Abstract
Background:
Silymarin is a flavonoid complex with nephro-protective properties. We evaluated the efficacy of silymarin in the prevention of contrast-induced nephropathy (CIN).
Methods:
This placebo-controlled clinical trial was conducted on 143 patients with chronic stable angina referring for elective coronary angiography. Patients with low to moderate risk for CIN were included and were randomized to receive silymarin (280 mg) or placebo 2 h before administration of the contrast material. A nonionic, iso-osmolar contrast material was used. Serum creatinine was measured before and 48 h after injection of the contrast material. CIN was defined as an increase in creatinine of ≥0.5 mg/dL or ≥25% from the baseline.
Results:
Serum creatinine was increased by 0.02 ± 0.07 mg/dL (P = 0.004) with silymarin and by 0.04 ± 0.15 mg/dL (P = 0.008) with placebo after contrast material injection (between group difference = 0.01 ± 0.02 mg/dL, P = 0.881). CIN was occurred less frequently, though statistically nonsignificant, with silymarin compared with placebo (2.9% vs. 10.8%, Odds ratio [OR] [95% confidence interval (CI)] = 0.246 [0.050–1.203], P = 0.099). In the logistic regression analysis controlling for patients characteristics and baseline creatinine level, silymarin was nonsignificantly associated with lower frequency of CIN (OR [95% CI] = 0.203 [0.037–1.117], P = 0.067).
Conclusions:
We found a trend toward the efficacy of silymarin in preventing contrast-induced renal dysfunction. Further trials with larger sample size and in patients with higher risk of CIN are warranted.
Keywords: Acute kidney injury, contrast media, coronary angiography, herbal medicine, silymarin
INTRODUCTION
Contrast-induced nephropathy (CIN) is a serious complication of angiographic procedures in which impaired renal function occurs within 48–72 h after administration of the intravascular contrast agent.[1] The mechanisms underlying CIN are not completely understood but seem to be multifactorial. Internal factors such as local hypoxia, oxidative stress, and direct cytotoxic effects of the contrast media interact with the external factors such as dehydration and decreased intravascular volume.[2] The incidence of CIN depends on several factors including contrast material type and volume, presence of comorbidities, and definition of CIN.[3] It ranges from 2% in patients who have no risk factor for CIN to up to 34% in those who are at a high risk for CIN.[4] The health burden associated with CIN is considerable.[5] Accordingly, effective preventive interventions are required to reduce the incidence of and burden associated with this complication.
Current evidence recommends a number of preventive interventions for CIN such as hydration before the angiographic procedure, minimizing the contrast material dose, and administration of nonionic contrast medium with iso- or low-osmolarity.[6] In addition, clinical trials have indicated efficacy of a number of medications including N-acetylcysteine,[7] theophylline,[8] and statins[9] in preventing CIN. These medications act via various mechanisms including increasing renal perfusion and anti-oxidative as well as anti-inflammatory effects.[7,8,9]
There are a number of herbal medicines shown to have nephro-protective effects such as Urtica dioica, Parietaria judaica, Rheum palmatum, and Silybum marianum. These herbal medicines have been used for nephropathy due to various insults including diabetes, drug and chemical toxicities, and chronic kidney disease in general.[10] Anti-oxidative effect of these phytomedicines is an important mechanism for their nephro-protective properties.[11] Other proposed mechanisms include an inhibiting angiotensin-converting enzyme and anti-inflammatory properties.[10]
S. marianum or milk thistle is a medicinal plant traditionally being used for the treatment of liver diseases.[12] Silymarin is an extract from the seeds of this plant which composed of silybin, silydianin, and silychrisin.[13] This flavonoid complex has potent anti-oxidative effects as well as anti-inflammatory properties.[14] Several studies have indicated the efficacy of silymarin as a complementary treatment for inflammatory liver conditions.[12] Studies also have shown the efficacy of silymarin for drug/chemical nephrotoxicity[15] and diabetic nephropathy.[16] Considering the role of oxidative stress and inflammation in CIN,[17] silymarin is a good candidate for the prevention of this complication. Accordingly, we aimed to evaluate the efficacy of silymarin in the prevention of CIN.
METHODS
Participant and study setting
This study was conducted on patients referring for elective coronary angiography between Jan and Mar 2015 to Noor University Hospital in Isfahan, Iran. Patients with mild to moderate risk for CIN were included in the study.[18] Patients with the following characteristics were not included in the study; unstable angina, myocardial infarction, cardiac arrhythmias, acute or chronic renal insufficiency/failure (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), acute or decompensate heart failure, diabetes, and intravascular administration of contrast material in the past month. The study was approved by the Ethics Committee of the Isfahan University of Medical Sciences, and informed consent was obtained from patients before entering the study.
Study design and sample size
The study was designed as a double-blind, placebo-controlled trial with two parallel arms including silymarin and placebo. An alphabetical code was assigned for each of the study arms (A and B). An independent investigator placed drugs in opaque and stapled drug pockets. Patients were consecutively entered into the study and were alternately assigned to the study arms. Blinding the attending physicians and patients was achieved by administering a placebo tablet identical in appearance with silymarin. The trial was registered in the Iranian Registry of Clinical Trials (www.irct.ir, registration code: IRCT2014051117648N1). Sample size was calculated as 84 cases in each group considering the significance level of 0.05, study power of 0.8, expecting 10% difference between the two groups in the frequency of CIN, and about 20% drop-out rate.
Intervention
Patients received a single dose of silymarin tablet (280 mg) or placebo tablet 2 h before administration of the contrast material. Silymarin tablets (Livergol®), containing standardized ethyl acetate extract of the S. marianum seeds, were purchased from Goldaru Pharmaceutical Company (Isfahan, Iran) and placebo tablets identical in shape and size were obtained from the Isfahan School of Pharmacy (Isfahan, Iran). All patients were hydrated with 0.9% sodium chloride (1 mL/kg/h) for 12 h, started 6 h before the operation and continued to 6 h after the procedure. Patients consuming nonsteroidal anti-inflammatory drugs were advised to discontinue medication from 48 h before to 48 h after angiography. Patients consuming metformin were advised to discontinue it from the day of the procedure to 48 h after angiography. Angiography was done according to the clinical standards, by trans-femoral or trans-radial approach. In all cases, Iodixanol (Visipaque™, Amersham Healthcare, Cork, Ireland) was used as a nonionic contrast media with iso-osmolarity. In average, 45 mL of the Iodixanol (320 mg/mL) was administered to each patient for during the angiographic procedure.
Measurements and study outcomes
Before the operation, all the patients underwent a detailed history and physical examination by a cardiologist. Age and gender were recorded, weight and height were measured, and body mass index was calculated (kg/m2). Fasting blood sugar was measured to rule out undiagnosed diabetes. Serum creatinine was measured before and 48 h after contrast material injection in the hospital laboratory. The CKD-EPI formula was applied for calculating the eGFR.[19]
The study outcomes were considered as (1) the amount of change in serum creatinine concentration after 48 h of contrast material injection and (2) CIN which was defined as an increase in serum creatinine of ≥0.5 mg/dL or ≥25% of the baseline creatinine after 48 h of contrast material injection.[18] The secondary outcome was considered as the amount of change in eGFR after 48 h of contrast material injection.
Statistical analyses
Data were analyzed using the SPSS software for windows version 16.0 (SPSS Inc., Chicago, IL., USA). Data are presented as mean ± standard deviation or number (%). The Chi-square test (or Fisher's exact test) was applied for comparison of qualitative data between the two groups. Quantitative data were checked if normally distributed in each group using the Kolmogorov–Smirnov test. Then, the independent sample t-test or the Mann–Whitney U-test was applied for comparison of data between the two groups. The Wilcoxon test was applied for within-group comparisons. In addition, logistic regression analysis was done for controlling the effects of possible confounders. A P < 0.05 was considered significant in all analyses. Odds ratio (OR) and confidence intervals (CI) are reported where relevant.
RESULTS
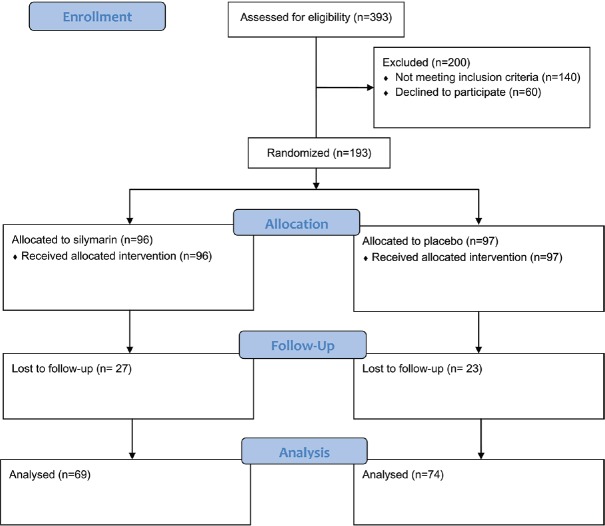
A total of 393 candidates of coronary angiography were evaluated during the study period; 253 patients were eligible for the study; 60 patients were unwilling to participate mostly due to living far from the study cite and being unable to attend the follow-up visit; 193 patients were allocated into the study groups. All patients received the assigned intervention, but 50 patients did not refer to the second visit [Figure 1]. Demographic data and baseline characteristics of the patients are summarized in Table 1. There was the difference between the study groups regarding baseline creatinine and eGFR [Tables 1 and 2].
Figure 1.
Patients’ flow diagram
Table 1.
Demographic data and baseline characteristics of the patients
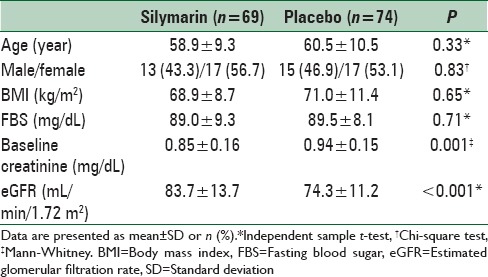
Table 2.
Change of serum creatinine level among the study groups
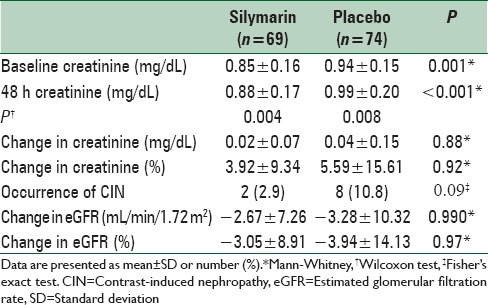
Creatinine level was significantly increased in both groups 48 h after angiography (both P < 0.01) [Table 2]. There was no difference between the study groups regarding the amount of changes in creatinine after angiography (mean difference = 0.01 ± 0.02 mg/dL, P = 0.881). Frequency of CIN was 2.9% and 10.8% with silymarin and placebo, respectively (OR [95% CI] = 0.246 [0.050–1.203], P = 0.099). No difference was observed between the two groups in change of eGFR (P > 0.05) [Table 2].
Considering the difference between the study groups in baseline creatinine, the logistic regression test was conducted controlling for baseline serum creatinine concentration as a covariate. Other baseline characteristics were also included into the analysis to find possible predictors of CIN. Compared with placebo, those who received silymarin were less likely, though statistically nonsignificant, to experience CIN after contrast injection (OR [95% CI] = 0.203 [0.037–1.117], P = 0.067) [Table 3]. Changes in serum creatinine from baseline to after angiography while controlling for baseline values are presented in Figure 2.
Table 3.
Logistic regression analysis of the possible predictors of contrast-induced nephropathy
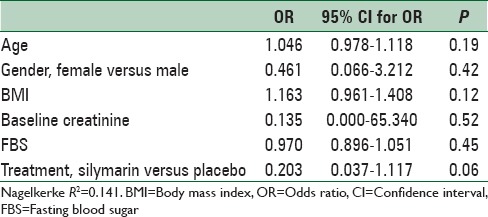
Figure 2.
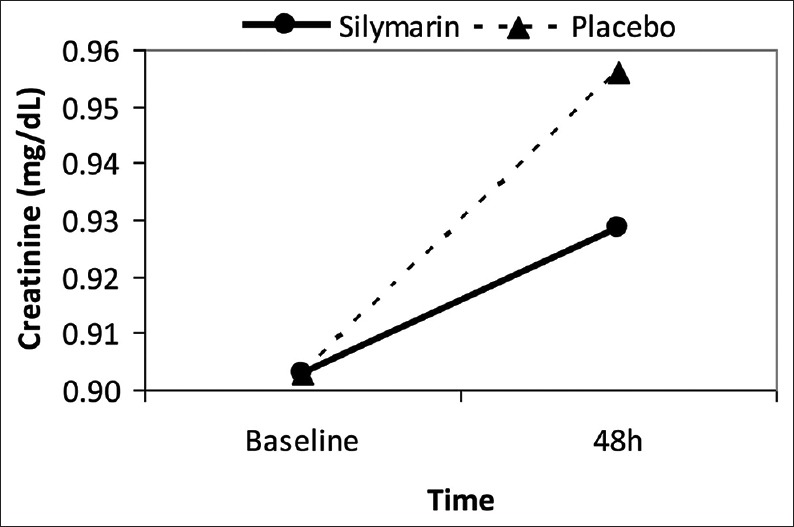
Change in serum creatinine level after contrast injection; baseline levels controlled
DISCUSSION
To our knowledge, this is the first study on the efficacy of silymarin for the prevention of CIN. Although we found no difference between silymarin and placebo in the amount of changes in serum creatinine after angiography, the incidence of CIN was lower with silymarin. However, the difference was not statistically significant due to small sample size of the study. Considering lower baseline serum creatinine in the silymarin compared with the placebo group, it is possible that the observed difference between the two groups in the frequency of CIN is confounded by the baseline creatinine. None of the patients had abnormal baseline creatinine level, and the logistic regression analysis controlling for this factor indicated an independent, albeit nonsignificant, association between silymarin and lower risk for CIN. These results are in favor of the effectiveness of silymarin in preventing CIN in patients with mild to moderate risk undergoing angiographic procedures.
Silymarin is a potent antioxidant and free radical scavenger that inhibits lipid peroxidation and stabilizes cell membrane.[14] It also increases intracellular glutathione[14] which plays a crucial role in the body's anti-oxidant capacity.[20] Silymarin also has anti-inflammatory properties inhibiting T-cell proliferation and cytokine secretion.[21,22] Although silymarin has been mostly under attention for its liver-protective effects, recent studies have indicated nephro-protective effects of this flavonoid complex as well.[12] Several studies showed that silymarin and its components prevent nephrotoxicity of various drugs such as methotrexate,[23] cisplatin,[24] adriamycin,[25] and gentamicin,[26] as well as chemicals such as arsenic[27] and ferric nitrilotriacetate.[28] In addition, animal and clinical studies have shown nephro-protective effects of silymarin for diabetic nephropathy via abrogating oxidative stress, inflammation, and apoptosis.[29,30,31,32,33] Considering the role of oxidative stress and inflammation in the pathophysiology of CIN, the observed beneficial effects of silymarin in our study can be attributed to its anti-oxidant and anti-inflammatory effects. Also, there is evidence, albeit scarce, that the same regenerative effects of silymarin on the liver tissue after injury[14] are also seen in renal tissue.[34] However, these possible preventive mechanisms of silymarin for CIN are required to be investigated in further studies.
Our study has a number of limitations. Our allocation method was not precisely randomized resulting in a difference in baseline characteristics of the study groups. However, we conducted a logistic regression analysis to solve this problem. Furthermore, the trial was a single-center study with small sample size and including only patients with mild to moderate risk for CIN, which may reduce the generalizability of the study results. Finally, we monitored our patients for 48 h, while longer follow-ups can provide more information on the efficacy of the preventive measures.
CONCLUSIONS
We found a trend toward the efficacy of silymarin in preventing contrast-induced renal dysfunction in patients undergoing coronary angiography with mild to moderate risk for CIN. Due to a small sample of patients we cannot draw a clear conclusion, and the study results should be interpreted cautiously considering the study limitations. Further trials with different dosage and treatment duration of silymarin, larger sample size, and longer follow-ups, and with including patients with higher risk of CIN are required.
ACKNOWLEDGEMENTS
This study was supported by the Isfahan University of Medical Sciences (grant number 393752). We are thankful to the Noor Hospital Angiography Unit staff for helping us in data gathering.
Footnotes
Source of Support: Isfahan University of Medical Sciences (grant number 393752)
Conflict of Interest: None declared.
REFERENCES
- 1.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–28. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 2.Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14–22. doi: 10.1111/j.1523-1755.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 3.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–15. doi: 10.1093/eurheartj/ehr494. [DOI] [PubMed] [Google Scholar]
- 4.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian S, Tumlin J, Bapat B, Zyczynski T. Economic burden of contrast-induced nephropathy: Implications for prevention strategies. J Med Econ. 2007;10:119–34. doi: 10.3111/200710119134. [DOI] [PubMed] [Google Scholar]
- 6.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–41. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 7.Wu MY, Hsiang HF, Wong CS, Yao MS, Li YW, Hsiang CY, et al. The effectiveness of N-Acetylcysteine in preventing contrast-induced nephropathy in patients undergoing contrast-enhanced computed tomography: A meta-analysis of randomized controlled trials. Int Urol Nephrol. 2013;45:1309–18. doi: 10.1007/s11255-012-0363-1. [DOI] [PubMed] [Google Scholar]
- 8.Dai B, Liu Y, Fu L, Li Y, Zhang J, Mei C. Effect of theophylline on prevention of contrast-induced acute kidney injury: A meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;60:360–70. doi: 10.1053/j.ajkd.2012.02.332. [DOI] [PubMed] [Google Scholar]
- 9.Ukaigwe A, Karmacharya P, Mahmood M, Pathak R, Aryal MR, Jalota L, et al. Meta-analysis on efficacy of statins for prevention of contrast-induced acute kidney injury in patients undergoing coronary angiography. Am J Cardiol. 2014;114:1295–302. doi: 10.1016/j.amjcard.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 10.Yarnell EL. Botanical medicines used for kidney disease in the United States. Iran J Kidney Dis. 2012;6:407–18. [PubMed] [Google Scholar]
- 11.Nasri H, Rafieian-Kopaei M. Protective effects of herbal antioxidants on diabetic kidney disease. J Res Med Sci. 2014;19:82–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Bahmani M, Shirzad H, Rafieian S, Rafieian-Kopaei M. Silybum marianum: Beyond Hepatoprotection. J Evid Based Complementary Altern Med. 2015;20:292–301. doi: 10.1177/2156587215571116. [DOI] [PubMed] [Google Scholar]
- 13.Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994;27:105–12. [PubMed] [Google Scholar]
- 14.Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest. 2002;22:51–65. [Google Scholar]
- 15.Dashti-Khavidaki S, Shahbazi F, Khalili H, Lessan-Pezeshki M. Potential renoprotective effects of silymarin against nephrotoxic drugs: A review of literature. J Pharm Pharm Sci. 2012;15:112–23. doi: 10.18433/j3f88s. [DOI] [PubMed] [Google Scholar]
- 16.Rafieian-Kopaie M, Nasri H. Silymarin and diabetic nephropathy. J Renal Inj Prev. 2012;1:3–5. doi: 10.12861/jrip.2012.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heyman SN, Rosen S, Khamaisi M, Idée JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45:188–95. doi: 10.1097/RLI.0b013e3181d2eed8. [DOI] [PubMed] [Google Scholar]
- 18.Mehran R, Nikolsky E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;69:S11–5. doi: 10.1038/sj.ki.5000368. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed DJ. Glutathione: Toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603–31. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- 21.Gharagozloo M, Velardi E, Bruscoli S, Agostini M, Di Sante M, Donato V, et al. Silymarin suppress CD4+T cell activation and proliferation: Effects on NF-kappaB activity and IL-2 production. Pharmacol Res. 2010;61:405–9. doi: 10.1016/j.phrs.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Gharagozloo M, Jafari S, Esmaeil N, Javid EN, Bagherpour B, Rezaei A. Immunosuppressive effect of silymarin on mitogen-activated protein kinase signalling pathway: The impact on T cell proliferation and cytokine production. Basic Clin Pharmacol Toxicol. 2013;113:209–14. doi: 10.1111/bcpt.12088. [DOI] [PubMed] [Google Scholar]
- 23.Dabak DO, Kocaman N. Effects of silymarin on methotrexate-induced nephrotoxicity in rats. Ren Fail. 2015;37:734–9. doi: 10.3109/0886022X.2015.1012984. [DOI] [PubMed] [Google Scholar]
- 24.Ninsontia C, Pongjit K, Chaotham C, Chanvorachote P. Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm Biol. 2011;49:1082–90. doi: 10.3109/13880209.2011.568506. [DOI] [PubMed] [Google Scholar]
- 25.El-Shitany NA, El-Haggar S, El-desoky K. Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem Toxicol. 2008;46:2422–8. doi: 10.1016/j.fct.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Ther. 2007;30:477–81. doi: 10.1111/j.1365-2885.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 27.Prabu SM, Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep. 2012;39:11201–16. doi: 10.1007/s11033-012-2029-6. [DOI] [PubMed] [Google Scholar]
- 28.Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Invest New Drugs. 2010;28:703–13. doi: 10.1007/s10637-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 29.Vessal G, Akmali M, Najafi P, Moein MR, Sagheb MM. Silymarin and milk thistle extract may prevent the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Ren Fail. 2010;32:733–9. doi: 10.3109/0886022X.2010.486488. [DOI] [PubMed] [Google Scholar]
- 30.Soto C, Pérez J, García V, Uría E, Vadillo M, Raya L. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine. 2010;17:1090–4. doi: 10.1016/j.phymed.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Fallahzadeh MK, Dormanesh B, Sagheb MM, Roozbeh J, Vessal G, Pakfetrat M, et al. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: A randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60:896–903. doi: 10.1053/j.ajkd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Sheela N, Jose MA, Sathyamurthy D, Kumar BN. Effect of silymarin on streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in rats. Iran J Kidney Dis. 2013;7:117–23. [PubMed] [Google Scholar]
- 33.Khazim K, Gorin Y, Cavaglieri RC, Abboud HE, Fanti P. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol. 2013;305:F691–700. doi: 10.1152/ajprenal.00028.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnenbichler J, Scalera F, Sonnenbichler I, Weyhenmeyer R. Stimulatory effects of silibinin and silicristin from the milk thistle Silybum marianum on kidney cells. J Pharmacol Exp Ther. 1999;290:1375–83. [PubMed] [Google Scholar]