–108. [DOI] [PubMed] [Google Scholar]
- 9. Vos AA, Posthumus AG, Bonsel GJ, Steegers EA, Denktaş S. Deprived neighborhoods and adverse perinatal outcome: a systematic review and meta‐analysis. Acta Obstetrica Gynecologica Scandinavia 2014; 93:727–740. [DOI] [PubMed] [Google Scholar]
- 10. Barker D, Barker M, Fleming T, Lampl M. Support mothers to secure future public health. Nature 2013; 504:209–211. [DOI] [PubMed] [Google Scholar]
- 11. Fielding JE, Teutsch SM. An opportunity map for societal investment in health. The Journal of the American Medical Association 2011; 305:2110–2111. [DOI] [PubMed] [Google Scholar]
- 12. Denktaş S, Poeran J, van Voorst SF, Vos AA, de Jong‐Potjer LC, Waelput AJM, et al Design and outline of the healthy pregnancy 4 all study. BMC Pregnancy and Childbirth 2014; 14:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brand A, Walker DK, Hargreaves M, Rosenbach M. Intermediate outcomes, strategies, and challenges of eight healthy start projects. Maternal and Child Health Journal 2010; 14:654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cramer ME, Chen LW, Roberts S, Clute D. Evaluating the social and economic impact of community‐based prenatal care. Public Health Nursing 2007; 24:329–336. [DOI] [PubMed] [Google Scholar]
- 15. Jackson MI. Early childhood WIC participation, cognitive development and academic achievement. Social Science & Medicine 2015; 126:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]


