Abstract
Alu elements represent one of the most common sources of homology and homeology in the human genome. Homeologous recombination between Alu elements represents a major form of genetic instability leading to deletions and duplications. Although these types of events have been studied extensively through genomic sequencing to assess the impact of Alu elements on disease mutations and genome evolution, the overall abundance of Alu elements in the genome often makes it difficult to assess the relevance of the Alu elements to specific recombination events. We recently reported a powerful new reporter gene system that allows the assessment of various cis and trans factors on the contribution of Alu elements to various forms of genetic instability. This allowed a quantitative measurement of the influence of mismatches on Alu elements and instability. It also confirmed that homeologous Alu elements are able to stimulate non-homologous end joining events in their vicinity. This appears to be dependent on portions of the mismatch repair pathway. We are now in a position to begin to unravel the complex influences of Alu density, mismatch and location with alterations of DNA repair processes in various tissues and tumors.
Keywords: Alu, DNA repair, genomic instability, non-homologous end joining, recombination
Introduction
Alu elements in the human genome provide a unique substrate that can influence DNA DSB repair and lead to specific forms of genetic instability. Alu elements are present at over 1 million copies in the human genome (about 11% of the overall mass of DNA) dispersed widely both within introns and between genes with a modest bias for increased density within genes.1,2 Alu elements are just under 300 bases in length, making them an adequate length to trigger non-allelic homologous recombination between nearby Alu elements, although this represents the lower end of lengths that can efficiently contribute to such recombination.3 Their relatively high divergence from one another (0%–30%) also is unique compared to most other recombination substrates and is expected to greatly influence homologous recombination.4 Thus, almost every Alu element has a number of other Alu elements within a few thousand bases with a range of sequence divergence of a few percent to more than 30% (homeologous Alu elements). In addition, because Alu elements continue to insert in the human genome, every genome has a portion of relatively recent polymorphic inserts.5,6
Despite their relatively short size and sequence divergence, non-allelic Alu/Alu recombination events have contributed to a great deal of genomic instability, including evolution of the human genome.7 and extensive DNA rearrangements associated with human disease.8,9 It is estimated that about 0.3% of new human genetic diseases are caused by homeologous Alu/Alu recombination and it is likely that they also represent an important form of somatic instability, particularly in tumors. This type of secondary genomic instability has a much bigger impact on gene disruption than the initial insertion of Alu elements. Non-allelic Alu/Alu recombination events can mediate deletion of segments from as small as 300 bases up to the tens of kilobases, as well as duplications of similar sizes. Of particular interest is the observation that several genes, such as LDLR,10 Mll-1,11 SPAST,12 MSH2, and MLH1,13 seem to be particularly prone to recurrent Alu/Alu recombination events leading to a high proportion of the disease-causing defects in those genes. The explanations for these recurrently affected genes include the relative Alu density within the genes as well as the potential for more regional chromatin influences on recombination.4,9 There have also been a number of suggestions that in addition to Alu/Alu recombination Alu elements may act as some kind of hotspot that can cause NHEJ-based rearrangements in their vicinity that may lead to segmental duplications14 or deletions.15
We set out to investigate the involvement of DSB repair pathways in homologous and homoeologous Alu/Alu recombination in mammalian cells. We have recently described a new reporter gene system that is able to efficiently measure both the rate of Alu/Alu recombination, as well as NHEJ deletions that cause a deletion in the vicinity of the 2 Alu elements in the system.16 This system improves on several previous reporters17,18 in that variations (e.g. introducing sequence variation between the 2 Alu copies) in the vector can be made and then returned to the identical, single-copy locus using Flp recombinase-mediated integration. This allows parallel measurements on the modified vectors for quantitative comparison. Our data suggested that Alu elements may contribute to several competing DNA repair pathways and that the degree of Alu sequence divergence may be a critical factor in prioritizing repair pathway choice.
Several DNA repair pathways may contribute to the types of deletions measured by our reporter system. For these approximately 1 kb deletions, it seems likely that most of the pathways would first require a 5′-end resection as shown in Figure 1A. Resection of the DSB ends produces 2 complementary single strands that are annealed. In the SSA pathway, this annealing is mediated by RAD52.19 This figure shows a classic SSA intermediate heteroduplex structure comprised of the 2 direct repeat homeologous Alu elements following Rad52-dependent annealing of exposed partially-complementary single strands. Although it is also possible that other mechanisms, such as invasion of the 3’ end of an exposed Alu element with the non-allelic Alu element present in the reporter on the sister chromatid, we believe that Figure 1A represents the likely intermediate common to many of the DNA repair outcomes in our reporter system.
Figure 1.
Alu/Alu-mediated genomic deletion mechanisms. (A) A DNA double-strand break (DSB) commonly undergoes 5’ to 3’ DNA-end resection that can expose nearby Alu elements as single-strand regions. The exposed single-strand Alu elements anneal to form a DNA heteroduplex with single-strand DNA 3’ flaps. This represents the typical intermediate for single-strand annealing (SSA) DNA repair that is subject to various competing forms of DNA repair resolution proposed in the other panels. (B) An Alu/Alu duplex DNA is formed between identical or nearly identical elements and non-homologous 3’ single-strand overhangs (3’ flaps) are cleaved to generate an annealed 3’ DNA end to be extended by a DNA repair polymerase. Ligation of the extended strands results in the deletion of the sequence that was previously between the direct repeat identical Alu elements. (C) A partial Alu/Alu heteroduplex is formed at a region of homology shared between homeologous direct repeat Alu elements. An unknown mechanism removes segments 3’ to a microhomology that represents ‘in register’ alignment of a region between the 2 Alu elements that is extended by DNA repair polymerases resulting in a chimeric Alu element as shown. (D) The entire Alu/Alu heteroduplex is formed, escaping heteroduplex rejection long enough to allow repair of the intermediate. The DNA mismatches are corrected through as yet unidentified mechanism(s) within segments of the heteroduplex, indiscriminate of strand-correction preference, to result in complex chimeric Alu elements bearing sequence signatures of each Alu element in multiple segments. (E) The entire Alu/Alu heteroduplex is formed and DNA mismatches are subject to DNA mismatch recognition and processing via MMR protein complexes, which are capable of inducing DNA nicks. The nicked DNA is subsequently processed as a new DNA end to undergo non-homologous end joining (NHEJ) repair mechanisms. The majority of these DNA ends are used to participate in microhomology-mediated end joining events. This pathway (homeology-influenced NHEJ) is particularly prone to occur between Alu elements with 15-30% sequence divergence and has the ability to induce genomic deletions in the vicinity of Alu elements.
We found when 2 direct repeat homologous (no sequence divergence) Alu elements flank a DNA DSB they almost exclusively created a classic SSA product resulting in homologous Alu/Alu recombination (as shown in Fig. 1B). This was a RAD52-dependent process as demonstrated by a drastic decrease in the rate of Alu/Alu recombination when RAD52 was knocked down with siRNA.20 We were able to test a wide range of sequence divergence between Alu elements, confirming previous studies on the influence of mismatches present on an Alu/Alu heteroduplex on the rate of recombination and providing the first quantitative view of how the Alu/Alu recombination rate responds to a range of Alu element sequence divergence.18,21,22 Mismatched Alu elements are likely to still undergo Rad52-dependent heteroduplex formation shown in Figure 1A. However, once formed, it is likely to be quickly dissociated by a process termed heteroduplex rejection. This process relies on DNA mismatch recognition by mismatch repair (MMR) sensor protein complexes (MutSα or MutSβ) which recruit a DNA helicase to unwind the DNA heteroduplex.21-23 Although Alu/Alu recombination events occur between diverged elements, the generation of a chimeric Alu element with a single sequence transition from the original upstream to the downstream direct repeat Alu elements (Fig. 1C) is most likely not due to SSA but instead represent products of alternate DNA repair pathways such as microhomology-mediated end-joining that just happens to occur between paralogous regions of the 2 Alu elements creating a complete, chimeric element.16,18 However, in our study we also noticed a portion of Alu/Alu recombination products that did not show the single transition from one Alu element to another as in Figure 1C, but instead had a patchwork arrangement of the mismatches from the 2 elements as shown in Figure 1D. These patchwork Alu/Alu recombination products went away with suppression of RAD52 by siRNA.16 We hypothesize that these Alu/Alu recombination products are the result of RAD52-dependent heteroduplex formation (an SSA intermediate), prior to DNA mismatch resolution in the patchwork manner by another DNA repair pathway (Fig. 1D). These types of complex chimeric Alu/Alu recombination products have been seen to occur in natural gene mutations as well.12
One of the most surprising findings with our new system was that the rate of genomic deletions in our reporter increased specifically within the 15–30% Alu element sequence divergence range.16 This was not due to any increase in Alu/Alu recombination, but instead resulted from NHEJ between sequences primarily found within the Alu elements (see schematic in Fig. 1E). The majority of these NHEJ junctions display evidence of microhomologies but do not use ‘in register’ microhomologies to generate a chimeric Alu element. These are presumably microhomology-mediated non-homologous end-joining events that are dependent on the overall homeology of the Alu elements, and we refer to them as homeology-influenced NHEJ (HI-NHEJ). Because this effect appeared to be dependent on a mismatched Alu/Alu heteroduplex, we tested whether it occurred in a mismatch repair-defective cell line-HCT116, which is deficient for the MMR protein Mlh1. We found these cells did not show the HI-NHEJ rate enhancement of NHEJ with Alu element sequence divergence in the range of 15-30%. We hypothesize the process in Figure 1E, in which a Rad52-dependent heteroduplex forms, but is degraded by mismatch repair, resulting in NHEJ events around the points of cleavage. The reporter used in our study recovered NHEJ events ranging from 897 -1881 bp and a previous studied showed events requiring 2.8 kb resection with a different vector.24 However, DNA resection about a DSB is able to expose up to 7 kb of sequence (M.E.M. and P.D. unpublished results) to reveal Alu elements that participate in SSA repair. Thus, Alu elements separated by several kilobases of sequence are likely able to form heteroduplexes to participate in DNA repair outcomes described in Figure 1.
Conclusions
Because Alu elements are spread ubiquitously throughout the genome,1 especially with their bias for presence in introns, they have a tremendous potential impact on genes. These impacts can include insertional mutagenesis upon insertion that commonly leads to new genetic diseases.8,25 It may also lead to more subtle influences on the genes in which they insert, such as alterations in gene expression26 and commonly through changes in splicing, a process termed Alu exonization.27 However, because they also provide the most common source of dispersed sequence homologies throughout the genome, perhaps the biggest impact of Alu elements is their ability to contribute to genetic rearrangements.25
The remarkable inhibition of Alu/Alu recombination by the accumulation of mismatches is critical to understanding how the high level of current Alu elements can be tolerated. At times when higher levels of Alu insertions were occurring, (i.e. 40 million years ago1) a higher proportion of consensus Alus would have been expected to contribute to more non-allelic homologous recombination.
The systematic analysis of HI-NHEJ provides experimental support for a number of previous anecdotal observations regarding rearrangements occurring in the vicinity of Alu elements. Many previous studies have noted non-homologous DNA rearrangements occurring in the vicinity of Alu elements. However, because of the ubiquitous presence throughout the genome, it has been difficult to assess whether Alu elements had any real contribution to the NHEJ events that occurred in their vicinity. Several studies have suggested, based on the frequency of rearrangements within specific genes,15 or the junctions of segmental duplications,14 that there is an enrichment for rearrangements in the vicinity of Alu elements. Although it has previously been proposed that Alu elements may have some sort of primary sequence that is prone to DSBs, our study suggests that much of this effect may be due to the homologies between the Alu elements themselves and their ability to form heteroduplexes during repair processes (Fig. 1).
All of these findings point to Alu elements contributing an intrinsic instability on the genome that is locally probably influenced by both the number of Alu elements as well as their relative mismatch to one another. Figure 2 shows a scenario where a new Alu inserts between 2 older, more divergent Alu elements. Because they all accumulate mutations randomly, the divergence between 2 Alu elements is approximately the sum of their divergences relative to the consensus sequence. The new Alu adds a series of different contributions to genetic instability in that region of the genome. If there is another near consensus element nearby, it may result in high levels of Alu/Alu recombination, while potentially contributing other opportunities for HI-NHEJ in the vicinity with other more divergent Alu elements. As the genome containing that new element evolves (millions of years) and all of the Alu elements in that region accumulate more random mutations, the previously high Alu/Alu recombination may turn into very low levels of Alu/Alu or NHEJ with that particularly pairing, while maintaining HI-NHEJ with other Alu elements in the region.
Figure 2.
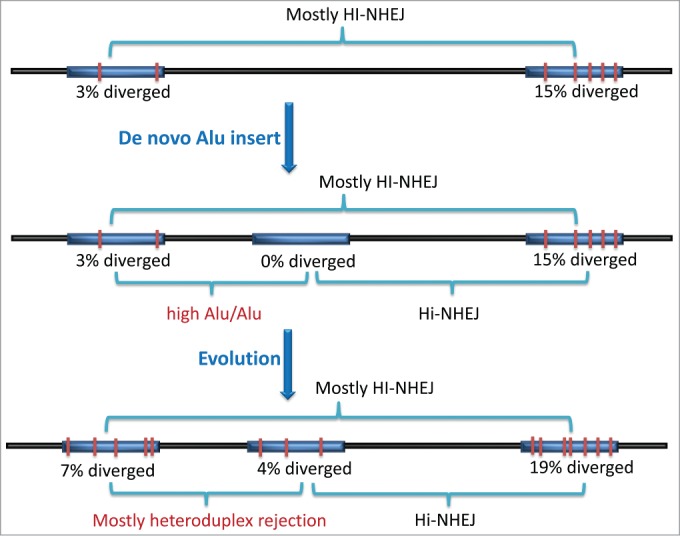
The impact of Alu elements on DNA repair of the genome. Homeologous Alu elements that are in proximity within the genome are shown with representative sequence divergence given as percentages relative to an actively mobile consensus Alu element. These sequence divergence values are generally additive between 2 elements such that a 3% and 15% diverged Alu element are ~18% diverged to one another. The level of sequence divergence between Alu elements at the top would be expected to stimulate high rates of homeology-influenced non-homologous end joining (HI-NHEJ) when DNA double-strand breaks (DSBs) occur between them. A de novo Alu retrotransposition event introduces a consensus Alu element between the 2 previously described Alu elements in the genome (middle drawing). Alu-mediated DNA repair about a DSB between this “0%” and a 3% sequence diverged Alu element will result primarily in Alu/Alu recombination whereas HI-NHEJ will be the primary Alu-mediated DNA repair result between this “0%” and a 15% sequence diverged Alu element. Genetic drift of the Alu elements during evolution of the genome results in neighboring Alu elements that diverge by 11% (the sum of the 7% and 4% sequence diverged elements) which are not expected to contribute effectively to Alu-mediated DNA repair of a DSB between them due to efficient heteroduplex rejection of a formed SSA intermediate. However, Alu-mediated DNA repair between the other Alu element combinations are expected to illicit high levels of HI-NHEJ.
A last key concept in terms of the contribution of Alu elements to the intrinsic instability of the genome relates to genetic background. Our data suggest that DNA repair pathways, such as mlh1 deficiency,16 may alter the ability of the genome to utilize HI-NHEJ. DSBs that would ordinarily be repaired near Alu elements by HI-NHEJ are then likely to be repaired more randomly. In addition, one study showed the potential of p53 defects to greatly stimulate Alu recombination.17 This was not carried out in the presence of mismatches, but certainly illustrates the potential of many DNA repair defects, particularly common in cancer, to greatly alter the use of Alu elements in genetic instability. Thus, it seems likely that Alu elements will contribute differentially to different forms of genetic instability in different genomic regions depending on the DNA repair background in the cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al.. Initial sequencing and analysis of the human genome. Nature 2001; 409:860-921; PMID:11237011; http://dx.doi.org/ 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- 2.Medstrand P, van de Lagemaat LN, Mager DL. Retroelement distributions in the human genome: variations associated with age and proximity to genes. Genome Res 2002; 12:1483-95; PMID:12368240; http://dx.doi.org/ 10.1101/gr.388902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundia MM, Desai V, Magwood AC, Baker MD. Nascent DNA synthesis during homologous recombination is synergistically promoted by the rad51 recombinase and DNA homology. Genetics 2014; 197:107-19; PMID:24583581; http://dx.doi.org/ 10.1534/genetics.114.161455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedges DJ, Deininger PL. Inviting instability: Transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat Res 2007; 616:46-59; PMID:17157332; http://dx.doi.org/ 10.1016/j.mrfmmm.2006.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witherspoon DJ, Zhang Y, Xing J, Watkins WS, Ha H, Batzer MA, Jorde LB. Mobile element scanning (ME-Scan) identifies thousands of novel Alu insertions in diverse human populations. Genome Res 2013; 23:1170-81; PMID:23599355; http://dx.doi.org/ 10.1101/gr.148973.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konkel MK, Walker JA, Batzer MA. LINEs and SINEs of primate evolution. Evol Anthropol 2010; 19:236-49; PMID:25147443; http://dx.doi.org/ 10.1002/evan.20283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nat Rev Genet 2009; 10:691-703; PMID:19763152; http://dx.doi.org/ 10.1038/nrg2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ade C, Roy-Engel AM, Deininger PL. Alu elements: an intrinsic source of human genome instability. Curr Opin Virol 2013; 3:639-45; PMID:24080407; http://dx.doi.org/ 10.1016/j.coviro.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belancio VP, Deininger PL, Roy-Engel AM. LINE dancing in the human genome: transposable elements and disease. Genome Med 2009; 1:97; PMID:19863772; http://dx.doi.org/ 10.1186/gm97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faiz F, Allcock RJ, Hooper AJ, van Bockxmeer FM. Detection of variations and identifying genomic breakpoints for large deletions in the LDLR by Ion Torrent semiconductor sequencing. Atherosclerosis 2013; 230:249-55; PMID:24075752; http://dx.doi.org/ 10.1016/j.atherosclerosis.2013.07.050 [DOI] [PubMed] [Google Scholar]
- 11.Strout MP, Marcucci G, Bloomfield CD, Caligiuri MA. The partial tandem duplication of ALL1 (MLL) is consistently generated by Alu-mediated homologous recombination in acute myeloid leukemia. Proc Natl Acad Sci U S A 1998; 95:2390-5; PMID:9482895; http://dx.doi.org/ 10.1073/pnas.95.5.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boone PM, Yuan B, Campbell IM, Scull JC, Withers MA, Baggett BC, Beck CR, Shaw CJ, Stankiewicz P, Moretti P, et al.. The Alu-rich genomic architecture of SPAST predisposes to diverse and functionally distinct disease-associated CNV alleles. Am J Hum Genet 2014; 95:143-61; PMID:25065914; http://dx.doi.org/ 10.1016/j.ajhg.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, McVety S, Younan R, Liang P, Du Sart D, Gordon PH, Hutter P, Hogervorst FB, Chong G, Foulkes WD. Distinct patterns of germ-line deletions in MLH1 and MSH2: the implication of Alu repetitive element in the genetic etiology of Lynch syndrome (HNPCC). Hum Mutat 2006; 27:388; PMID:16541406; http://dx.doi.org/ 10.1002/humu.9417 [DOI] [PubMed] [Google Scholar]
- 14.Bailey JA, Liu G, Eichler EE. An Alu transposition model for the origin and expansion of human segmental duplications. Am J Hum Genet 2003; 73:823-34; PMID:14505274; http://dx.doi.org/ 10.1086/378594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Smith AJ, Walters RG, Coin LJ, Steinfeld I, Yakhini Z, Sladek R, Froguel P, Blakemore AI. Small deletion variants have stable breakpoints commonly associated with alu elements. PLoS One 2008; 3:e3104; PMID:18769679; http://dx.doi.org/ 10.1371/journal.pone.0003104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales ME, White TB, Streva VA, DeFreece CB, Hedges DJ, Deininger PL. The contribution of alu elements to mutagenic DNA double-strand break repair. PLoS Genet 2015; 11:e1005016; PMID:25761216; http://dx.doi.org/ 10.1371/journal.pgen.1005016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebow D, Miselis N, Liber HL. Homologous and nonhomologous recombination resulting in deletion: effects of p53 status, microhomology, and repetitive DNA length and orientation. Mol Cell Biol 2000; 20:4028-35; PMID:10805745; http://dx.doi.org/ 10.1128/MCB.20.11.4028-4035.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott B, Richardson C, Jasin M. Chromosomal translocation mechanisms at intronic alu elements in mammalian cells. Mol Cell 2005; 17:885-94; PMID:15780943; http://dx.doi.org/ 10.1016/j.molcel.2005.02.028 [DOI] [PubMed] [Google Scholar]
- 19. Van Dyck E, Stasiak AZ, Stasiak A, West SC. Visualization of recombination intermediates produced by RAD52-mediated single-strand annealing. EMBO Rep 2001; 2:905-9; PMID:11571269; http://dx.doi.org/ 10.1093/embo-reports/kve201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales ME, Servant G, Ade C, Roy-Engel AM. Altering Genomic Integrity: Heavy Metal Exposure Promotes Transposable Element-Mediated Damage. Biol Trace Elem Res 2015; 166(1):24-33; PMID:25774044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugawara N, Goldfarb T, Studamire B, Alani E, Haber JE. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc Natl Acad Sci U S A 2004; 101:9315-20; PMID:15199178; http://dx.doi.org/ 10.1073/pnas.0305749101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfarb T, Alani E. Distinct roles for the Saccharomyces cerevisiae mismatch repair proteins in heteroduplex rejection, mismatch repair and nonhomologous tail removal. Genetics 2005; 169:563-74; PMID:15489516; http://dx.doi.org/ 10.1534/genetics.104.035204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larocque JR, Jasin M. Mechanisms of recombination between diverged sequences in wild-type and BLM-deficient mouse and human cells. Mol Cell Biol 2010; 30:1887-97; PMID:20154148; http://dx.doi.org/ 10.1128/MCB.01553-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark JM, Pierce AJ, Oh J, Pastink A, Jasin M. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol Cell Biol 2004; 24:9305-16; PMID:15485900; http://dx.doi.org/ 10.1128/MCB.24.21.9305-9316.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deininger PL, Batzer MA. Alu repeats and human disease. Mol Genet Metab 1999; 67:183-93; PMID:10381326; http://dx.doi.org/ 10.1006/mgme.1999.2864 [DOI] [PubMed] [Google Scholar]
- 26.Schmitz J. SINEs as driving forces in genome evolution. Genome Dyn 2012; 7:92-107; PMID:22759815; http://dx.doi.org/ 10.1159/000337117 [DOI] [PubMed] [Google Scholar]
- 27.Sorek R, Ast G, Graur D. Alu-containing exons are alternatively spliced. Genome Res 2002; 12:1060-7; PMID:12097342; http://dx.doi.org/ 10.1101/gr.229302 [DOI] [PMC free article] [PubMed] [Google Scholar]



