Abstract
BACKGROUND
Plasma is commonly used for vitamin K antagonist (VKA) reversal, but observational studies suggest that it is associated with transfusion‐related adverse reactions (e.g., volume overload). However, this issue has not previously been addressed in a randomized controlled trial (RCT).
STUDY DESIGN AND METHODS
Factors associated with volume overload were examined using data from two Phase IIIb RCTs comparing plasma with four‐factor prothrombin complex concentrate (4F‐PCC, Beriplex/Kcentra, CSL Behring) for urgent VKA reversal. VKA‐treated patients with major bleeding (NCT00708435) or requiring an urgent surgical or invasive procedure (NCT00803101) were randomly assigned (1:1) to receive either plasma or 4F‐PCC, concomitant with vitamin K. Adverse events (AEs) and serious AEs were prospectively captured up to Day 10 and 45, respectively. Volume overload predictors were evaluated on a univariate and multivariate basis.
RESULTS
A total of 388 patients (4F‐PCC, n = 191; plasma, n = 197) were enrolled. Volume overload occurred in 34 (9%) patients (4F‐PCC, n = 9; plasma, n = 25). In univariate analyses, use of plasma (vs. 4F‐PCC), use of nonstudy plasma and/or platelets, race, history of congestive heart failure (CHF), and history of renal disease were associated with volume overload. In multivariate analyses, use of plasma (vs. 4F‐PCC), history of CHF, and history of renal disease were independent volume overload predictors. In an additional analysis restricted to volume overload events recorded up to Day 7, only use of plasma (vs. 4F‐PCC) was an independent volume overload predictor.
CONCLUSIONS
After adjusting for other potential risk factors, plasma use was independently associated with a greater risk of volume overload than 4F‐PCC in patients requiring urgent VKA reversal.
ABBREVIATIONS
- 4F‐PCC
four‐factor prothrombin complex concentrate
- AE(s)
adverse event(s)
- CAD
coronary artery disease
- CHF
congestive heart failure
- INR
international normalized ratio
- MedDRA
Medical Dictionary for Regulatory Activities
- RCT(s)
randomized controlled trial(s)
- SAE(s)
serious adverse event(s)
- VKA(s)
vitamin K antagonist(s)
Vitamin K antagonists (VKAs), such as warfarin, are widely used for the primary and secondary prevention of venous and arterial thrombotic events.1 In 2013, approximately 3.4 million patients were prescribed warfarin in the United States.2 While interruption of VKA treatment may be sufficient to prevent excessive bleeding in patients requiring elective surgery, rapid VKA reversal is required before urgent surgical procedures or in patients with acute major bleeding. Major bleeding episodes have been reported in 1.7% to 3.4% of VKA‐treated patients and, in the United States, approximately two‐thirds of the more than 60,000 annual emergency department visits by VKA‐treated patients are associated with acute hemorrhaging.3, 4
Plasma (typically fresh‐frozen plasma) is commonly used for urgent VKA reversal, but its use has been associated with complications such as volume overload.1, 5, 6, 7 This is particularly significant in patients with cardiac or renal disease, who are at increased risk of developing volume overload,8 and in whom volume overload is associated with poorer outcomes.9, 10, 11, 12 In an observational study of 23,347 patients with acute bleeding requiring urgent VKA reversal, the number of plasma units administered was highly correlated with the incidence of volume overload (r > 0.91) and was associated with admission to the intensive care unit, inpatient mortality, and discharges to nursing home facilities and hospices.6 However, interpretation of these results is limited by lack of a control treatment arm.
Current American and European treatment guidelines recommend four‐factor prothrombin complex concentrate (4F‐PCC) for urgent VKA reversal.13, 14, 15 4F‐PCCs are lyophilized, nonactivated concentrates of vitamin K–dependent coagulation factors II, VII, IX, and X and proteins C and S.1, 16 This study analyzed predictors of volume overload based on data collected during two recently completed randomized controlled trials (RCTs) that evaluated the efficacy of 4F‐PCC versus plasma in patients requiring urgent VKA reversal for acute major bleeding or before an urgent surgical or invasive procedure.17, 18
MATERIALS AND METHODS
Study design
This study is a post hoc analysis of patient‐level data collected from two international, multicenter, Phase IIIb, prospective, active‐controlled, open‐label, randomized noninferiority trials. These two RCTs evaluated VKA‐treated patients presenting with acute major bleeding (NCT00708435) or requiring an urgent surgical or invasive procedure (NCT00803101) who were randomly assigned to receive plasma or 4F‐PCC (Beriplex/Kcentra, CSL Behring, Marburg, Germany), concomitant with vitamin K; methods and results have been reported previously.17, 18 Both RCTs were sponsored by CSL Behring, conducted according to local legal requirements and registered at www.clinicaltrials.gov; written informed consent was obtained for all patients.
Participants and interventions
Patients enrolled in the RCTs and considered in this post hoc analysis were at least 18 years of age, were receiving VKA therapy, presented with acute major bleeding or required an urgent surgical or invasive procedure within 24 hours of the start of study treatment infusion, and had an international normalized ratio (INR) of at least 2 during the 3 hours before the start of study treatment.17, 18
On Day 1, patients received a single intravenous infusion of their assigned study treatment (plasma or 4F‐PCC); dose was based on baseline INR and body weight (Table S1, available as supporting information in the online version of this paper).17, 18 4F‐PCC was administered at an infusion rate of not more than 3 international units (IU)/kg/min. In both RCTs, the infusion rate for plasma was at the discretion of the clinical team; however, in the acute bleeding study, an infusion rate of 1 unit per 30‐minute interval was recommended by the protocol. All patients were also to receive vitamin K according to American College of Chest Physicians guidelines (2008)19 or local practice if different.
Data collection
Adverse events (AEs) observed by the investigator or reported by the study patient were prospectively captured and recorded up to Day 10 (visit window Days 7‐11), and coded according to the Medical Dictionary for Regulatory Activities (MedDRA) Version 12.0. Serious AEs (SAEs) were recorded up to Day 45 (visit window Days 43–51). Safety was evaluated by an independent data and safety monitoring board; in addition, SAEs were reviewed by a blinded safety adjudication board. For this post hoc analysis, volume overload events were analyzed by patient (for patients with more than one event, only the first occurring volume overload event was considered), considered up to Day 51, and identified using the following investigator‐defined AE terms: fluid overload, pulmonary edema, cardiac failure congestive, cardiac failure chronic, and cardiac failure. Subsequent to the publication of the results of the acute bleeding study,17 an additional volume overload event, previously omitted from the volume overload event count due to a coding error (but included in the AE count), was reported in the 4F‐PCC group. This event is included in the analysis of fluid overload predictors reported here.
Statistical analysis
Data were analyzed using computer software (SAS, Version 9.3, SAS Institute, Inc., Cary, NC). The analysis was conducted using the intent‐to‐treat safety population, comprising all randomized patients who received any portion of study product; patients with missing values for the potential predictor variables were to be excluded from the analysis. No power calculation was performed for this post hoc analysis. All variables used in the model were dichotomous except for age, which was used as a continuous variable measured to the nearest year. Potential predictors of volume overload were evaluated on a univariate basis (chi‐square, Wilcoxon rank‐sum tests); variables demonstrating a p value of less than 0.05 for individual prediction of volume overload were entered into a forward stepwise logistic regression algorithm to select the best predictors for the final multivariate model. The selected predictors were then evaluated by multivariate logistic regression using an exact model fitting algorithm where necessary to deal with small stratum sizes. Significant predictors in this model were defined based on a p value of less than 0.05. In addition to the analysis of volume overload events occurring during the full SAE reporting period (up to Day 51), a multivariate analysis was also carried out on volume overload events occurring up to Day 7.
The possible predictors of volume overload evaluated by univariate analysis were: study treatment (plasma vs. 4F‐PCC), race (white vs. nonwhite), history of congestive heart failure (CHF; defined according to MedDRA high‐level group term “heart failures”), history of coronary artery disease (CAD; defined according to MedDRA high‐level group term “coronary artery disorders”), history of renal disease (defined according to MedDRA high‐level term “renal failure and impairment”), age, sex, red blood cell (RBC) usage, use of nonstudy plasma (i.e., any plasma administered in addition to the per‐protocol specified study plasma infusion) or platelets (PLTs), and volume expander usage. RBC usage included RBCs and whole blood. Volume expanders included hetastarch, albumin, gelofusin, dextran, gelafundin, and hyetellose with sodium chloride. Administration of RBCs, nonstudy plasma and/or PLTs, and volume expanders was considered up to 24 hours after study product infusion in patients without volume overload (including transfusions that started before study product infusion and were ongoing at the start of study product infusion) and up to the start day and time of the first volume overload event in patients with volume overload. The multivariate analysis was performed with and without use of nonstudy plasma and/or PLTs as a predictor variable. Use of crystalloids was not analyzed as data were not uniformly captured. An additional analysis also evaluated the potential association between plasma infusion rate and history of CHF, CAD, or renal disease using the Wilcoxon rank‐sum test.
RESULTS
Demographics
The pooled safety population comprised 388 patients (plasma, n = 197; 4F‐PCC, n = 191; Table 1). Baseline data and characteristics were similar between treatment groups. A large proportion of patients in the plasma and 4F‐PCC groups had a prior history of CHF (41.1 and 36.7%, respectively), CAD (50.3 and 50.3%, respectively), and/or renal disease (22.8 and 24.6%, respectively). Baseline characteristics for patients who did or did not experience a volume overload event are also presented in Table 1.
Table 1.
Demographic and baseline characteristics (pooled safety population)a
| Characteristic | Total | Without volume overload | With volume overload | |||
|---|---|---|---|---|---|---|
| Plasma | 4F‐PCC | Plasma | 4F‐PCC | Plasma | 4F‐PCC | |
| (n = 197) | (n = 191) | (n = 172) | (n = 182) | (n = 25) | (n = 9) | |
| Gender | ||||||
| Female | 89 (45.2) | 87 (45.5) | 77 (44.8) | 82 (45.1) | 12 (48.0) | 5 (55.6) |
| Male | 108 (54.8) | 104 (54.5) | 95 (55.2) | 100 (55.0) | 13 (52.0) | 4 (44.4) |
| Age (years) | 68.8 (±13.3) | 69.8 (±13.5) | 68.3 (±13.6) | 69.4 (±13.7) | 72.4 (±9.9) | 76.6 (±9.1) |
| Age group (years) | ||||||
| <65 | 71 (36.0) | 66 (34.6) | 66 (38.4) | 66 (36.3) | 5 (20.0) | 0 |
| ≥65 to <75 | 48 (24.4) | 49 (25.7) | 40 (23.3) | 45 (24.7) | 8 (32.0) | 4 (44.4) |
| ≥75 | 78 (39.6) | 76 (39.8) | 66 (38.4) | 71 (39.0) | 12 (48.0) | 5 (55.6) |
| Race | ||||||
| White | 175 (88.8) | 179 (93.7) | 154 (89.5) | 173 (95.1) | 21 (84.0) | 6 (66.7) |
| Nonwhite | 22 (11.2) | 12 (6.3) | 18 (10.5) | 9 (5.0) | 4 (16.0) | 3 (33.3) |
| History of CHF | 81 (41.1) | 70 (36.7) | 64 (37.2) | 65 (35.7) | 17 (68.0) | 5 (55.6) |
| History of CAD | 99 (50.3) | 96 (50.3) | 82 (47.7) | 91 (50.0) | 17 (68.0) | 5 (55.6) |
| History of renal disease | 45 (22.8) | 47 (24.6) | 32 (18.6) | 44 (24.2) | 13 (52.0) | 3 (33.3) |
Data are reported as number (%) or mean (±SD).
Interventions
The median (range) 4F‐PCC dose, volume, and infusion time were 25 (15.5–50.5) IU/kg, 90 (48–230) mL, and 17 (7–288) minutes, respectively. The median (range) plasma dose, volume, and infusion time were 10 (3.9–17.7) mL/kg, 800 (353–1525) mL, and 120 (22–928) minutes, respectively. The volume of study plasma and 4F‐PCC administered to patients with or without volume overload is presented in Fig. 1. In each treatment group, infusion volumes and rates were similar between patients who did or did not experience a volume overload event. Of note, the median volume of study plasma administered was approximately nine times greater than that of 4F‐PCC.
Figure 1.
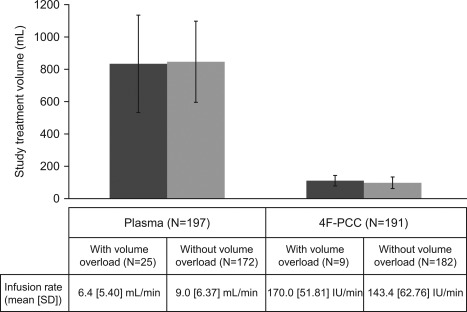
Study product infusion volume and rate in patients with ( ) and without (
) and without ( ) volume overload. Nominal potency of 4F‐PCC is 500 or 1000 IU of Factor IX per vial, approximately 25 IU/mL after reconstitution (range, 20–31 IU/mL; Kcentra Highlights of Prescribing Information, 2014).
) volume overload. Nominal potency of 4F‐PCC is 500 or 1000 IU of Factor IX per vial, approximately 25 IU/mL after reconstitution (range, 20–31 IU/mL; Kcentra Highlights of Prescribing Information, 2014).
Volume overload events
Volume overload occurred in 25 (12.7%) patients in the plasma group and nine (4.7%) patients in the 4F‐PCC group (Tables 1 and 2). Some patients experienced more than one volume overload event during the full SAE reporting period (up to Day 51) and the total number of volume overload events recorded was 40 (plasma, n = 29; 4F‐PCC, n = 11; Table 2). In the 4F‐PCC group “cardiac failure congestive” was the most common volume overload event (6/11; 54.5%) whereas there were no events listed as “fluid overload.” In the plasma group, the most common volume overload event was “(acute) pulmonary edema” (10/29; 34.5%) and seven events (24.1%) were listed as “fluid overload.” The majority (14/25; 56%) of patients with volume overload events in the plasma group received the 10 mL/kg dose. This majority is proportionate to the overall planned dose groups in the study: 62% of patients in the plasma group were assigned to receive 10 mL/kg (Table S2, available as supporting information in the online version of this paper).
Table 2.
Volume overload events
| Patient | Age (years) | Onset day | Preferred term | SAE | VKA indication | Actual dose (IU/kg [4F‐PCC] or mL/kg [plasma]) | Death (day) | Relationship of volume overload event to treatmenta | History of disease | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CHF | CAD | Renal disease | |||||||||
| 4F‐PCC group | |||||||||||
| K1 | 85 | 6 | Pulmonary edema | No | AF | 25 | Not related | Yes | Yes | No | |
| 9 | Pulmonary edema | No | Not related | ||||||||
| K2 | 88 | 5 | Pulmonary edema | No | AF | 25 | Not related | No | No | No | |
| K3 | 66 | 4 | CF congestive | No | AF | 25 | Not related | Yes | Yes | Yes | |
| K4 | 75 | 4 | CF | Yes | Cardiomyopathy | 50 | 5 | Not related | No | Yes | No |
| K5 | 69 | 6 | CF congestive | No | AF | 50 | Not related | Yes | No | No | |
| K6 | 72 | 6 | Pulmonary edema | No | AF | 50 | Not related | No | No | Yes | |
| K7 | 67 | 46 | CF congestive | Yes | AF | 35 | Not related | Yes | Yes | No | |
| K8 | 77 | 12 | CF congestive | Yes | AF | 50 | Not related | Yes | Yes | Yes | |
| K9 | 90 | 6 | CF congestive | No | AF | 25 | Not related | No | No | No | |
| 25 | CF congestive | Yes | Not related | ||||||||
| Plasma group | |||||||||||
| P1 | 77 | 3 | Pulmonary edema | No | AF | 10 | Not related | Yes | Yes | Yes | |
| P2 | 70 | 2 | Pulmonary edema | No | AF | 10 | Possibly related | Yes | Yes | Yes | |
| P3 | 74 | 3 | Pulmonary edema | No | AF | 12 | Probably related | No | No | No | |
| P4 | 80 | 2 | Pulmonary edema | No | AF | 10 | Probably related | Yes | Yes | No | |
| P5 | 74 | 3 | Pulmonary edema | No | AF | 10 | Not related | Yes | No | No | |
| P6 | 85 | 4 | Pulmonary edema | No | AF | 12 | 13 | Not related | Yes | Yes | No |
| 4 | CF congestive | Yes | Not related | ||||||||
| P7 | 81 | 2 | Fluid overload | No | AF | 12 | Possibly related | No | No | No | |
| P8 | 65 | 19 | CF congestive | Yes | AF | 10 | Not related | Yes | Yes | Yes | |
| P9 | 80 | 1 | Fluid overload | No | CVA | 10 | Related | Yes | Yes | Yes | |
| 8 | CF chronic | Yes | Not related | ||||||||
| 40 | CF chronic | Yes | Not related | ||||||||
| P10 | 75 | 24 | CF congestive | Yes | AF | 15 | Not related | Yes | Yes | Yes | |
| P11 | 83 | 49 | CF congestive | Yes | AF | 15 | Not related | Yes | Yes | Yes | |
| P12 | 56 | 1 | Fluid overload | No | AF | 12 | Possibly related | Yes | Yes | Yes | |
| P13 | 79 | 2 | Fluid overload | No | AF | 12 | Not related | Yes | Yes | No | |
| P14 | 61 | 2 | Acute pulmonary edema | Yes | AF | 10 | 8 | Related | No | No | No |
| P15 | 69 | 6 | CF congestive | Yes | AF | 10 | Not related | Yes | Yes | No | |
| 31 | CF congestive | Yes | Not related | ||||||||
| P16 | 72 | 9 | Fluid overload | Yes | Embolism | 10 | Possibly related | No | Yes | No | |
| P17 | 79 | 1 | CF congestive | No | Embolism | 15 | Possibly related | Yes | Yes | Yes | |
| P18 | 55 | 3 | Fluid overload | No | AF | 10 | Not related | Yes | No | Yes | |
| P19 | 74 | 5 | Pulmonary edema | No | Atrial flutter | 15 | Related | No | No | Yes | |
| P20 | 77 | 14 | CF | Yes | PE | 10 | Not related | Yes | Yes | Yes | |
| P21 | 76 | 2 | Pulmonary edema | No | DVT | 12 | Possibly related | No | No | Yes | |
| P22 | 63 | 1 | Pulmonary edema | No | AF | 10 | Not related | No | Yes | No | |
| P23 | 66 | 3 | CF congestive | No | AF | 10 | Probably related | No | Yes | No | |
| P24 | 90 | 3 | CF congestive | No | AF | 10 | Possibly related | Yes | Yes | Yes | |
| P25 | 50 | 1 | Fluid overload | No | AF | 12 | Related | Yes | No | No | |
Investigator assessed.
AF = atrial fibrillation; CF = cardiac failure; CVA = cerebrovascular accident; DVT = deep vein thrombosis; PE = pulmonary embolism.
Timings of volume overload events are presented in Fig. 2. The majority of volume overload events occurred within the first 7 days after study product infusion (21/29 [72.4%] in the plasma group and 7/11 [63.6%] in the 4F‐PCC group). There were no volume overload events on Days 1 to 3 in the 4F‐PCC group, compared with 17 events in the plasma group. Fourteen volume overload events in the plasma group (48.3%), and none in the 4F‐PCC group, were considered treatment‐related by the investigator (Fig. 2). Additional details of patients who experienced a volume overload event, including nonstudy product transfusions, are presented in Table S3 (available as supporting information in the online version of this paper). Data regarding plasma infusion rate (Figs. S1 and S2, available as supporting information in the online version of this paper) and use of diuretics (Fig. S3, available as supporting information in the online version of this paper) are also reported in the supplementary materials.
Figure 2.
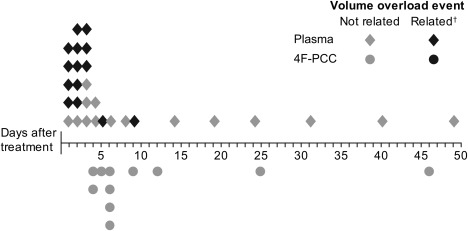
Timing of volume overload event (days). All volume overload events listed for patients with one or more event; some patients had more than one volume overload event; †related defined as volume overload events that were at least possibly related to study treatment in the opinion of the investigator. The majority of volume overload events occurred on Days 1 to 7: 21 of 29 (72.4%) in the plasma group and seven of 11 (63.6%) in the 4F‐PCC group. There were no treatment‐related volume overload events in the 4F‐PCC group, compared with 14 (48.3%) in the plasma group.
Predictors of volume overload (univariate analysis)
Treatment with plasma (vs. 4F‐PCC), race (white vs. nonwhite), use of nonstudy plasma and/or PLTs, history of CHF, and history of renal disease were significantly associated with volume overload (Table 3). All other variables, including history of CAD, RBCs, age, and volume expander usage were not significant (p > 0.05; Table 3).
Table 3.
Predictors of volume overload:a univariate and multivariate analyses
| Predictor | OR | 95% CI | p value |
|---|---|---|---|
| Univariate analysis | |||
| History of renal disease | 3.25 | 1.58, 6.68 | 0.0008b |
| History of CHF | 3.20 | 1.53, 6.68 | 0.001b |
| Plasma vs. 4F‐PCC | 2.93 | 1.33, 6.48 | 0.006b |
| Race (white vs. nonwhite) | 3.14 | 1.25, 7.87 | 0.01b |
| Use of nonstudy plasmac and/or PLTs | 2.53 | 1.14, 5.62 | 0.02b |
| Age | 1.03 | 1.00, 1.06 | 0.05 |
| History of CAD | 1.92 | 0.92, 4.00 | 0.08 |
| RBC usage | 1.53 | 0.75, 3.15 | 0.24 |
| Volume expanderd usage | 2.04 | 0.57, 7.40 | 0.27 |
| Gender (female vs. male) | 1.23 | 0.61, 2.48 | 0.57 |
| Multivariate analysis (with nonstudy plasmac and/or PLTs) | |||
| Plasma vs. 4F‐PCC | 2.74 | 1.21, 6.19 | 0.02† |
| History of renal disease | 2.46 | 1.12, 5.39 | 0.02† |
| History of CHF | 2.36 | 1.07, 5.21 | 0.03† |
| Multivariate analysis (without nonstudy plasmac and/or PLTs) | |||
| Plasma vs. 4F‐PCC | 2.96 | 1.32, 6.62 | 0.008† |
| History of renal disease | 2.58 | 1.19, 5.61 | 0.02† |
| History of CHF | 2.36 | 1.07, 5.18 | 0.03† |
Volume overload events recorded over the entire SAE reporting period (up to Day 51).
Significant p values (p < 0.05).
That is, any plasma administered in addition to the per‐protocol specified study plasma infusion.
Volume expanders included hetastarch, albumin, gelofusin, dextran, gelafundin, and hyetellose with sodium chloride. Table ordered by increasing p values within each analysis section.
Predictors of volume overload (multivariate analyses)
Plasma versus 4F‐PCC use, history of CHF, and history of renal disease were the only significant predictors of volume overload (p < 0.05; Table 3) regardless of the model used for the analysis (i.e., with or without use of nonstudy plasma and/or PLTs in the model). All other variables, including use of nonstudy plasma and/or PLTs and race, were not significant (p > 0.05; data not shown).
An additional post hoc analysis, restricted to volume overload events that occurred up to Day 7 (4F‐PCC, n = 7; plasma, n = 21), was conducted. In multivariate analyses, only use of plasma (vs. 4F‐PCC) was found to be a predictor of volume overload in this time‐restricted model.
DISCUSSION
This study used a post hoc statistical analysis approach to identify factors associated with volume overload, based on data collected in two RCTs that compared plasma and 4F‐PCC for urgent VKA reversal. Volume overload occurred in 25 of 197 (12.7%) patients in the plasma group and nine of 191 (4.7%) patients in the 4F‐PCC group. Based on multivariate analysis, use of plasma versus 4F‐PCC, history of CHF, and history of renal disease were the only significant predictors of volume overload, regardless of the model used. Although use of nonstudy plasma and/or PLTs and race were risk factors for volume overload in univariate analyses, these effects were nonsignificant after controlling for medical comorbidities.
Based on the estimated number of patients prescribed warfarin each year, and the percentage of those patients affected by major bleeding episodes,2, 3 approximately 57,000 to 116,000 VKA‐treated patients could experience major bleeding episodes requiring urgent VKA reversal every year, an estimate that does not take into account patients who require urgent VKA reversal before emergency surgery. Understanding factors that may be associated with development of volume overload following VKA reversal therapy would thus be highly relevant to a large number of patients and physicians. In addition, the identification of alternative VKA reversal agents that are at least as effective as plasma, but potentially associated with a lower risk of volume overload, is of particular importance. 4F‐PCC has been shown in RCTs to be an effective alternative to plasma for VKA reversal in patients with acute major bleeding or in need of an urgent surgical or invasive procedure. Although these RCTs were not powered for safety, the safety profile of 4F‐PCC was found to be generally similar to that of plasma and no significant differences in the incidence of AEs, SAEs, thromboembolic events, and deaths were observed between treatment groups.17, 18
The main post hoc analysis included volume overload events reported over the entire SAE reporting period. However, when evaluating VKA reversal agents as potential predictors of volume overload, it may be more relevant to assess only those volume overload events that occurred soon after product infusion. In the additional post hoc analysis restricted to volume overload events that occurred up to Day 7, only use of plasma (vs. 4F‐PCC) was found to be a predictor of volume overload. In this time‐restricted model, history of CHF and renal disease were no longer found to be significant. It may be that this loss of significance is due to the reduced statistical power (smaller number of events). Despite this loss of significance, it should be noted that a large proportion of patients who developed volume overload during this 7‐day period had a history for at least one of the three comorbidities (CHF, CAD, or renal disease; five of seven [71.4%] patients in the 4F‐PCC group and 17 of 21 [81.0%] patients in the plasma group; Table 2).
It is of interest to understand why certain patients in the 4F‐PCC group experienced volume overload. It is possible that pre‐existing comorbidities predisposed these patients to a volume overload event.20, 21 In this regard, it is notable that the predominant indication for VKA treatment in these patients was atrial fibrillation (Table 2), a medical condition that is known to be associated with CHF,22 which was, in turn, the most common volume overload event listing in these patients (six of 11 [54.5%] volume overload events; Table 2). Additionally, most of these patients were elderly (mean [±SD] age, 76.6 [±9.1] years) with a relatively long history of cardiac problems (mean [±SD], 3.6 [±1.8] years). Although blood product usage might be expected to be a contributing factor to volume overload, this was not found to be the case in this study; the effect of using RBCs was nonsignificant in the univariate analysis and the effect of using nonstudy plasma and/or PLTs, although significant in the univariate analysis, was nonsignificant in the multivariate analysis.
Diuretics are commonly given to patients at high risk of volume overload, such as the elderly and patients with CHF, CAD, renal dysfunction, pulmonary disorders, and/or a previous history of volume overload. In this study, a higher proportion of plasma‐treated patients with a history of CHF, CAD, or renal disease were given diuretics during VKA reversal, compared with patients who did not have such comorbidities (Fig. S3). Similar observations were made for 4F‐PCC–treated patients (Fig. S3). Despite these preventative measures, it should be noted that a large proportion of the patients who developed volume overload had received diuretics (21/25 [84.0%] and seven of nine [77.8%] in the plasma and 4F‐PCC groups, respectively), illustrating the fragile fluid balance that these patients need to maintain.
In practice, plasma is usually administered at lower rates in patients with a high risk of volume overload. In this study, no significant differences in the plasma infusion rate were observed in all plasma‐treated patients based on their prior history of disease (Fig. S2). However, lower infusion rates were observed in plasma‐treated patients with a history of CHF, CAD, or renal disease who developed fluid overload compared with those who did not. Volume overload events in the plasma group occurred over a large range of plasma infusion rates and volumes (Fig. S1), indicating that these factors may not have been as relevant to the onset of volume overload in our studies compared with previously published results.5 Alternatively, local providers may have adjusted the infusion rate based on information that was not captured, such as patient response to the initial unit of plasma, physical examination findings, or other data; as a result, they may have provided more rapid infusion to lower risk patients and slower infusions for higher risk patients.
One of the study limitations is that this is a post hoc analysis. Furthermore, the RCTs used for this analysis were designed as efficacy trials, and not safety studies, and were therefore not powered to analyze between‐treatment group differences in terms of safety outcomes. However, this limitation would not apply in cases where significant between‐group differences were observed. Finally, although this analysis did take into account the transfusion of nonstudy fluids such as RBCs, nonstudy plasma, PLTs, and colloids, it could not account for all fluids administered (e.g., crystalloids) as data were not uniformly captured.
In summary, this study shows that the use of plasma (vs. 4F‐PCC), history of CHF, and history of renal disease appear to be independently predictive of volume overload. Careful consideration should be given to the use of plasma for urgent VKA reversal and alternative VKA reversal therapies, such as 4F‐PCC, should be considered.
CONFLICT OF INTEREST
MR—consultancy (CSL Behring), membership on an entity's board of directors, speakers bureau, or its advisory committees (CSL Behring); JG—consultancy (CSL Behring), research funding (CSL Behring); ML—consultancy (CSL Behring); BD—employment (CSL Behring); TJM—consultancy (CSL Behring), membership on an entity's board of directors, speakers bureau, or its advisory committees (CSL Behring); and RS—consultancy (CSL Behring), honoraria directly received from an entity (CSL Behring)
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1. Dose of study treatment per baseline INR
Table S2. Planned dose groups (pooled safety population)
Table S3. Details of patients with volume overload (pooled safety population)
Fig. S1. Plot of study plasma infusion rate versus total volume for plasma‐treated patients with and without volume overload
Fig. S2. Infusion rate distribution versus history of disease
Fig S3. Proportion of patients receiving diuretics based on prior history of CHF, CAD, or renal disease
ACKNOWLEDGMENT
Medical writing assistance was provided by Chrystelle Rasamison of Fishawack Communications Ltd, funded by the sponsor.
The study was sponsored by CSL Behring. The sponsor was responsible for data collection, management, and analysis of the data according to a predefined statistical analysis plan. Preparation and review of the manuscript as well as decision to submit the manuscript for publication was performed by a publication steering committee that included academic medical experts and representatives of the sponsor.
* Address reprint requests to: Kevin Kovaleski, CSL Behring, 1020 First Avenue, PO Box 61501, King of Prussia, PA 19406; e‐mail: Kevin.Kovaleski@cslbehring.com.
REFERENCES
- 1. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e44S‐88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US National Prescription Audit . Parsippany (NJ): IMS Institute for Healthcare Informatics; 2013.
- 3. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:257S–98S. [DOI] [PubMed] [Google Scholar]
- 4. Shehab N, Sperling LS, Kegler SR, et al. National estimates of emergency department visits for hemorrhage‐related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med 2010;170:1926–33. [DOI] [PubMed] [Google Scholar]
- 5. Li G, Rachmale S, Kojicic M, et al. Incidence and transfusion risk factors for transfusion‐associated circulatory overload among medical intensive care unit patients. Transfusion 2011;51:338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magee G, Peters C, Zbrozek A. Analysis of inpatient use of fresh frozen plasma and other therapies and associated outcomes in patients with major bleeds from vitamin K antagonism. Clin Ther 2013;35:1432–43. [DOI] [PubMed] [Google Scholar]
- 7. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion 2012;52 Suppl 1:65S–79S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy EL, Kwaan N, Looney MR, et al. Risk factors and outcomes in transfusion‐associated circulatory overload. Am J Med 2013;126:357.e29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bagshaw SM, Cruz DN. Fluid overload as a biomarker of heart failure and acute kidney injury. Contrib Nephrol 2010;164:54–68. [DOI] [PubMed] [Google Scholar]
- 10. Bouchard J, Mehta RL. Fluid balance issues in the critically ill patient. Contrib Nephrol 2010;164:69–78. [DOI] [PubMed] [Google Scholar]
- 11. Costanzo MR, Agostoni P, Marenzi G. Extracorporeal fluid removal in heart failure patients. Contrib Nephrol 2010;164:173–98. [DOI] [PubMed] [Google Scholar]
- 12. Kalantar‐Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long‐term hemodialysis. Circulation 2009;119:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holbrook A, Schulman S, Witt DM, et al. Evidence‐based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141:e152S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin—fourth edition. Br J Haematol 2011;154:311–24. [DOI] [PubMed] [Google Scholar]
- 15. Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013;17:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holland L, Warkentin TE, Refaai M, et al. Suboptimal effect of a three‐factor prothrombin complex concentrate (Profilnine‐SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009;49:1171–7. [DOI] [PubMed] [Google Scholar]
- 17. Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4‐factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma‐controlled, phase IIIb study. Circulation 2013;128:1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldstein JN, Refaai MA, Milling TJ Jr, et al. Four‐factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open‐label, non‐inferiority, randomised trial. Lancet 2015; DOI: http://dx.doi.org/10.1016/S0140-6736(14)61685‐8 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:160S–98S. [DOI] [PubMed] [Google Scholar]
- 20. Bolton‐Maggs PH, Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol 2013;163:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lieberman L, Maskens C, Cserti‐Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion‐associated circulatory overload. Transfus Med Rev 2013;27:206–12. [DOI] [PubMed] [Google Scholar]
- 22. Lubitz SA, Benjamin EJ, Ellinor PT. Atrial fibrillation in congestive heart failure. Heart Fail Clin 2010;6:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1. Dose of study treatment per baseline INR
Table S2. Planned dose groups (pooled safety population)
Table S3. Details of patients with volume overload (pooled safety population)
Fig. S1. Plot of study plasma infusion rate versus total volume for plasma‐treated patients with and without volume overload
Fig. S2. Infusion rate distribution versus history of disease
Fig S3. Proportion of patients receiving diuretics based on prior history of CHF, CAD, or renal disease


