Contents
Ultrasound is one of the most promising forms of non‐invasive contraception and has been studied in several animal models. The objective of the current investigation was to determine the most practical and effective application protocol for dog sterilization. A total of 100 dogs were divided into five equal groups. Group A received 5‐min applications three times performed at 48‐hr intervals and covering the entire testicular area at frequency of 1 MHz; Group B received 5‐min applications three times performed at 48‐hr intervals over the dorso‐cranial area of the testis at frequency of 3 MHz; Group C received three sequential 5‐min applications (at 5‐min intervals between applications) covering the entire testicular area at frequency of 1 MHz; Group D received 15‐min applications two times performed at 48‐hr intervals and covering the entire testicular area at frequency of 1 MHz. The experimental groups' ultrasound had an intensity of 1.5W/cm2. The Control Group had the same procedure as Group A, but with the transducer switched‐off. Dogs were surgically castrated 40 days following the treatment for histological examination. Azoospermia, testicular volume reduction and apparently irreversible testicular damage were achieved by Group A. No effects were noticed in the other groups. Testosterone levels remained within physiological range with all application protocols. A regimen of three applications of ultrasound at 1 MHz, and 1.5 W/cm2, lasting 5 min with an interval of 48 h was effective as permanent sterilization in the dog without hormonal impact.
Introduction
Canine overpopulation has become a global problem with significant public health impacts. Current population control methods are expensive and/or have questionable efficacy, with continued reliance on euthanasia of large numbers of animals. Male dog contraceptive approaches should be long‐acting or irreversible, highly effective and safe; produce few or no side effects; require limited or no need for significant action to be applied; necessitate no continuing treatment; and be low cost (Verstegen 2010). Other desirable qualities would include no need for invasive surgical procedures or hormonal treatments.
Ultrasound (U/S) is a promising canine contraceptive. U/S consists of inaudible high frequency (greater than 20 kHz) mechanical vibrations (pressure waves). The waves are transmitted by propagation through molecular collision and vibration, with a progressive loss of the intensity of the energy during passage through the tissue. When absorbed by tissue, U/S energy waves are converted to heat and it is very effective in deep heating. The temperature of the scrotal testis of mammals is normally several degrees (3–4°C) below that of the body core. An elevation of testicular temperature results in impairment of spermatogenesis (Jung and Schuppe 2007). Thus, it is possible to affect sperm production by raising testicular temperature.
Another effect of U/S is mechanical. U/S markedly alters the permeability of the membrane to ions and other substances: U/S increased the sodium concentration and decreased the potassium concentration in the fluid of the seminiferous tubules and decreased the sodium concentration and increased the potassium concentration in the fluid of the rete testis (Fahim 1978). Substances passing from the blood into the seminiferous tubules must pass through the walls of the testicular capillaries, through the tubular wall or into the rete testis and then into the tubular lumen (Setchell 1974). Transport through the capillary wall is passive but is comparatively unrestricted. In contrast, after U/S, there is great variation in the rate at which substances enter the seminiferous tubules and, for some substances, specific transport systems may be involved. These actions have been shown to impact spermatogenesis.
Early research on the use of U/S as a suppressant of spermatogenesis found it to be effective even with a small increase in temperature because of its combined thermal and mechanical effects (Fahim et al. 1977).
Ultrasound's potential as a male dog contraceptive was first reported in 1975 (Fahim et al. 1975). In a series of publications in the 1970s by Fahim and colleagues, it was shown that a single application of U/S to the testes could result in a dramatic loss of germ cells that was reversible. No notable side effects other than infertility were reported during studies with rats, dogs and monkeys (Fahim et al. 1977). This method was tested on several human subjects, and the authors reported that U/S is a pain‐free procedure that only creates a gentle feeling of warmth (Fahim et al. 1977; Fahim 1978, 1980).
The literature over the years highlights different effects on fertility among species.
From the studies conducted to date, U/S treatment appears to have a transient contraceptive effect in human and non‐human primates (VandeVoort and Tollner 2012), while in rats (Tsuruta et al. 2012) and dogs (Leoci et al. 2009, 2011), U/S seems to be permanent at the dosages used. Apart from testicular size, frequency and duration also varied among studies. Leoci reported that in dogs treated with three applications of U/S at an intensity setting of 1.5 Watt/cm2, a treatment frequency of 1 MHz on alternating days for 5 min on each testicle was required to obtain suppression of spermatogenesis, but no histological examination was performed. Tsuruta reported that in rats treated with one application of 10 min, treatment frequency of 1 MHz or 3 MHz, and intensity setting of 1–2.2 W/cm2, therapeutic U/S was capable of inducing a nearly complete loss of germ cells. VandeVoort treated four monkeys at 2.5 W/cm2 for 30 min every other day for three treatments total, which resulted in a 91% decrease in sperm count when applied through saline but not for application directly to the testicles.
Despite previous promising studies, a standard U/S treatment for contraceptive purposes has not yet been identified, which has limited the usefulness of this approach. Identification of the treatment parameters, including the smallest number of applications needed, the shortest interval among applications, the most effective area of the testis to treat, the speed of the sound head, and the ultrasonic variables (frequency, intensity, duty cycle, etc.), to produce durable contraception has yet to be determined.
Thus, the objective of this study was to determine the optimal treatment regime for permanent canine sterilization and identify the nature of testicular tissue damage. Our focus was to find a method that was effective yet easy to perform by investigating various combinations of treatment duration, application number, intensity and frequency parameters.
Materials and Methods
Animals
For the study, 100 mature, healthy mixed‐breed male dogs, 3–6 years of age (mean = 4.5 years, SD = 1.1 years), weighing 24–39 kilograms (kg) (mean = 32.3 kg, SD = 4.2 kg) were selected. They were housed indoors at night and had access to outdoor runs each day. They were fed a commercially available ration once per day and had access to water ad libitum. The study was conducted at a private shelter with cooperation of the shelter owner. Dogs remained at the shelter throughout the study.
Prior to initiation of the study, potential subjects were given a complete physical examination, including palpation of the testes and bloodwork, and at least three semen evaluations. Dogs that produced ejaculates with at least 80% sperm motility and 80% morphologically normal sperm cells were selected for the study. The pre‐treatment characteristics of the ejaculates of the selected animals were subsequently used to define ‘normal ejaculates’ for comparison with samples taken after the ultrasonic treatments.
Investigations were conducted in accordance with the Principles for the Care and Use of Research Animals, promulgated by the European Union. The Italian Ministry of Health (Progetto di Ricerca Corrente 2009 IZS SI 11/09: ‘Randagismo applicazione e valutazione di metodi innovativi per il controllo delle nascite’) approved this study.
Experiment
At day 0 (T0), the testicles were scanned by ultrasonography and testicular volume was calculated. Semen collection and blood samples were performed to check semen quality, semen volume and blood testosterone concentration. Dogs were randomly divided into five equal groups (n = 20) named A, B, C, D and Control Group. Each group was similar in terms of age and weight. Both testes of each subject were exposed to U/S as indicated in Table 1. Dogs in the Control Group received similar treatment as Group A with the probe of a switched‐off device to control for any effect of handling and the procedure.
Table 1.
Details on U/S treatments in each experimental group
| Control | Group A | Group B | Group C | Group D | |
|---|---|---|---|---|---|
| Weight | 32.7 ± 3.91 | 32.7 ± 4.08 | 32.3 ± 4.25 | 33.0 ± 4.39 | 31.1 ± 4.59 |
| Age | 4.4 ± 0.99 | 4.5 ± 1.10 | 4.5 ± 1.19 | 4.6 ± 1.05 | 4.5 ± 1.15 |
| U/S Power (W/cm2) | NA | 1.5 | 1.5 | 1.5 | 1.5 |
| U/S Frequency (MHz) | NA | 1 | 3 | 1 | 1 |
| Length of application (min) | 5 | 5 | 5 | 5 | 15 |
| Number of applications | 3 | 3 | 3 | 3 | 2 |
| Interval between applications | 48 h | 48 h | 48 h | 5 min | 48 h |
| Testicular surface treated | All over | All over | Dorso‐cranial area | All over | All over |
| Transducer size (cm2) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
NA, not available.
Dogs of Group B received U/S at a different frequency. Frequency determines depth of penetration. A frequency of 1 MHz heats at a depth of 2–5 cm (Shulthies 1995). As the frequency increases, penetration decreases. Therefore, 3 MHz U/S has a more superficial beam. Our intent with this group was to damage the testicular dorso‐cranial area where ductus deferens are located.
At day 30 (T1), testicular measurement, sperm evaluation and blood testosterone were performed. At days 40‐47 (T2), dogs were surgically castrated and testicles were collected for histological evaluation. Throughout the trial, the dogs were under clinical observation.
Ultrasound treatment
Subjects were positioned awake in right lateral recumbency using manual restraint. To facilitate U/S penetration into underlying tissues, scrotal hairs were clipped and a water soluble U/S gel was spread over the skin just before placing the probe on the surface of the gonads. The U/S transducer was applied to the skin with circular movements. Both testes of each subject were exposed to U/S (Vetri‐son Clinic, Physiomed® Elektromedizin AG, Shnaittack, Germany: 2.5 cm2 transducer).
Morphometric procedures
Testicular size and the presence of any tenderness were examined before and 4 weeks after the treatment. Testicular length (L) and width (Wi) were echographically measured (Sonoace Pico Medison‐Korea) and saved on removable hard disk for further evaluations. Testicular volume was calculated according to the formula for a prolate spheroid: L Wi2 0.52 (Paltiel et al. 2002).
Assay for serum testosterone
At the T0 and T1 time points, blood was collected from the saphenous vein of each dog between 8:00 a.m. and 8:30 a.m. A portion of blood was allowed to stand for 10‐15 min at 4°C, and then centrifuged at 1.500 × g for 10 min at 4°C prior to aspiration of serum. Serum samples were stored at −20°C until thawed and assayed for testosterone concentrations by a chemiluminescence technique (Immulite Immunoassay System, Siemens Healthcare, Munich, Germany).
Semen collection and evaluation
Semen was collected by digital manipulation in the presence of a teaser bitch using a plastic semen collection cone (Artificial vagina: IMV Technologies, Italia) (Freshman 2002).
Sterile centrifuge tubes were used to collect semen as it was ejaculated in the three fractions (Kutzler 2005). The second sperm‐rich fraction of the ejaculate was immediately examined using an integrated visual optical system for semen analysis (IVOS version 12.2; Hamilton Thorne Biosciences Inc., Beverly, MA, USA). A sperm drop was mounted on a 4‐chamber Leja® slide at 37°C, and six different randomly selected microscopic fields were counted. Each sample was measured for volume, motility (%), sperm concentration and abnormal morphology (%) (Rijsselaere et al. 2012) (Table 2).
Table 2.
Effect of U/S treatment on sperm quality
| Ultrasonic treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Semen volume (ml)a | Sperm concentration (× 106 spz/ml) | Motility (%) | Abnormal sperm (%) | |||||
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |
| Control | 2.71 ± 0.65 | 2.61 ± 0.66 | 704.25 ± 105.30 | 676.45 ± 135.88 | 86.15 ± 3.09 | 84.98 ± 3.59 | 2.85 ± 1.53 | 2.65 ± 1.04 |
| A | 2.56 ± 0.70 | 0b | 719.9 ± 92.5 | 0b | 86.52 ± 3.67 | 0b | 2.95 ± 1.19 | 0b |
| B | 2.49 ± 0.64 | 2.42 ± 0.62 | 692.15 ± 90.68 | 671.90 ± 101.50 | 85.93 ± 2.77 | 83.60 ± 4.59b | 2.50 ± 1.0 | 2.2 ± 0.70 |
| C | 2.70 ± 0.67 | 2.61 ± 0.68 | 662.45 ± 112.69 | 667.55 ± 118.17 | 85.87 ± 3.84 | 84.60 ± 3.91 | 3.00 ± 1.08 | 2.55 ± 0.76 |
| D | 2.67 ± 0.77 | 2.54 ± 0.76 | 664.0 ± 124.81 | 665.15 ± 123.31 | 86.64 ± 3.10 | 84.17 ± 3.66b | 2.70 ± 1.17 | 2.55 ± 1.10 |
Semen characteristics were generally conserved over the treatment period except for Group A at T1.
All values are mean ± SD.
Indicates the volume of the second fraction of the ejaculate.
There is a significant difference from T0 to T1.
Routine clinical observation
All animals were subjected to routine clinical observation during the T0–T3 period: parameters considered included body weight, blood counts, general attitude, appetite, rectal temperature, heart and respiratory rates.
Histological evaluation
Dogs were anesthetized lege artis and surgically castrated. Removed testicles were preserved in Bouin's fixative. Sections obtained were stained with haematoxylin–eosin and observed under light microscopy to describe any histopathology. Representative microscopic fields were selected and digitized using software for image analysis. Morphometric measurements were performed on scanned images to identify and quantify the extent of the damage, by estimating changes in ratios of different cell types (including germ cells and stroma) and between intracellular components (nucleus/cytoplasm, volume and morphology).
Statistical analysis
All data were summarized for each individual animal by parameter measured (age, weight, testosterone level, sperm count, sperm motility, sperm volume, abnormal sperm) and time point (T0–T3) using the Microsoft Excel 2011 program (Microsoft Corporation, Redmond, WA, USA). These data were described in terms of the average and standard deviation (SD) (mean ± SD) in the text for brevity.
Statistical analyses were conducted using Statistica (StatSoft, Inc., Tulsa, OK, USA). Repeated‐measures analysis of variance (anova), with Time as the within factor and Group as the between factor, was used to evaluate the measurements in the five groups across the two time points (T0, T1). Measures were evaluated for violations of assumptions of normality and homogeneity of variance. If the overall test had statistical significance, then planned comparisons were conducted. Dunnett's test for comparison to a Control Group was used, as well as univariate planned comparisons to determine whether the measures changed after treatment and whether the treated groups differed from the Control Group. A two‐tailed significance level of p < 0.05 was identified.
Results
During the experiment, dogs did not show signs of discomfort. No local or systemic adverse effect was noticed. Light scrotal redness was observed in treated groups, which disappeared after 24–48 h. No variations were noticed in body weight, blood counts, urinalysis, rectal temperature, urethral blood discharge, heart and respiratory rates or general attitude through the clinical trial. At the end of the study, Group A dogs' testicles showed homogenous tenderness at palpation.
Testicular volume
Testicular volume was consistent before and after treatment in all groups (range 8604–9294 cm3) except Group A which declined following treatment (see Fig. 1). Statistical analysis revealed a significant Group × Time effect (F = 227.4, p < 0.001). This was due to a significant difference between T0 and T1 for Group A (F = 1185.2, p < 0.001). All other measures were not significantly different from the Control Group.
Figure 1.
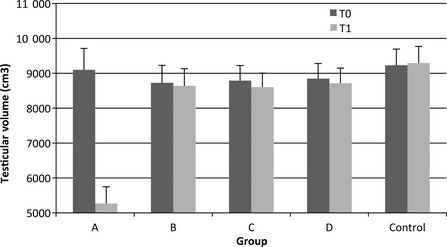
Testicular volume throughout the study. Significant reduction in Group A following treatment
Semen quality
Semen characteristics were generally conserved over the treatment period except for Group A which was azoospermic at T1 (see Table 2). Semen volume ranged from 2.42 to 2.71 ml at other time points, and no significant differences between groups or over time were found except for Group A (comparison of T0–T1: F = 976.03, p < 0.001). Sperm concentration (×106) showed a significant Time × Group effect (F = 167.5, p < 0.001) due to the drop in Group A at T1 as compared to T0 (T = 860.8, p < 0.001). Several measures of sperm motility had a skewed distribution, but this does not have an appreciable effect on the F statistic in anova. The overall Time × Group analysis for motility percentage was significant (F = 2496, p < 0.001). While there were no differences between groups at T0, there were significant decreases in sperm motility at T1 as compared to T0 for Group A (F = 13 013, p < 0.001), Group B (F = 9.48, p < 0.027) and Group D (F = 10.6, p < 0.002). Evaluation of the percentage of abnormal sperm indicated a Time × Group interaction (F = 21.1, p < 0.001). As with most other measures, there was no difference between the groups at T0, but Group A was significantly lower at T1 (F = 127.3, p < 0.001).
Testosterone
Testosterone levels were at physiological range throughout the study (range 1.5–5.0 ng/dl) (Fig. 2). There was no significant difference in testosterone over time and no differences between the Control Group and other groups (all p > 0.05).
Figure 2.
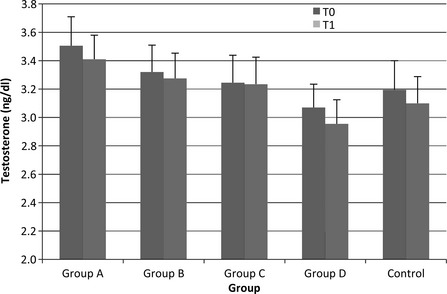
Testosterone levels throughout the study. There was no significant difference in testosterone over time and no differences between the Control Group and other groups (all p > 0.05)
Histological evaluation
Macroscopically, testes showed serious hypoplasia in Group A. The histological examination (Fig. 3) revealed general testicular degeneration with a widespread tubular atrophy and a significant decrease of the testicular parenchyma. Seminiferous tubules were covered by one or two rows of cells with epithelial vacuolization. The basal membrane appeared with slightly reduced diameter and irregular profile. Sertoli cells appeared normal, while irreversible damage was due to the complete lack of spermatogonia and results in azoospermia.
Figure 3.
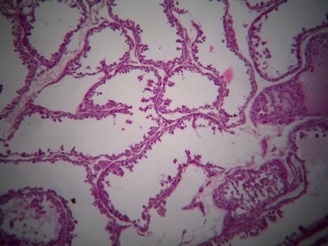
General testicular degeneration with a widespread tubular atrophy and a significant decrease of the testicular parenchyma. Seminiferous tubules were covered by one or two rows of cells with epithelial vacuolization. The basal membrane appeared with slightly reduced diameter and irregular profile. Sertoli cells appeared normal, while there was a complete lack of spermatogonia
No changes were detectable in Groups B–D and the Control Group (Fig. 4).
Figure 4.
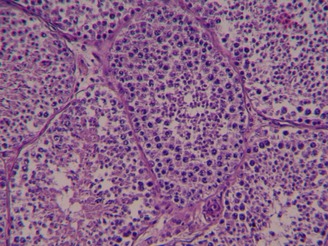
No pathological changes of testicular tissues
Discussion
Results from our study indicate that U/S treatment elicits no local or systemic side effects. The treatment procedure was easy to administer and the dogs remained calm during treatment. As the first study to compare various treatment regimens for canine contraception, we identified the most effective regimen. Group A treatment, which included three 5‐min treatments on alternate days at 1 MHz, 1.5 W/cm2, and treating all of the testicular surface, led to azoospermia and a significant reduction in the volume and consistency of the testicles. Histopathological evaluation indicated that these changes were due to irreversible testes damage and widespread tubular atrophy.
No other combination of U/S treatment variables (frequency, length and number of applications, time between applications) resulted in a similar effect. From our study, it appears that brief treatment needs to be conducted several times over a longer period of time to produce permanent sterilization. While higher intensity results in more serious damage and likely sterilization, this must be balanced against the risk of skin burns and pain.
The mechanism of action of U/S contraception was first supposed by Fahim et al. (1975). They proposed that the combined thermal and mechanical (non‐thermal) effect of U/S application could cause an ion exchange between the fluid in the seminiferous tubules and rete testis, creating an environment not suitable for spermatogenesis which might explain the spermatogenesis suppression we observed. Alternatively, Tsuruta et al. (2012) reported that therapeutic U/S treatment depleted developing germ cells from the testis. They also reported that a combination of elevated temperature, high power and high frequency is the key to reducing sperm count. The right combination of these characteristics is the most challenging aspect of sterilization by U/S. The goal is increasing the testicular temperature and having a mechanical effect on the tissue. But the higher the intensity, the greater and faster the temperature increase. Burns may occur if intensity is too high or the transducer is held stationary, thereby concentrating energy in a small area (Millis et al. 2004).
Generally, intensities required to increase tissue temperature to a range of 40–45°C may vary from 1.0 to 2.0 W/cm2 continuous wave for 5–10 min. To optimize a contraception protocol, U/S intensity and time of application should be considered first. It has been reported that a physiotherapy treatment at the above intensity can last no more than 5 or 10 min or dogs start to whine or act uncomfortable (Millis et al. 2004). Protocols used in this study did not cause pain or discomfort even with longer applications as U/S was not continuous. However, discomfort is not easily evaluated and may arise from prolonged restrain.
In our experience, a relevant point was how to cause a homogeneous temperature increase. We treated the entire testicular surface for the chosen amount of time, but the testicle size was not considered. Longer treatment time was possible in the rhesus monkey, which has larger testicles, and an alternative treatment method of applying the probe to a saline‐filled cup holding the testicle was found to be more effective in decreasing sperm (VandeVoort and Tollner 2012). Future studies may evaluate the correlation between treatment duration and application method with testicular size to clarify this relationship.
This study shows that sterilization by U/S is a feasible alternative to traditional surgery. Depleting spermatocytes and spermatids from testes non‐invasively with therapeutic U/S has multiple applications. By histological examination, the effect seems to be irreversible, but more studies on long‐term sperm production should be considered. If the method proves to be reversible, it would provide a new method to be used in breeding dogs where temporary contraception is desired and preservation of testosterone is required. On the contrary, if the damage proves to be irreversible as the testis damage in our histological examination seems to indicate, a new approach to sterilizing dogs non‐invasively will be available. This may appeal to many dog owners who are reluctant to consider surgery (Soto et al. 2005).
A negative aspect of the application of U/S as an irreversible method for dog population control is that it may not be practical. U/S is not feasible for large‐scale stray dog population control because the effective protocol identified in this study is too time intensive. Stray dogs would have to be kept in shelters for at least 5 days to be treated three times in a week. Moreover, this treatment method does not affect testosterone production by Leydig cells. Histological examination showed no damage to this cell type and also blood testosterone remained within physiological range for all subjects, in agreement with previous work by Fahim et al. (1977). Thus, U/S may not be a useful sterilization method when reduction of marking and aggression is also desired, but may be an appealing alternative for working‐dog owners who do not want the behaviour change accompanying loss of testosterone that occurs with traditional neutering. Additionally, more dog owners are turning to hormone‐sparing sterilization methods to avoid long‐term health problems, such as joint disorders and cancer (Torres de la Riva et al. 2013).
Conclusions
Three treatments 48 h apart at 1 MHz, 1.5 W/cm2 lasting 5 min each, treating the entire testicular area, lead to irreversible testis damage and azoospermia in the dog. No notable side effects other than infertility and no influence on testosterone production were reported during this study. Confirming its apparent permanence through longer observational studies is the next required step to establish whether therapeutic U/S can serve as the basis for a new, irreversible male dog contraceptive.
Conflict of interest
The authors declare that they have no competing interests.
Authors contributions
RL was involved in the concept and design of the study, interpretation of results, semen sampling and evaluation, and preparation of this manuscript. RL performed the treatments. RL, GA and FS performed the ultrasonography. FS performed the acquisition of data. RL, GML and EL were involved in revision of the manuscript. All authors have read and approved the manuscript.
Acknowledgements
This work was supported by Parsemus Foundation. The authors are deeply grateful to Parsemus Foundation, Berkeley, CA, USA, for financial assistance, continuous support and interest in this study. Also, the authors sincerely acknowledge Linda Brent for analysis and interpretation of data and language revision. The authors are also grateful to Physiomed Elektromedizin AG, Germany, for supplying Vetrison® and for technical support.
References
- Fahim MS, 1978: Apparatus useful in suppression of spermatogenesis. U.S.P.T.O, US Patent No. 4,078,556. Available: http://www.freepatentsonline.com/4078556.html (accessed 28 March 2015)
- Fahim MS, 1980. Male fertility regulation by means of ultrasound In Hafez ESE, Schill WB, Cunningham GR. (eds), Regulation of Male Fertility. The Haguel, Boston London, 5:234. [Google Scholar]
- Fahim MS, Fahim Z, Der R, Hail DG, Harma J, 1975: Heat in male contraception (hot water 60 degrees C, infrared, microwave, and ultrasound). Contraception 11, 549–562. [DOI] [PubMed] [Google Scholar]
- Fahim MS, Fahim Z, Harman J, Thompson I, Montie J, Hall DG, 1977: Ultrasound as a new method of male contraception. Fertil Steril 28, 823–831. [PubMed] [Google Scholar]
- Freshman JL, 2002: Semen collection and evaluation. Clin Technol Small Anim Pract 17, 7. [DOI] [PubMed] [Google Scholar]
- Jung A, Schuppe HC, 2007: Influence of genital heat stress on semen quality in humans. Andrologia 39, 203–215. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, 2005: Semen collection in the dog. Theriogenology 64, 747–754. [DOI] [PubMed] [Google Scholar]
- Leoci R, Aiudi G, De Sandro SA, Silvestre F, Binetti F, Lacalandra GM, 2009: Ultrasound as a mechanical method for male dog contraception. Reprod Dom Anim 44, 326–328. [DOI] [PubMed] [Google Scholar]
- Leoci R, Aiudi G, Silvestre F, Forte A, Lacalandra GM, 2011: Dog mechanical castration by ultrasound: comparison of application protocols. IX National S.I.R.A. Congress, Bari, Italy: 23rd‐24th June. Il Qudrifoglio, Bari, 178–181. [Google Scholar]
- Millis D, Levine D, Taylor M, 2004: Therapeutic modalities In: Sounders WB. (ed), Canine Rehabilitation and Physical Therapy. Elsevier, Philadelphia, pp 328 [Google Scholar]
- Paltiel HJ, Diamond DA, Di Canzio J, Zurakowski D, Borer JG, Atala A, 2002: Testicular volume: comparison of orchidometer and US measurements in dogs. Radiology 222, 114–119. [DOI] [PubMed] [Google Scholar]
- Rijsselaere T, Van Soom A, Maes D, Nizanski W, 2012: Computer‐assisted sperm analysis in dogs and cats: an update after 20 years. Reprod Dom Anim 47, 204–207. [DOI] [PubMed] [Google Scholar]
- Setchell BP, 1974: The entry of substances into seminiferous tubules ln: Mancini RE, Martini L. (eds), Male Fertility and Sterility. Academic, New York, pp. 37. [Google Scholar]
- Shulthies SS, 1995: Interview with Dr David O Draper. Sports Phys Ther Sect Newslett,Am Phys Ther Assoc, Winter pp. 12–13 [Google Scholar]
- Soto FRM, Ferreira F, Pinheiro SR, Nogari F, Risseto MR, de Souza O, Amaku M, 2005: Adoption of shelter dogs in a Brazilian community: assessing the caretaker profile. J Appl Anim Welf Sci 8, 105–116. [DOI] [PubMed] [Google Scholar]
- Torres de la Riva G, Hart BL, Farver TB, Oberbauer AM, Messam LLM, Willits N, Hart LA, 2013: Neutering Dogs: effects on Joint Disorders and Cancers in Golden Retrievers. PLoS ONE 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta JK, Dayton PA, Gallippi CM, O'Rand MG, Streicker MA, Gessner RC, Gregory TS, Silva EJR, Hamil KG, Moser GJ, Sokal DC, 2012: Therapeutic ultrasound as a potential male contraceptive: power, frequency and temperature required to deplete rat testes of meiotic cells and epididymides of sperm determined using a commercially available system. Reprod Biol Endocrinol 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeVoort CA, Tollner TL, 2012: The efficacy of ultrasound treatment as a reversible male contraceptive in the rhesus monkey. Reprod Biol Endocrinol 10, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstegen , 2010: Session I: Non‐reproductive Effects of Spaying and Neutering. Proceedings of the Third International Symposium on Non‐Surgical Contraceptive Methods for Pet Population Control. Available: www.acc-d.org (accessed 28 March 2015)


