Abstract
Background :
The use of long-chain polyunsaturated fatty acids has been studied in the context of healing and tissue regeneration mainly due to its anti-inflammatory, immunoregulatory and antioncogenic properties. Previous studies have demonstrated beneficial effects with the use of enteral immunonutrition containing various farmaconutrients such as L-arginine, omega-3, trace elements, but the individual action of each component in the healing of colonic anastomosis remains unclear.
Aim :
To evaluate the influence of preoperative supplementation with omega-3 fatty acids on the healing of colonic anastomoses of well-nourished rats.
Methods :
Forty Wistar adult male rats, weighing 234.4±22.3 g were used. The animals were divided into two groups: the control group received for seven days olive oil rich in omega-9 oil through an orogastric tube, while the study group received isocaloric and isovolumetric omega-3 emulsion at a dose of 100 mg/kg/day, also for seven days. Both groups were submitted to two colotomies followed by anastomosis, in the right and left colon, respectively. Parameters evaluated included changes in body weight, anastomotic complications and mortality, as well as maximum tensile strength by using a tensiometer and collagen densitometry at the anastomotic site.
Results :
There were no differences in body weight or mortality and morbidity between groups. The value of the maximum tensile strength of the control group was 1.9±0.3 N and the study group 1.7±0.2, p=0.357. There was, however, a larger amount of type I collagen deposition in the study group (p=0.0126). The collagen maturation índex was 1.74±0.71 in the control group and 1.67±0.5 in the study group; p=0,719).
Conclusions :
Preoperative supplementation of omega-3 fatty acid in rats is associated with increased collagen deposition of type I fibers in colonic anastomoses on the 5th postoperative day. No differences were observed in the tensile strength or collagen maturation index.
Keywords: Omega-3, Wound healing, Colonic anastomosis, Rats
Abstract
Racional:
O uso de ácidos graxos poliinsaturados de cadeia longa tem sido estudado no contexto de cicatrização e regeneração do tecido, principalmente devido a suas propriedades imunorreguladoras, antioncogênicas e anti-inflamatórias. Estudos anteriores demonstraram efeitos benéficos com o uso de imunonutrição enteral contendo vários farmaconutrientes (L-arginina, ômega-3, oligoelementos), mas a ação individual de cada componente na cicatrização de anastomose colônica permanece incerto.
Objetivo:
Avaliar a influência da suplementação no pré-operatório com ácidos graxos ômega-3 na cicatrização de anastomoses colônicas de ratos eutróficos.
Método:
Quarenta ratos Wistar, machos adultos, pesando 234,4±22,3 g foram divididos em dois grupos: grupo controle recebeu a suplementação de azeite de oliva (rico em ômega-9) por gavagem por sete dias e o grupo estudo recebeu ômega-3 em forma de emulsão isocalórica e isovolumétrica, também por gavagem, na dose de 100 mg/kg/dia por sete dias no pré-operatório. Ambos os grupos foram submetidos à duas colotomias seguidas de anastomose colônica, em cólon direito e cólon esquerdo, respectivamente. Foram avaliados a evolução do peso dos animais, morbimortalidade e realizados testes tensiométrico e de densitometria do colágeno dos corpos de prova.
Resultados:
Não houve diferenças na evolução do peso e na morbimortalidade entre os grupos. O valor da força de tração máxima do grupo controle foi de 1,9±0,3N e no grupo estudo de 1,7±0,2, p=0,357. Houve, no entanto, maior quantidade de colágeno tipo I (p=0,0126) na anastomose no grupo estudo. O índice de maturação do colágeno foi cálculado para os grupos (1,74±0,71 - grupo controle; 1,67±0,5 - grupo estudo, p=0,719).
Conclusões:
A suplementação pré-operatória com ácido graxo ômega-3 está associada ao aumento da deposição de colágeno do tipo I em anastomoses de cólon em ratos no 5º dia do pós-operatório, mas não exerce influência na resistência tênsil de colorrafia ou no índice de maturação do colágeno.
INTRODUCTION
Immunonutrition is defined as the use of specific nutrients in formulas which promote through different mechanisms of action, effects on the inflammatory and imune systems4 , 10 , 14 , 22.
Initially tested in animal models, these formulas were started to be used in patients previously malnourished that were to be submitted to surgical procedures. Clear benefits were obtained on postoperative recovery, with reduced morbidity rate, length of hospital stay and duration of mechanical ventilation in intensive care units21. However, the use of these formulas has spread and studies began to show positive effect also in well-nourished individuals8 , 15 , 23 , 24 , 25 , 26.
However, due to the complex mechanism of interaction between absorption processes, membranes attachment and intracellular signaling, it is known that formulas consisting of amino acids, essential fatty acids, trace elements, vitamins, nucleotides and nucleosides, were effective when using together, while the use of specific nutrients alone was associated with less clear benefits and with divergent results5.
The use of long-chain polyunsaturated fatty acids have been studied in the context of healing and tissue regeneration, mainly due to its anti-inflammatory, immunoregulatory and antioncogenics properties. However, studies performed in rat models of induced colitis resulted in negative results, in which the use of omega-3 was detrimental, resulting in deterioration of colitis16 , 29.
Its anti-inflammatory properties arise from processes such as the partial replacement of arachidonic acid in cell membranes, and thus, a reduction in the production of derivatives considered pro-inflammatory, such as prostaglandin E2. There is also a reduction in the chemotaxis of monocytes and neutrophils, modulating the inflammatory response in its earliest stages. Also notable are its immunoregulatory effects by reducing the proliferation of cytotoxic T lymphocytes in response to proinflammatory cytokines, the production of mediators such as nitric oxide and tumor necrosis factor1 , 2 , 13 , 28.
Thus, despite the fact that omega-3 fatty acids play a clear role in the inflammatory response, the supplementation with omega-3 and its role in the overall context of colonic anastomosis healing is still unknown4.
The aim of this study was to evaluate the influence of preoperative supplementation with omega-3 fatty acids on the healing of colonic anastomoses in rats.
METHODS
Forty Wistar adult male rats ( Rattus norvegicusalbinus , Rodentia mammalia), weighing 234.4±22.3 g obtained from the Universidade Estadual de Maringá were acclimatized individually receiving ad libitum standard rat chow prior to the surgical procedure. Seven days before surgery, the control group (GAO) received daily olive oil supplementation while the study group (GOM), received omega-3 fatty acid emulsion as an isocaloric and isovolumetric emulsions at a dose of 100 mg/kg/day through an rigid orogastric tube.
Both groups received the fatty acids suplementation through an orogastric gavage without anesthesia and were submitted to two colotomies followed by anastomosis with interrupted 6-0 nylon in the right and left colon, respectively. On the 5th postoperative day, rats were killed. Parameters evaluated included changes in body weight, anastomotic complications and mortality, as well as maximum tensile strength by using a tensiometer and collagen densitometry at the anastomotic site. Statistical analysis was performed using the Student t test, Mann-Whitney test, Fisher's exact test and Z-test to compare proportions, with p<0.05.
RESULTS
Anastomotic leakages occurred in four animals in each group. The mortality rate in the GAO group was 35.0% (n=7) and 10.0% (n=2) in the GOM group, p=0.1973. There were no diferences in the average weight at the beginning of the experiment, on the 7th, 14th, 21st day, the day of surgery and the day of sacrifice, respectively (Table 1).
TABLE 1. - Average weight±standard error (SE) per group (g) .
| Days | GAO (n=20) | GOM (n=20) | p | ||||
|---|---|---|---|---|---|---|---|
| Average | ± | SE | Average | ± | SE | ||
| D0 | 292,3 | ± | 4,4 | 284,3 | ± | 5,3 | 0,343 |
| D7 | 322,3 | ± | 5,0 | 313,1 | ± | 5,6 | 0,208 |
| D14 | 348,4 | ± | 5,5 | 339,0 | ± | 6,5 | 0,323 |
| D21 | 369,7 | ± | 6,2 | 363,4 | ± | 6,9 | 0,695 |
| DCx | 349,7 | ± | 6,4 | 344,0 | ± | 7,2 | 0,561 |
| DSx | 321,0 | ± | 8,8 | 316,1 | ± | 3,8 | 0,857 |
D0=first day of study; DCx=day of surgery; DSx=day of sacrifice; GOM=omega-3 group; GAO=olive oil group; p=statistical signicance (Mann-Whitney test)
The value of the maximum tensile strength of the GAO group was 1.9±0.3 N and the GOM group 1.7±0.2, p=0.357 (Figure 1). The rupture force of groups GAO and GOM were also similar (1,5+0,2 vs 1,4+0,2 respectively, p=0,3572).
FIGURE 1. - Comparison between maximum tensile strength of the GAO and GOM groups.
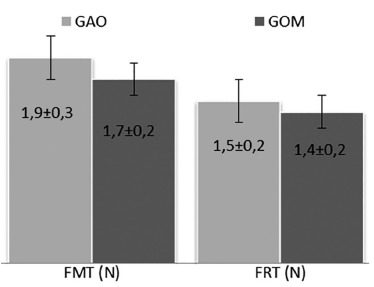
p=statistical significance; FMT=maximum strenght rupture force (p=0,6028); FRT=total rupture force (p=0,3572); N=newtons; GAO=olive oil group; GOM=omega-3 group (Mann-Whitney test)
There was, however, a larger amount of type I collagen deposition (p = 0.0126) in the GOM group (Figure 2).
FIGURE 2. - Type I and III collagen distribuition among GOM and GAO groups.
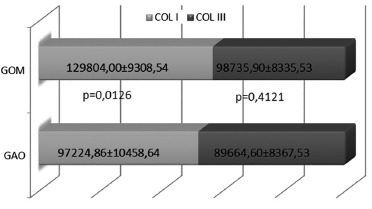
COL I=type I collagen;COL III=type III collagen; GAO=olive oil group; GOM=omega-3 group; SEM=standard error; p=statistical significance
The collagen maturation index (CMI) at the anastomotic site was calculated for both groups (GAO - 1.74±0.71; GOM - 1.67±0.5; p=0,719) and no difference were detected (Table 2).
TABLE 2. - Average±standard error for collagen maturation index for GAO and GOM groups.
| Groups | CMI | p | |||
|---|---|---|---|---|---|
| n | Média | ± | EP | ||
| GAO | 13 | 1,74 | ± | 0,71 | 0,719 |
| GOM | 18 | 1,67 | ± | 0,5 | |
CMI=collagen maturation index; GAO=olive oil group; GOM=omega-3 group; SEM=standard error; p=statistical significance (Mann-Whitney test)
DISCUSSION
The wound healing of the intestine after an anastomosis depends on balanced reconstitution, proliferation and differentiation processes.
The intestinal cells can be injured by surgery, inflammatory diseases, or toxic luminal substances, despite of its barrier function. The healing process starts with de surrounding epithelial cells that loses their columnar polarity, becoming a flattered cell that migrates into the denude area to restore the intestinal barrier12 and despite where the healing process is located, the phases of healing tends to occur in a certain sequence: inflammatory, proliferation and maturation. Each phase has its own particular duration and cell-type envolved.
Cellular types such as neutrophils, macrophages, fibroblasts and even platelets play a fundamental role through secretion of chemoatrative factors, cell-to-cell interations and are vunerable to a vaste type of interferences, promoted mostly by the nutritional status of the host, presence or not of inflammatory process and oxidative stress. In this case the primary proinflammatory cytokines such interleukin-1 (IL-1), IL-6 and tumor necrosis factor-α (TNF- α) and also the autocrine, paracrine and endocrine events can be affect by such factors17.
All these interations of proinflammatory cytokines assist in controlling infection and prepare tissue for repair by promoting and enhacing phagocytic activity, stimulating keratinocyte migration, fibroblast chemotaxis and by regulating the remodulation of the extracellular matrix proteins12 , 17.
In this scenario, the role of immunonutrition on the colonic anastomotic healing depends on physiological performance and specific molecular interactions of the nutrients involved. Both macro and micronutrients with immuno-modulating activities have been identified, and the rationale for the use of such formulations in surgical patients is the need to reduce or mitigate the inflammatory response at specific times during surgical recovery22.
Inflammation is part of the healing process.The omega-9 olive oil was chosen as a isocaloric control because of its neutral activity as inflammation is concerned. The use of the more usual soybean oil, presented in most lipid emulsions, could result in an stimulation of the inflammatory process due to its rich content of linoleic acid, a pro-inflammatory precursor.
Many studies have shown clear benefit of the use of immune- modulating enteral formulas by various mechanisms, because they enhancing the immune status in its various aspects11. However, the use of individual components present in these formulas did not bring such positive evidences and literature is divergent as regards to its results4 , 5. The omega-3 polyunsaturated fatty acids have been shown to play an important role in the mechanisms involving cellular signaling pathways that culminate with an anti-inflammatory effect. For example, they reduce TNF-α production by macrophages, as well as the production of pro-inflammatory cytokines and nitric oxide1 , 7 , 9 , 18.
The immune response ability to generate an inflammatory response is eventually modified by the addition of omega-3 polyunsaturated fatty acids in the diet. The cell membrane modifies its characteristics, showing better fluidity, lipid domain areas, and a greater or lesser affinity for certain pathways which ultimately modulates especially leukocyte function. As a result, there is a competition for the same enzymatic pathways as arachidonic acid (AA), but generating less inflammatory eicosanoids. It also reduces the ability of macrophages to present antigens1 , 3 , 6 , 13 , 17 , 20 , 27 , 30. One possible consequence of the administration of omega-3 fatty acids would be the reduction of the healing process, particularly in the breaking strength, because it attenuates the inflammatory phase of the healing process. Therefore our finding that the administration of omega-3 did not reduce the tensile strength is remarcable.
The omega-3 can act also by competing with LTB4 receptors occupancy, blocking the transduction signals for synthesis of core protein G, or even in cell signaling through inhibition of phospholipase C activation induced by TNF-α, hampering or delaying the cellular signs1 , 27 , 30.
This network involving pro and antiinflamatory factors among a nutricional status and exogenous administration of omega-3 fatty acids affects the balance of intestinal healing process in its various aspects that are still object of study by many researchers throughout the world.
CONCLUSIONS
Preoperative supplementation with omega-3 is associated with increased collagen deposition of type I fibers in colonic anastomoses in rats on the 5thpostoperative day. No differences were observed in the breaking strength or collagen maturation index.
Footnotes
Financial source: Conselho Nacional de Pesquisa (CNPQ)
REFERENCES
- 1.Calder PC. Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz J Med Biol Res 1998;31(4):467–490. doi: 10.1590/S0100-879X1998000400002. [DOI] [PubMed] [Google Scholar]
- 2.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids 2001;36(9):1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. Polyunsaturated fatty acids, inflammatory process and inflammatory bowel diseases. Mol Nutr Food Res 2008;52(8):885–897. doi: 10.1002/mnfr.200700289. [DOI] [PubMed] [Google Scholar]
- 4.Chow O, Barbul A. Immunonutrition: Role in Wound Healing and Tissue Regeneration. Advances in Wound Care 2014;3(1):46–48. doi: 10.1089/wound.2012.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrêa-Neto MP, Campos ACL, Branco AB, Matias JEF. Efeito da suplementação dietética de arginina na cicatrização das anastomoses colônicas em ratos. ABCD Arq Bras Cir Dig. 2009;22(1):7–14. [Google Scholar]
- 6.Eisner F, Jacob P, Frick JS, Feilitzsch M. Immunonutrition with long-chain fatty acids prevents activation of macrophages in the gut wall. J Gastrointest Surg 2011;15(5):853–859. doi: 10.1007/s11605-011-1431-z. [DOI] [PubMed] [Google Scholar]
- 7.Endres S, Reza Ghorbani BS, Kelley VE, Georgilis K, Lonnemann G, Van Der Meer JWM. The Effect of Dietary Supplementation with n-3 Polyunsaturated Fatty Acids on the Synthesis of Interleukin-1 and Tumor Necrosis Factor by Mononuclear Cells. N Engl J Med 1989;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 8.Flesch AG, Poziomyck AK, Damin DC. The therapeutic use of symbiotics. Arq Bras Cir Dig. 2014 Jul-Sep;27(3):206–209. doi: 10.1590/S0102-67202014000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm H, Mayer K, Mayser P, Eigenbrodt E. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. British Journal of Nutrition. 2002;87(Suppl.1):S59–S67. doi: 10.1079/BJN2001457. [DOI] [PubMed] [Google Scholar]
- 10.Guimarães MV, Moreira GH, Rocha LP, Nicoluzzi JE, de Souza CJ, Repka JC. L-arginine action in cutaneous flap evolution under nicotine exposure in rats. Rev Col Bras Cir. 2013 Jan-Feb;40(1):49–54. doi: 10.1590/s0100-69912013000100009. [DOI] [PubMed] [Google Scholar]
- 11.Heys SD, Walker LG, Smith I, Ermin O. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg. 1999;229(4):467–477. doi: 10.1097/00000658-199904000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol 2011;17(7):2161–2171. doi: 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuratko CN. Proliferation of colonic lymphocytes in response to inflammatory cytokines is lower in mice fed with fish oil than in mice fed with corn oil. Cancer Lett 2000;148(1):27–32. doi: 10.1016/S0304-3835(99)00266-9. [DOI] [PubMed] [Google Scholar]
- 14.Magalhães CR, Malafaia O, Torres OJ, Moreira LB, Tefil SC, Pinherio Mda R, Harada BA. Liver regeneration with l-glutamine supplemented diet: experimental study in rats. Rev Col Bras Cir. 2014 Mar-Apr;41(2):117–121. doi: 10.1590/s0100-69912014000200008. [DOI] [PubMed] [Google Scholar]
- 15.Marimuthu K, Varadhan K, Ljungqvist O, Lobo D. A Meta-Analysis of the Effect of Combinations Of Immune Modulating Nutrients on Outcome in Patients Undergoing Major Open Gastrointestinal Surgery. Annals Of Surgery 2012;6(255):1060–1068. doi: 10.1097/SLA.0b013e318252edf8. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K, Miura S. Omega-3 fatty acids exacerbates DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis 2008;10(14):1348–1357. doi: 10.1002/ibd.20491. [DOI] [PubMed] [Google Scholar]
- 17.McDaniel JC, Belury M, Ahijevych K, Blakely W. ?-3 fatty acids effect on wound healing. Wound Repair Regen. 2008 May-Jun;16(3):337–345. doi: 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Physiol 2003;284(1):L84–L84. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 19.Paschoal VA, Vinolo MA, Crisma AR, Magdalon J, Curi R. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid differentially modulate rat neutrophil function in vitro. Lipids 2013;48(2):93–103. doi: 10.1007/s11745-012-3726-6. [DOI] [PubMed] [Google Scholar]
- 20.Sherrington EJ, Sanderson P, Calder PC. The effect of dietary lipid manipulation on macrophage cell surface molecule expression. Biochemical Society Transactions. 1995;23(2):272S–272S. doi: 10.1042/bst023272s. [DOI] [PubMed] [Google Scholar]
- 21.Silveira TM, Sousa JB, Stringhini ML, Freitas AT, Melo PG. Nutritional assessment and hand grip strength of candidates for surgery of the gastrointestinal tract. Arq Bras Cir Dig. 2014 Apr-Jun;27(2):104–108. doi: 10.1590/S0102-67202014000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suchner U, Kuhn KS, Fürst P. The scientific basis of immunonutrition. Proc Nutr Soc 2000;59(4):553–563. doi: 10.1017/S0029665100000793. [DOI] [PubMed] [Google Scholar]
- 23.Thieme RD, Cutchma G, Chieferdecker ME, Campos AC. Nutritional risk index is predictor of postoperative complications in operations of digestive system or abdominal wall? Arq Bras Cir Dig. 2013 Nov-Dec;26(4):286–292. doi: 10.1590/s0102-67202013000400007. [DOI] [PubMed] [Google Scholar]
- 24.Turnock A, Calder PC, West AL, Izzard M, Morton RP, Plank LD. Perioperative Immunonutrition in Well-Nourished Patients Undergoing Surgery for Head and Neck Cancer: Evaluation of Inflammatory and Immunologic Outcomes. Nutrients 2013;5(4):1186–1199. doi: 10.3390/nu5041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract. 2009 Aug-Sep;24(4):487–499. doi: 10.1177/0884533609339071. [DOI] [PubMed] [Google Scholar]
- 26.Weimann A, Braga M, Harsanyi L, Laviano A, Ljungvist O, Soeters P, Kemen JM, Hiesmayr JM, Horbach T, Kuse ER, Vestweber KH. ESPEN Guidelines on Enteral Nutrition: Surgery including Organ Transplantation. Clinical Nutrition 2006;25(2):224–244. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Werner S, Grose R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 28.Whiting CV, Bland PW, Tarlton JF. Dietary n-3 polyunsaturated fatty acids reduce disease and colonic proinflamamtory cytokines in a mouse model for colitis. Inflamm Bowel Dis 2005;11(4):340–349. doi: 10.1097/01.MIB.0000164016.98913.7c. [DOI] [PubMed] [Google Scholar]
- 29.Woodwoth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model inflammatory colitis. Cancer Res 2010;20(70):7960–7969. doi: 10.1158/0008-5472. [DOI] [PubMed] [Google Scholar]
- 30.Yagaloff KA, Franco L, Simko B, Burghardt B. Essential fatty acids are antogonists of the leukotriene B4 receptor. Prostaglandins, Leukotrienes and Essencial Fatty Acids 1995;52(5):293–297. doi: 10.1016/0952-3278(95)90029-2. [DOI] [PubMed] [Google Scholar]


