Abstract
A polysulfide material was synthesized by the direct reaction of sulfur and d‐limonene, by‐products of the petroleum and citrus industries, respectively. The resulting material was processed into functional coatings or molded into solid devices for the removal of palladium and mercury salts from water and soil. The binding of mercury(II) to the sulfur‐limonene polysulfide resulted in a color change. These properties motivate application in next‐generation environmental remediation and mercury sensing.
Keywords: limonene, polysulfide, sulfur, sustainable materials, waste valorization
The exploration of sustainable feedstocks is important in the synthesis of functional materials.1 Herein, we report the utility of a polysulfide synthesized directly from two industrial by‐products: sulfur2 and d‐limonene3 (Scheme 1). This study was inspired by classic reports on the reaction of sulfur and limonene,4 the use of limonene as a renewable monomer,5 and the recent and innovative applications of “inverse vulcanization” to access a variety of advanced materials with high sulfur content.6 We found that the sulfur‐limonene polysulfide can be processed into coatings and solid devices that remove metal salts such as palladium(II) and mercury(II) from water and soil. We also report the discovery of a chromogenic response when the polysulfide is exposed to mercury(II). As sulfur is produced annually in excess of 60 million tons as a by‐product of petroleum refining2 and more than 70 thousand tons of limonene are isolated each year from orange zest in the citrus industry,3 the sulfur‐limonene polysulfide is inexpensive—further motivating its use in metal sequestration, sensing, and environmental remediation.
Scheme 1.
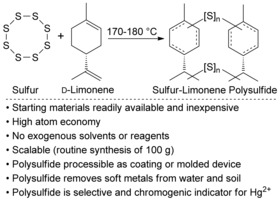
Synthesis and applications of a sulfur‐limonene polysulfide.
As a starting point, sulfur was melted (T>120 °C) and then heated to 170 °C. Above 150 °C, S−S bond scission occurs,7 thereby generating thiyl radicals that could add to limonene. An equal mass of limonene was added to the molten sulfur, which produced a two‐phase mixture that becomes a single, dark red phase upon reaction. An equal mass of sulfur and limonene was chosen to maximize the content of both industrial by‐products in the final material. 1H NMR analysis of the reaction mixture indicated limonene's exocyclic alkene was consumed more rapidly than its endocyclic alkene, with complete consumption of all olefins within 90 min (see Figures S6–S8 in the Supporting Information). Little change was observed by 1H NMR spectroscopy on further heating.
The emergence of aromatic signals in the 1H NMR spectrum indicated the oxidation of limonene. The conversion of limonene into p‐cymene by reaction with sulfur has been reported,8 but the complicated 1H NMR signals between 6.9 and 7.6 ppm suggested other aromatic material was also present in the product (Scheme 2). Prolonged distillation of the product mixture allowed isolation of p‐cymene as well as volatile thiol and sulfide by‐products (see Figures S11 and S12 in the Supporting Information).8a Good mass balance was observed, with the volatile fraction typically constituting 20 % of the product and the nonvolatile portion 80 % of the mass.
Scheme 2.
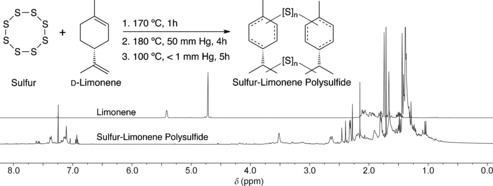
Synthesis of the sulfur‐limonene polysulfide on a 100 g scale. 1H NMR spectra of technical grade limonene (top) and the sulfur‐limonene polysulfide (bottom).
Cooling the nonvolatile component to room temperature produced a red waxlike material. Differential scanning calorimetry (DSC) revealed a glass transition (T g) at −21 °C and simultaneous thermal analysis (STA) indicated substantial thermal decomposition above 200 °C (see Figure S32–S35 in the Supporting Information). The material is insoluble in water, sparingly soluble in methanol, and fully soluble in dichloromethane, chloroform, and tetrahydrofuran. A band with a λ max=420 nm was observed in the UV/Vis spectrum of a solution of the material in dichloromethane. Combustion analysis revealed an elemental composition of 38.97 % C, 4.97 % H, and 56.6 % S, consistent with the high sulfur content envisioned for this product. Less than 4 % of this sulfur was unreacted S8, as determined by GC‐MS (see Figures S16–S18 in the Supporting Information), thus indicating a high conversion in the reaction between sulfur and limonene. The product was also optically active, which indicates that at least a portion of the product derived from limonene maintained its stereochemical integrity ([α]D=−27.3 (c=1.0, CHCl3).
Expecting a polymeric product, the sulfur‐limonene material was examined by size‐exclusion chromatography (SEC). The SEC trace of the sulfur‐limonene material revealed a higher molecular volume than limonene (see Figures S21 and S22 in the Supporting Information). Mass spectrometry, however, indicated a lower molecular weight product than expected. In this analysis, the sulfur‐limonene material was chemically ionized by coordination to silver(I) prior to infusion into the MS source.9 A cluster of signals from m/z=495 to 886 was observed and assigned as [M+Ag]+ ions, based on the doublets present for the two abundant isotopes of Ag (106.9 and 108.9 Da; see Figures S29–S31 in the Supporting Information). This result suggested that the mass range of the limonene‐sulfur material that ionized under these conditions varied between 386 and 777 Da. Unlike recent reports of inverse vulcanization where sulfur is cross‐linked with dienes,6 the reaction of sulfur and limonene did not appear to form a high‐molecular‐weight polymer. Instead, the material is more appropriately described as a low‐molecular‐weight polysulfide. Raman spectroscopy of the material revealed a dominant signal at 464 cm−1, which corresponds to various stretching modes of S−S bonds,10 and additional bands at 149, 215, 589, and 2918 cm−1. Further evidence for S−S bonds was provided by the reaction of the material with LiAlH4. SEC and GC‐MS analysis after the reaction with LiAlH4 revealed a product with a lower molecular weight, consistent with decomposition of S−S cross‐links in the sulfur‐limonene material (see Figures S23–S28 in the Supporting Information). This experiment illustrated that the polysulfide can be broken down by a reducing agent. We also note that the STA analysis mentioned previously is consistent with a polysulfide structure, where significant S−S scission occurs above 200 °C. Interestingly, this thermal depolymerization proceeded similarly in both air and nitrogen, thus suggesting that the polysulfide is not prone to aerobic oxidation (see Figures S32–S34 in the Supporting Information).
The sulfur‐limonene polysulfide can be synthesized on a large scale. We have prepared several kilograms of the material, typically in 50 and 100 g batches. The sulfur‐limonene polysulfide can then be processed as a coating or molded into a desired shape. For the former, the distillation step was omitted and the p‐cymene and other volatile materials generated during the synthesis conveniently served as the solvent. Figure 1 A shows the interior of a flask spin‐coated at 70 °C. Heat, vacuum, or a stream of nitrogen was used to drive off the residual solvent. For molding, the sulfur‐limonene polysulfide was melted (>100 °C) and poured into a silicone cast (Figure 1 B) or glass petri dish (Figure 1 C). In Figure 1 C, it is notable that the polysulfide is transparent at a thickness of 3 mm.
Figure 1.
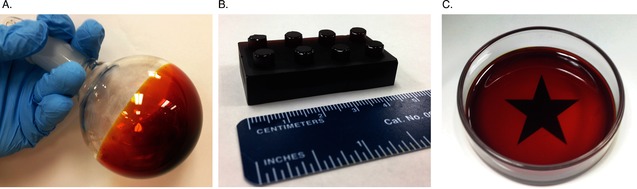
Processing the sulfur‐limonene polysulfide into a coating (A) or molded object (B,C). The polysulfide is transparent at a thickness of 3 mm (C).
We next assessed the ability of the polysulfide to sequester metals from water. Elemental sulfur has been used in mercury disposal,11 but it is difficult to process into useful devices because of its high crystallinity. The sulfur‐limonene polysulfide, in contrast, can be converted into a coating or solid object (Figure 1). Should the polysulfide have a high affinity for metals, this property would motivate applications in environmental remediation.
As a starting point, the removal of palladium(II) from water was studied. Palladium is a popular catalyst in organic synthesis,12 and its use in water leads to waste streams from which the metal must be removed. Palladium pollution from catalytic converter exhausts is another motivation for developing new sequestration technologies.13 To test the affinity of the sulfur‐limonene polysulfide for palladium, an aqueous solution of Na2PdCl4 (0.35 mm) was incubated on a 28 cm2 plate of the polysulfide. The concentration of Na2PdCl4 in the water was then monitored by UV/Vis spectroscopy.14 In the event, the palladium concentration dropped rapidly over the first hour, with 42 % of the palladium removed from solution within 2 h (see Figures S37 and S38 in the Supporting Information). After this time, the palladium binding appeared to reach equilibrium. Importantly, this experiment demonstrated that the sulfur‐limonene polysulfide could remove soft metal salts from water. Furthermore, a control experiment demonstrated that the sulfur‐limonene polysulfide trapped palladium as quickly and effectively as elemental sulfur (see Figures S38 and S39 in the Supporting Information).
Encouraged by this preliminary result, we moved to sequestration studies of HgCl2. Mercury(II) exposure can result in a compromised immune system, kidney damage, and embryotoxic effects.15 Therefore, effective and inexpensive technologies are needed to remove mercury(II) from the environment.15a, 16 When an aqueous solution of HgCl2 (10 mm) was added to the surface of the polysulfide, the result was a surprise: a bright yellow deposit formed that remained immobilized on the polysulfide (Figure 2). The deposit typically appeared within 30 min and remained on the polysulfide, even after washing with water. Remarkably, this deposit was only formed when the polysulfide was exposed to Hg2+. No color change or deposit was observed when the polysulfide was treated with Li+, Fe3+, Ca2+, Cu2+, Pb2+, Mg2+, Zn2+, Ni2+, K+, Mn2+, or deionized water (Figure 2 and see Figure S46 in the Supporting Information).
Figure 2.
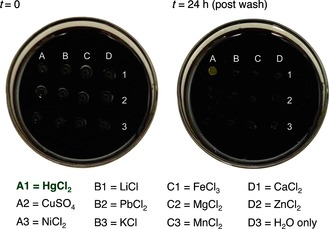
10 mm solutions of metal salts were added to the sulfur‐limonene polysulfide and incubated for 24 h. A selective color change was observed upon exposure to HgII.
This result does not necessarily mean that the other metals did not bind to the polysulfide, but only that the color change is unique to mercury(II). Importantly, this color change was not observed when S8 was exposed to Hg2+ under the same conditions (see Figure S43 in the Supporting Information), thus revealing another advantage of the sulfur‐limonene polysulfide over elemental sulfur in metal sequestration.
Analyzing this yellow deposit by scanning electron microscopy (SEM) and energy‐dispersive X‐ray (EDX) spectroscopy revealed the presence of mercury (Figure 3 and see Figures S47–S63 in the Supporting Information). The polysulfide appeared to form rippled sheets and ridges upon exposure to Hg2+ (Figure 3, bottom). The most distinctive feature, however, was the formation of nano‐ and microparticles that adhered to the surface, even after washing with water (Figure 3, bottom images). These particles contained high levels of mercury, up to 50 wt % as determined by EDX analysis. Interestingly, several of these particles penetrated the surface of the polysulfide (Figure 3, bottom right)—likely the result of the high density of the mercury particles and the malleable nature of the polysulfide. We anticipate this particle entrapment will be useful for the sequestration and disposal of inorganic mercury.
Figure 3.
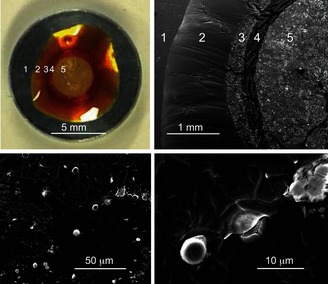
Top: SEM pin mount coated with the polysulfide. Region 5 was exposed to HgCl2. Regions 1–4 did not contain mercury, as determined by EDX. Bottom: Representative area in region 5, up to 50 wt % mercury was detected in the nano‐ and microparticles formed.
Motivated by these intriguing results, we examined how the polysulfide responded to HgII in complex mixtures. We spiked river water and a suspension of pond soil with ≥2 mg mL−1 HgCl2. Remarkably, the same insoluble mercury deposit was formed on the polysulfide in both cases (see Figures S42–S44 in the Supporting Information). For the soil suspension, the silt and pond debris were removed by washing with water, while the yellow mercury deposit remained adhered to the polysulfide. This result demonstrated that the sulfur‐limonene polysulfide can remove HgII from the complex mixtures encountered in environmental remedyation.
While mercury sensing is an obvious application of the polysulfide's response to HgII, we noted that the yellow deposit was only visible for HgII concentrations of 1 mm or higher (see Figure S45 in the Supporting Information). Therefore, rather than use this chromogenic response to detect low levels of inorganic mercury, we envision using it to indicate that a threshold level of mercury has bound to the polysulfide. This response could be used to monitor the lifetime of remediation devices made from the polysulfide. It is important to point out, however, that mercury binds to the polysulfide at lower concentrations, even when there is no chromogenic response. For example, an aqueous solution of 2000 ppb HgCl2 was incubated on a 28 cm2 plate of the polysulfide for 24 h. After this time, the water was removed and analyzed by cold vapor atomic absorption spectroscopy. A final concentration of 910 ppb was measured, thus indicating approximately 55 % of the inorganic mercury was removed with a single treatment. While alternative, and highly effective, mercury sensors17 and adsorbents18 have been reported, their deployment in environmental remediation is often limited due to the challenges and cost of their large‐scale synthesis.15a, 16 We note that the sulfur‐limonene polysulfide is comparatively inexpensive, easy to produce on a large scale, and displays a useful chromogenic response.
We envision using the polysulfide to remove inorganic mercury from water and soil at the site of contamination. Before the polysulfide can be deployed directly in natural waterways and ecosystems, however, an assessment of its toxicity must be completed. Initiating these studies, we treated hepatic cell lines HepG2 and Huh7 with water that had been exposed to the sulfur‐limonene polysulfide for 24 h. Even when this water made up 50 % of the culture medium, no difference in cell viability was observed between the treated cells and a negative control sample that was treated with pure, sterile water (see Figure S64 in the Supporting Information). This experiment indicates that the polysulfide does not release harmful materials into water, thus motivating further studies in soil and water remediation. These investigations are ongoing.
In conclusion, we have explored the properties of a polysulfide synthesized entirely from the industrial by‐products sulfur and limonene. The polysulfide is easy to synthesize on a large scale and requires no exogenous reagents or solvents. The polysulfide removes PdII and HgII from water and soil and turns yellow when exposed to mercury(II). This response is selective for mercury, a discovery that may find use in sensing applications. We plan to develop the sulfur‐limonene polysulfide as an inexpensive material for environmental remediation, where it will be used to sequester toxic metals from complex mixtures. More generally, this research is part of a growing effort to identify new and useful properties of materials with high sulfur content6 and to synthesize them in an efficient and sustainable fashion.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank Flinders University, The Australian Research Council (DE150101863, J.M.C), the Australian National Fabrication Facility (A.D.S), and the Tulsa Undergraduate Research Challenge (M.P.C., A.M.E) for financial support. We also acknowledge the support of the Australian Microscopy and Microanalysis Research Facility at Flinders University. We thank Evan Shrestha for preliminary experiments and Jennifer Holland for assistance with mass spectrometry. We gratefully acknowledge the SEC analysis provided by Polymer Solutions Incorporated (Blacksburg, VA), an ISO‐17025 accredited laboratory.
M. P. Crockett, A. M. Evans, M. J. H. Worthington, I. S. Albuquerque, A. D. Slattery, C. T. Gibson, J. A. Campbell, D. A. Lewis, G. J. L. Bernardes, J. M. Chalker, Angew. Chem. Int. Ed. 2016, 55, 1714.
References
- 1.
- 1a.“ACS Symposium Series, Vol. 921” Feedstocks for the Future: Renewables for the Production of Chemicals and Materials (Eds.: J. J. Bozell, M. K. Patel), American Chemical Society, Washington, DC, 2006; [Google Scholar]
- 1b. Meier M. A. R., Metzger J. O., Schubert U. S., Chem. Soc. Rev. 2007, 36, 1788–1802; [DOI] [PubMed] [Google Scholar]
- 1c. Gandini A., Macromolecules 2008, 41, 9491–9504; [Google Scholar]
- 1d. Wilbon P. A., Chu F., Tang C., Macromol. Rapid Commun. 2013, 34, 8–37. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Meyer B., Kharasch N., Elemental Sulfur - Chemistry and Physics, Interscience Publishers, New York, 1965; [Google Scholar]
- 2b. Kutney G., Sulfur: History, Technology, Applications & Industry, 2nd ed., ChemTec Publishing, Toronto, 2013. [Google Scholar]
- 3.
- 3a. Braddock R. J., Handbook of Citrus By-Products and Processing Technology, Wiley, New York, 1999; [Google Scholar]
- 3b. Kerton F. M., in Alternative Solvents for Green Chemistry, 1st ed. (Eds.: J. H. Clark, G. A. Kraus), RSC Green Chemistry, Cambridge, 2009, p. 109. [Google Scholar]
- 4.
- 4a. Nakatsuchi A., J. Soc. Chem. Ind. Jpn. 1930, 33, 408; [Google Scholar]
- 4b. Nakatsuchi A., J. Soc. Chem. Ind. Jpn. 1932, 35, 376; [Google Scholar]
- 4c. Weitkamp A. W., J. Am. Chem. Soc. 1959, 81, 3430–3434; [Google Scholar]
- 4d. Weitkamp A. W., J. Am. Chem. Soc. 1959, 81, 3434–3437; [Google Scholar]
- 4e. Weitkamp A. W., J. Am. Chem. Soc. 1959, 81, 3437–3439; [Google Scholar]
- 4f. Currell B. R., Williams A. J., Mooney A. J., Nash B. J., in New Uses of Sulfur, Vol. 140 (Ed.: J. West), American Chemical Society, Washington, DC, 1975, pp. 1–17. [Google Scholar]
- 5.
- 5a. Byrne C. M., Allen S. D., Lobkovsky E. B., Coates G. W., J. Am. Chem. Soc. 2004, 126, 11404–11405; [DOI] [PubMed] [Google Scholar]
- 5b. Firdaus M., de Espinosa L. M., Meier M. A. R., Macromolecules 2011, 44, 7253–7262; [Google Scholar]
- 5c. Firdaus M., Meier M. A. R., Green Chem. 2013, 15, 370–380; [Google Scholar]
- 5d. Nejad E. H., Paoniasari A., van Melis C. G. W., Koning C. E., Duchateau R., Macromolecules 2013, 46, 631–637. [Google Scholar]
- 6.
- 6a. Chung W. J., Simmonds A. G., Griebel J. J., Kim E. T., Suh H. S., Shim I.-B., Glass R. S., Loy D. A., Theato P., Sung Y.-E., Char K., Pyun J., Angew. Chem. Int. Ed. 2011, 50, 11409–11412; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 11611–11614; [Google Scholar]
- 6b. Chung W. J., Griebel J. J., Kim E. T., Yoon H., Simmonds A. G., Ji H. J., Dirlam P. T., Glass R. S., Wie J. J., Nguyen N. A., Guralnick B. W., Park J., Somogyi Á., Theato P., Mackay M. E., Sung Y.-E., Char K., Pyun J., Nat. Chem. 2013, 5, 518–524; [DOI] [PubMed] [Google Scholar]
- 6c. Griebel J. J., Li G., Glass R. S., Char K., Pyun J., J. Polym. Sci. Part A 2015, 53, 173–177; [Google Scholar]
- 6d. Griebel J. J., Namnabat S., Kim E. T., Himmelhuber R., Moronta D. H., Chung W. J., Simmonds A. G., Kim K.-J., van der Laan J., Nguyen N. A., Dereniak E. L., Mackay M. E., Char K., Glass R. S., Norwood R. A., Pyun J., Adv. Mater. 2014, 26, 3014–3018; [DOI] [PubMed] [Google Scholar]
- 6e. Kim E. T., Chung W. J., Lim J., Johe P., Glass R. S., Pyun J., Char K., Polym. Chem. 2014, 5, 3617–3623; [Google Scholar]
- 6f. Simmonds A. G., Griebel J. J., Park J., Kim K. R., Chung W. J., Oleshko V. P., Kim J., Kim E. T., Glass R. S., Soles C. L., Sung Y.-E., Char K., Pyun J., ACS Macro Lett. 2014, 3, 229–232; [DOI] [PubMed] [Google Scholar]
- 6g. Lim J., Pyun J., Char K., Angew. Chem. Int. Ed. 2015, 54, 3249–3258; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 3298–3308. [Google Scholar]
- 7.
- 7a. Parker A. J., Kharasch N., Chem. Rev. 1959, 59, 583–628; [Google Scholar]
- 7b. Meyer B., Chem. Rev. 1976, 76, 367–388. [Google Scholar]
- 8.
- 8a. Illa O., Namutebi M., Saha C., Ostovar M., Chen C. C., Haddow M. F., Nocquet-Thibault S., Lusi M., McGarrigle E. M., Aggarwal V. K., J. Am. Chem. Soc. 2013, 135, 11951–11966; [DOI] [PubMed] [Google Scholar]
- 8b. Ruzicka L., Meyer J., Mingazzini M., Helv. Chim. Acta 1922, 5, 345–368. [Google Scholar]
- 9. Wang K., Groom M., Sheridan R., Zhang S., Block E., J. Sulfur Chem. 2013, 34, 55–66. [Google Scholar]
- 10. Oh Y., Morris C. D., Kanatzidis M. G., J. Am. Chem. Soc. 2012, 134, 14604–14608. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a.Sulfur Polymer Stabilization/Solidification (SPSS) Treatment of Mixed-Waste Mercury Recovered from Environmental Restoration Activities at BNL, P. D. Kalb, J. W. Adams, L. W. Milian, Brookhaven National Laboratory, Upton, New York, 2001, BNL-52614;
- 11b. Fuhrmann M., Melamed D., Kalb P. D., Adams J. W., Milian L. W., Waste Manage. 2002, 22, 327–333. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Nicolaou K. C., Bulger P. G., Sarlah D., Angew. Chem. Int. Ed. 2005, 44, 4442–4489; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 4516–4563; [Google Scholar]
- 12b. Torborg C., Beller M., Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar]
- 13. Barbante C., Veysseyre A., Ferrari C., Van De Velde K., Morel C., Capodaglio G., Cescon P., Scarponi G., Boutron C., Environ. Sci. Technol. 2001, 35, 835–839. [DOI] [PubMed] [Google Scholar]
- 14. Tait C. D., Janecky D. R., Rogers P. S. Z., Geochim. Cosmochim. Acta 1991, 55, 1253–1264. [Google Scholar]
- 15.
- 15a. Wang J., Feng X., Anderson C. W. N., Xing Y., Shang L., J. Hazard. Mater. 2012, 221–222, 1–18; [DOI] [PubMed] [Google Scholar]
- 15b. Inorganic Mercury TEACH Chemical Summary, U.S. Environmental Protection Agency, 2007, 1–19. http://www.epa.gov/teach/chem summ/mercury inorg summary.pdf. Accessed June 29, 2015.
- 16. Treatment Technologies for Mercury in Soil, Waste, and Water U.S. Environmental Protection Agency, Washington, DC, 2007 http://www.epa.gov/tio/download/remed/542r07003.pdf. Accessed June 29, 2015.
- 17. Chen G., Guo Z., Zeng G., Tang L., Analyst 2015, 140, 5400–5443. [DOI] [PubMed] [Google Scholar]
- 18. Li B., Zhang Y., Ma D., Shi Z., Ma S., Nat. Commun. 2014, 5, 5537, and references therein. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


