Abstract
Background:
Renal ischemia-reperfusion injury (IRI) is one of the most important causes of kidney injury, which is possibly gender-related. This study was designed to investigate the role of γ-aminobutyric acid (GABA) against IRI in ovariectomized estradiol-treated rats.
Methods:
Thirty-five ovariectomized Wistar rats were used in six experimental groups. The first three groups did not subject to estradiol treatment and assigned as sham-operated, control, and GABA-treated groups. GABA (50 μmol/kg) and saline were injected in the treated and control groups 30 min before the surgery, respectively. The second three groups received the same treatments but received estradiol valerate (500 μg/kg, intramuscularly) 3 days prior to the surgery. The IRI was induced in the control and treated groups by clamping the renal artery for 45 min and then 24 h of reperfusion. All animals were sacrificed for the measurements.
Results:
The serum levels of creatinine and blood urea nitrogen, kidney weight, and kidney tissue damage score significantly increased in the IRI rats (P < 0.05). GABA significantly decreased the aforementioned parameters (P < 0.05). The uterus weight increased significantly in rats that received estradiol (P < 0.05). Serum and kidney levels of nitrite (nitric oxide metabolite) did not alter significantly. Serum level of malondialdehyde increased significantly in the ovariectomized rats exposed to IRI (P < 0.05).
Conclusions:
It seems that GABA improved IRI in ovariectomized rats. Estradiol was also nephroprotective against IRI. However, co-administration of estradiol and GABA could not protect the kidney against IRI.
Keywords: Estradiol, γ-aminobutyric acid, ovariectomized rats, renal ischemia-reperfusion
INTRODUCTION
Acute kidney injury (AKI) caused by ischemia may lead to the development of chronic kidney disease (CKD).[1] CKD may increase the risk of cardiovascular and cerebral disturbances.[2,3] Kidney ischemia is a common problem in the perioperative period, which increases the rate of morbidity and mortality.[4,5] The condition can also be a consequence of arterial occlusion, shock, and organ transplantation. AKI is accompanied with a complex and interconnected series of events, leading to death of renal cells.[6] Reperfusion is necessary for the survival of ischemic tissue, but causes extracellular injury.[7,8] In addition, ischemia-reperfusion (I/R) disturbs central organ systems such as heart, brain, and gut.[9] It is reported that intravenous treatment with γ-aminobutyric acid (GABA), as a main inhibitory neurotransmitter in the mammalian brain, has inhibitory effect on I/R-induced renal failure in rats.[10,11,12,13,14] Renal sympathetic nerve activity is enhanced during ischemic period,[15,16] and GABA inhibits renal sympathetic nerve activity during ischemia and norepinephrine overflow from renal sympathetic nerve.[16] That is why renal sympathetic hyperactivity is effective in the treatment of kidney ischemia.[15]
Ovariectomy causes greater uterine sympathetic nerve density, and this can be decreased by estradiol administration.[17] Estradiol decreases kidney damage in animal models.[18] It is well-documented that women are more at risk of renal diseases after menopause[19] while male sex hormone, testosterone, is also involved in progression of renal diseases.[20]
The role of GABA against I/R complication in the presence of estradiol is not documented. Therefore, the present study was designed to determine the effect of GABA in ovariectomized rats treated with estradiol and were subjected to I/R.
METHODS
Thirty-five adult female Wistar rats (Animal Centre, Isfahan University of Medical Science) weighing 169.58 ± 2.93 g were used in the study. The rats were housed at the temperature of 23–25°C with a 12-h light/12-h dark cycle and had free access to water and rat chow. The Ethics Committee of Isfahan University of Medical Sciences approved the experimental procedures in advance.
Drugs
GABA (code A2129-10G) and estradiol valerate were provided from Sigma (St. Louis, MO, USA) and Aburaihan Co., (Tehran, Iran), respectively.
Animals
The animals were anesthetized with ketamine (75 mg/kg, i.p). A 2 cm incision was made in the subabdominal area, and the ovaries were removed.
After 1-week of recovery, the animals were randomly assigned to six experimental groups as follows:
Group 1 (n = 5, named OV), received sesame oil and saline (0.5 ml) as negative control group
Group 2 (n = 6, named OVI), treated as Group 1 but 4 days later they were subjected to the I/R surgery
Group 3 (n = 6, named OVIG), treated as Group 2 but received GABA (50 μmol/kg; intravenously) 15 min before I/R surgery
Group 4 (n = 7, named OVE), received estradiol valerate (500 μg/kg, solved in sesame oil; intramuscularly) and saline (0.5 ml)
Group 5 (n = 6, named OVEI), treated as Group 4 but 4 days later they were subjected to the I/R surgery
Group 6 (n = 6, named OVEIG), received treatment similar to Group 5, and also received GABA (50 μmol/kg; intravenously) 15 min before the I/R surgery.
Ischemia-reperfusion injury
The I/R was induced by bilateral I/R in rat kidneys. To accomplish I/R, the rats were anesthetized by chlorohydrate (450 mg/kg) and were placed on the surgical platform in the dorsal position. The cuts were made on both sides of the dorsal wall of the abdominal cavity. The kidneys were exposed and subjected to ischemia by occluding renal pedicles on both sides for 45 min. Then, the clamp was removed to ensure the establishment of blood flow, and the surgical site was sutured, and the kidneys were allowed to have normal perfusion for 24 h.
After 24 h, the animals were anesthetized again, blood sample was taken via heart puncture, and the animals were sacrificed. The samples were centrifuged, and the serum levels of blood urea nitrogen (BUN), creatinine (Cr), nitrite, and malondialdehyde (MDA) were measured. The kidneys were also removed. The right kidney was homogenized and centrifuged at 15,000 × g for 2 min, and the supernatant was used for MDA and nitrite measurements. The left kidney was fixed in formalin for histopathological investigations.
Measurements
Serum levels of Cr and BUN were measured using quantitative kits (Pars Azmoon, Iran). Serum and kidney levels of nitrite (stable metabolite of nitric oxide [NO]) were measured using an ELISA assay kit (Promega Corporation, USA). Assessment of kidney and serum MDA levels was performed according to the manual method.[21,22]
Histopathological procedures
The kidneys removed were fixed in 10% formalin solution and embedded in paraffin for histopathological staining. Hematoxylin and Eosin staining was applied to examine the tubular injury. The intensity of tissue damage was scored from 1 to 3 while score zero was assigned to the normal tissue without damage. The score was given by a pathologist who was blind to the study protocol.
Statistical analysis
The data are presented as mean ± standard error. The quantitative data in the groups were compared by the one-way analysis of variance, followed by the least significant difference. The Mann–Whitney or Kruskal–Wallis test was used to compare the groups with regard to the pathological damage scores. P < 0.05 was considered as statistically significant.
RESULTS
Effect of ischemia-reperfusion on serum levels of blood urea nitrogen and creatinine
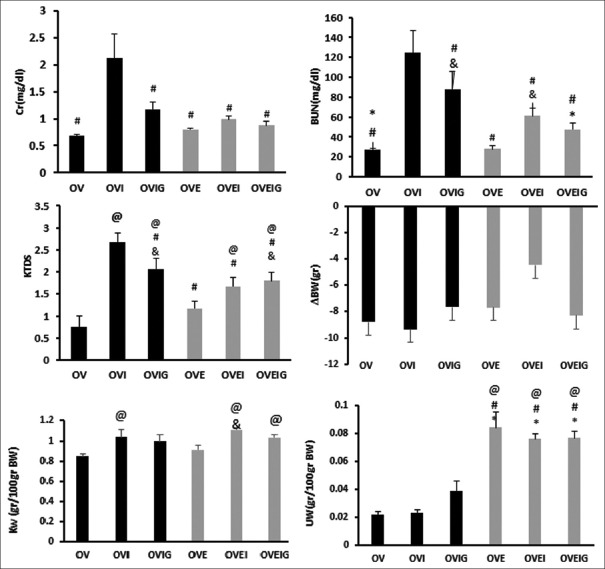
The serum level of Cr and BUN increased significantly in the OVI group when compared with the other groups (P < 0.05). Also, serum level of BUN increased significantly in the OVIG group compared to the OVEIG, OVE, and OV groups (P < 0.05). The data also showed that the serum level of BUN in the OVEI group is statistically higher than that in the OVE group (P < 0.05) [Figure 1].
Figure 1.
Serum levels of blood urea nitrogen (BUN) and creatinine (Cr); kidney weight (KW); body weight change (ΔBW), kidney tissue damage score (KTDS), and uterus weight (UW). The *, #, &, and @ indicate significant difference from the OVIG, OVI, OVE, and OV groups, respectively
Effect of ischemia-reperfusion on kidney tissue damage score
The results showed that the kidney tissue damage score (KTDS) increased significantly in the OVI, OVIG, OVEI, and OVEIG groups compared to the OV group (P < 0.05). As it was observed, estradiol or GABA or both could attenuate the tissue damage induced by the I/R injury. Also, the tissue damage in the OVI group was significantly more severe than that in other groups (P < 0.05). Also, KTDS in the OVIG and OVEIG groups was significantly higher than that obtained for the OVE group (P < 0.05) [Figures 1 and 2].
Figure 2.

Images of kidney tissue (magnification ×400). OV = Ovariectomized + Saline, OVI = Ovariectomized + Ischemia, OVIG = Ovariectomized + Ischemia + GABA, OVE = Ovariectomized + Estradiol, OVEI = Ovariectomized + Estradiol + Ischemia, OVEIG = Ovariectomized + Estradiol + Ischemia+ GABA. Higher tissue damage was observed in the OVI group
Effect of ischemia-reperfusion on body weight, kidney weight, and uterus weight changes
In general, body weight decreased in all the groups, and the groups are not significantly different in this regard. Kidney weight (KW) increased significantly in the OVI, OVEI, and OVEIG groups that underwent ischemia when compared with the OV group (P < 0.05). Also, KW in the OVEI group increased significantly in comparison with the OVE group (P < 0.05).
The results showed that uterus weight (UW) in all estradiol-treated groups (OVE, OVEI, and OVEIG groups) increased significantly compared to the groups that did not received estradiol (OV, OVI, and OVIG groups) (P < 0.05) [Figure 1].
Effect of ischemia-reperfusion on kidney and serum nitrite and malondialdehyde levels
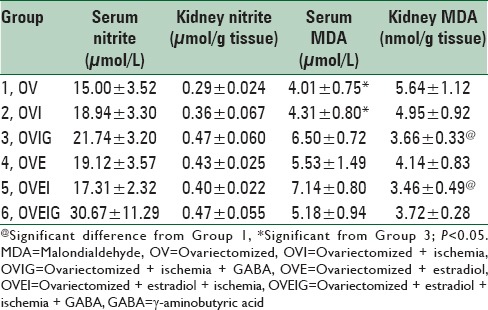
The groups were not significantly different with regard to the changes occurred in the serum and kidney levels of nitrite after the I/R injury [Table 1]. Serum level of MDA increased significantly in the OVIG group compared to the OV and OVI groups (P < 0.05). Kidney level of MDA decreased in groups that received estradiol (OVE, OVEI, and OVEIG). Kidney level of MDA also decreased significantly in the OVIG and OVEI groups when compared with the OV group (P < 0.05) [Table 1].
Table 1.
Serum and kidney levels of nitrite and MDA in the experimental groups
DISCUSSION
In this study, we considered the effect of GABA in kidney ischemia in ovariectomized estradiol-treated rats. The major finding of the study is that the administration of GABA or estradiol alone possibly has better nephroprotective effect against the I/R side effects when compared with co-administration of GABA and estradiol.
Ischemia is one of the most important factors of AKI that leads to kidney cell injury and impairs the kidney function.[23] Free radicals produced by damaged cells[24] through lipid peroxidation of cell and organelle membranes[25] lead to kidney function impairment. It is reported that the primary damage of ischemia is increased by reperfusion in response to the production of free radical.[26] Therefore, the vascular and tubular damages induced by ischemia reduce glomerular filtration rate[27] and change the serum levels of Cr, BUN, nitrite, and MDA.[20,21,22,28,29] In our study, the serum level of Cr increased in the ischemia group, and the level decreased by GABA or estradiol or both. As Kim et al. have mentioned, GABA reduces Cr clearance and prevents progression of renal failure.[11] This is while estrogen could decrease Cr clearance to prevent progression of renal failure.[30] Our results showed that serum level of BUN increased in the groups that were exposed to ischemia; and estrogen, GABA, or both could decrease the serum level of BUN, similar to what Kim et al. reported.[11]
Our results also indicate that KW increased in all the ischemia groups that may be related to retention of water and nutrients in the kidney. This leads to increased kidney weight.[31]
UW increased in all the groups that received estradiol, as the hormone secreted by the ovaries affects the uterus lining and enhances the uterus growth.[32]
The results obtained regarding MDA in the current study is in agreement with the findings of Papaconstantinou et al.[33] MDA is the final product of lipid peroxidation of cell membrane while decreasing the oxidative stress condition.[34] Kidney level of MDA is increased by I/R.[34,35] Deng et al. observed that the reaction between GABA and fatty acids and consumption of GABA can trap the reactive intermediates during lipid peroxidation and reduce the MDA level.[36] Also, Shen et al. reported that the degree of lipid peroxidation (determined by MDA formation within the kidney) is reduced by estrogen.[37]
The nitrite level is important to distinguish the function of NO;[38] 17β-estradiol increases the NO production.[39] In our study, we did not find any significant differences in the nitrite level among the groups. However, the serum and kidney levels of nitrite in the OVIG, OVE, OVEI, and OVEIG groups were higher than those in other groups possibly due to the effect of estradiol, GABA, or both. Finally, there is one limitation in our study. After blood sampling, most animals were died before kidneys were removed. Therefore, the tubules may collapse. However, the conditions for all groups were the same.
CONCLUSIONS
It seems that GABA and estradiol could decrease the injury induced by I/R. However, in the presence of estradiol, GABA may not be nephroprotective against I/R. According to our results, the role of estradiol seems to be more effective than GABA.
ACKNOWLEDGEMENTS
This research was supported by Isfahan University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 2.Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO. Renal ischemia: Does sex matter? Anesth Analg. 2008;107:239–49. doi: 10.1213/ane.0b013e318178ca42. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarten M, Gehr T. Chronic kidney disease: Detection and evaluation. Am Fam Physician. 2011;84:1138–48. [PubMed] [Google Scholar]
- 4.Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: The role of the kidney. Curr Opin Crit Care. 2009;15:481–7. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 5.Gu J, Sun P, Zhao H, Watts HR, Sanders RD, Terrando N, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karaman A, Turkmen E, Gursul C, Tas E, Fadillioglu E. Prevention of renal ischemia/reperfusion-induced injury in rats by leflunomide. Int J Urol. 2006;13:1434–41. doi: 10.1111/j.1442-2042.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh D, Chander V, Chopra K. Protective effect of catechin on ischemia-reperfusion-induced renal injury in rats. Pharmacol Rep. 2005;57:70–6. [PubMed] [Google Scholar]
- 8.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–99. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 9.Daemen MA, van ’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–9. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobuchi S, Tanaka R, Shintani T, Suzuki R, Tsutsui H, Ohkita M, et al. Mechanisms underlying the renoprotective effect of GABA against ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther. 2011;338:767–74. doi: 10.1124/jpet.111.180174. [DOI] [PubMed] [Google Scholar]
- 11.Kim HY, Yokozawa T, Nakagawa T, Sasaki S. Protective effect of gamma-aminobutyric acid against glycerol-induced acute renal failure in rats. Food Chem Toxicol. 2004;42:2009–14. doi: 10.1016/j.fct.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Brar R, Singh JP, Kaur T, Arora S, Singh AP. Role of GABAergic activity of sodium valproate against ischemia-reperfusion-induced acute kidney injury in rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:143–51. doi: 10.1007/s00210-013-0928-2. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: Role of GABA. Am J Physiol. 1998;275:R728–34. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 15.Vink EE, Blankestijn PJ. Evidence and consequences of the central role of the kidneys in the pathophysiology of sympathetic hyperactivity. Frontiers in Physiology. 2012;3:29. doi: 10.3389/fphys.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobuchi S, Shintani T, Sugiura T, Tanaka R, Suzuki R, Tsutsui H, et al. Renoprotective effects of gamma-aminobutyric acid on ischemia/reperfusion-induced renal injury in rats. Eur J Pharmacol. 2009;623:113–8. doi: 10.1016/j.ejphar.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Zoubina EV, Smith PG. Sympathetic hyperinnervation of the uterus in the estrogen receptor alpha knock-out mouse. Neuroscience. 2001;103:237–44. doi: 10.1016/s0306-4522(00)00549-2. [DOI] [PubMed] [Google Scholar]
- 18.Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol. 2012;303:F377–85. doi: 10.1152/ajprenal.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanes LL, Sartori-Valinotti JC, Reckelhoff JF. Sex steroids and renal disease: Lessons from animal studies. Hypertension. 2008;51:976–81. doi: 10.1161/HYPERTENSIONAHA.107.105767. [DOI] [PubMed] [Google Scholar]
- 20.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279:52282–92. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 21.Moeini M, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Azarkish F, et al. Protective role of recombinant human erythropoietin in kidney and lung injury following renal bilateral ischemia-reperfusion in rat model. Int J Prev Med. 2013;4:648–55. [PMC free article] [PubMed] [Google Scholar]
- 22.Azarkish F, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Pezeshki Z, et al. N-acetylcysteine prevents kidney and lung disturbances in renal ischemia/reperfusion injury in rat. Int J Prev Med. 2013;4:1139–46. [PMC free article] [PubMed] [Google Scholar]
- 23.Basile DP. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007;72:151–6. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 24.Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. 2010;55:2024–33. doi: 10.1016/j.jacc.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 25.Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab. 1991;17:124–32. [PubMed] [Google Scholar]
- 26.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 27.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–9. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 28.Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–26. doi: 10.1016/s0022-2143(98)90060-9. [DOI] [PubMed] [Google Scholar]
- 29.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, et al. Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology. 2010;112:395–405. doi: 10.1097/ALN.0b013e3181c98da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 32.Chang CC, Kuan TC, Hsieh YY, Ho YJ, Sun YL, Lin CS. Effects of diosgenin on myometrial matrix metalloproteinase-2 and -9 activity and expression in ovariectomized rats. Int J Biol Sci. 2011;7:837–47. doi: 10.7150/ijbs.7.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: Role of the estrogen receptor. Toxicol Sci. 2000;56:332–9. doi: 10.1093/toxsci/56.2.332. [DOI] [PubMed] [Google Scholar]
- 34.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Paller MS, Neumann TV. Reactive oxygen species and rat renal epithelial cells during hypoxia and reoxygenation. Kidney Int. 1991;40:1041–9. doi: 10.1038/ki.1991.312. [DOI] [PubMed] [Google Scholar]
- 36.Deng Y, Xu L, Zeng X, Li Z, Qin B, He N. New perspective of GABA as an inhibitor of formation of advanced lipoxidation end-products: It's interaction with malondiadehyde. J Biomed Nanotechnol. 2010;6:318–24. doi: 10.1166/jbn.2010.1130. [DOI] [PubMed] [Google Scholar]
- 37.Shen SQ, Zhang Y, Xiong CL. The protective effects of 17beta-estradiol on hepatic ischemia-reperfusion injury in rat model, associated with regulation of heat-shock protein expression. J Surg Res. 2007;140:67–76. doi: 10.1016/j.jss.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: An overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 39.Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori M. Amelioration of ischemia- and reperfusion-induced myocardial injury by 17beta-estradiol: Role of nitric oxide and calcium-activated potassium channels. Circulation. 1997;96:1953–63. doi: 10.1161/01.cir.96.6.1953. [DOI] [PubMed] [Google Scholar]