Abstract
By cellulose acetate or acrylamide gel electrophoresis it is possible to separate these alkaline phosphatase isoenzymes from serum: [anode] fast liver, slow liver, placenta/Regan, bone, intestine, bile [cathode]. Heat or chemical inhibition can confirm the differentiation.
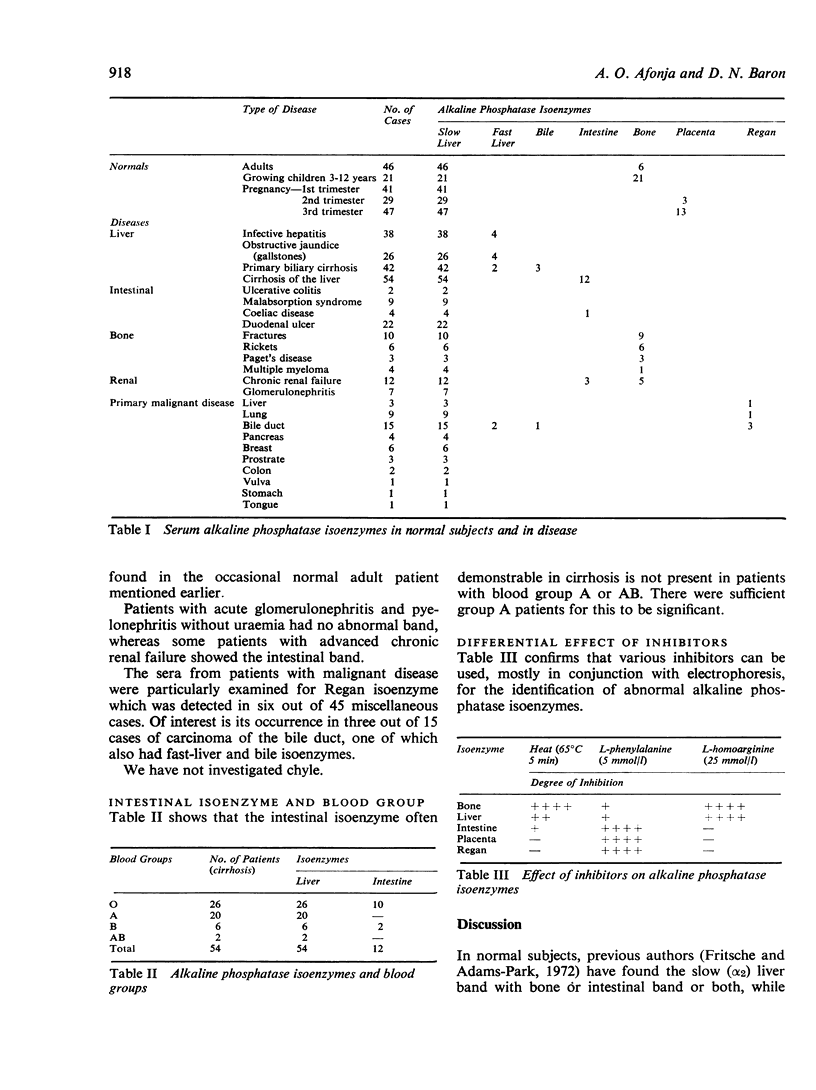
Normal adult serum always contains slow-liver isoenzyme, and sometimes bone isoenzyme: the latter is always present in serum of children. In hepatobiliary disease slow-liver isoenzyme was always increased: intestinal isoenzyme appeared in many cases of cirrhosis (of blood groups B and 0) but fast-liver and bile isoenzymes were occasionally seen in miscellaneous cases. The findings in other diseases included Regan isoenzyme in six out of 45 cases of malignant disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARFORS K. E., BECKMAN L., LUNDIN L. G. FURTHER STUDIES ON THE ASSOCIATION BETWEEN HUMAN SERUM PHOSPHATASES AND BLOOD GROUPS. Acta Genet Stat Med. 1963;13:366–368. doi: 10.1159/000151820. [DOI] [PubMed] [Google Scholar]
- Aoba H., Hariu Y., Yamaguchi R. Serum heat-stable alkaline phosphatase in normal and abnormal pregnancy. Tohoku J Exp Med. 1967 Feb;91(2):201–207. doi: 10.1620/tjem.91.201. [DOI] [PubMed] [Google Scholar]
- BAMFORD K. F., HARRIS H., LUFFMAN J. E., ROBSON E. B., CLEGHORN T. E. SERUM-ALKALINE-PHOSPHATASE AND THE ABO BLOOD-GROUPS. Lancet. 1965 Mar 6;1(7384):530–531. doi: 10.1016/s0140-6736(65)92027-1. [DOI] [PubMed] [Google Scholar]
- BECKMAN L. ASSOCIATIONS BETWEEN HUMAN SERUM ALKALINE PHOSPHATASES AND BLOOD GROUPS. Acta Genet Stat Med. 1964;14:286–297. [PubMed] [Google Scholar]
- Canapa-Anson R., Rowe D. J. Electrophoretic separation of tissue-specific serum alkaline phosphatases. J Clin Pathol. 1970 Sep;23(6):499–508. doi: 10.1136/jcp.23.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DEUXCHAISNES C. N., KRANE S. M. PAGET'S DISEASE OF BONE: CLINICAL AND METABOLIC OBSERVATIONS. Medicine (Baltimore) 1964 May;43:233–266. [PubMed] [Google Scholar]
- Fishman W. H., Bardawil W. A., Habib H. G., Anstiss C. L., Green S. The placental isoenzymes of alkaline phosphatase in sera of normal pregnancy. Am J Clin Pathol. 1972 Jan;57(1):65–74. doi: 10.1093/ajcp/57.1.65. [DOI] [PubMed] [Google Scholar]
- Fishman W. H. Carcinoplacental isoenzyme antigens. Adv Enzyme Regul. 1973;11:293–321. doi: 10.1016/0065-2571(73)90021-6. [DOI] [PubMed] [Google Scholar]
- Fishman W. H., Inglis N. I., Stolbach L. L., Krant M. J. A serum alkaline phosphatase isoenzyme of human neoplastic cell origin. Cancer Res. 1968 Jan;28(1):150–154. [PubMed] [Google Scholar]
- Fox H. Villous immaturity in the term placenta. Obstet Gynecol. 1968 Jan;31(1):9–12. doi: 10.1097/00006250-196801000-00002. [DOI] [PubMed] [Google Scholar]
- Fritsche H. A., Jr, Adams-Park H. R. Cellulose acetate electrophoresis of alkaline phosphatase isoenzymes in human serum and tissue. Clin Chem. 1972 May;18(5):417–421. [PubMed] [Google Scholar]
- Green S., Cantor F., Inglis N. R., Fishmann W. H. Normal serum alkaline phosphatase isoenzymes examined by acrylamide and starch gel electrophoresis and by isoenzyme analysis using organ-specific inhibitors. Am J Clin Pathol. 1972 Jan;57(1):52–64. doi: 10.1093/ajcp/57.1.52. [DOI] [PubMed] [Google Scholar]
- Inglis N. R., Fishman L., Stolbach L. L., Warshaw J. B., Fishman W. H. A comparison of chyle isoenzymes of alkaline phosphatase in chyle and hypophosphatasemic sera. Clin Chim Acta. 1972 Apr;38(1):67–73. doi: 10.1016/0009-8981(72)90209-4. [DOI] [PubMed] [Google Scholar]
- Jacoby B., Bagshawe K. D. Placental-type alkaline phosphatase from human tumour tissue. Clin Chim Acta. 1971 Dec;35(2):473–481. doi: 10.1016/0009-8981(71)90223-3. [DOI] [PubMed] [Google Scholar]
- KORNER N. H. Distribution of alkaline phosphatase in serum protein fractions. J Clin Pathol. 1962 May;15:195–199. doi: 10.1136/jcp.15.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Rogers L. Separation of human serum-alkaline-phosphatase isoenzymes by polyacrylamide gel electrophoresis. Lancet. 1969 Nov 15;2(7629):1029–1031. doi: 10.1016/s0140-6736(69)90640-0. [DOI] [PubMed] [Google Scholar]
- Langman M. J., Leuthold E., Robson E. B., Harris J., Luffman J. E., Harris H. Influence of diet on the "intestinal" component of serum alkaline phosphatase in people of different ABO blood groups and secretor status. Nature. 1966 Oct 1;212(5057):41–43. doi: 10.1038/212041a0. [DOI] [PubMed] [Google Scholar]
- Moss D. W. A note on the spectrophotometric estimation of alkaline phosphatase activity. Enzymologia. 1966 Oct 31;31(4):193–202. [PubMed] [Google Scholar]
- POSEN S., NEALE F. C., CLUBB J. S. HEAT INACTIVATION IN THE STUDY OF HUMAN ALKALINE PHOSPHATASES. Ann Intern Med. 1965 Jun;62:1234–1243. doi: 10.7326/0003-4819-62-6-1234. [DOI] [PubMed] [Google Scholar]
- Rhone D. P., Mizuno F. M. Profiles of alkaline phosphatase isoenzymes in serum using cellulose acetate electrophoresis and organ-specific inhibitors. Am J Clin Pathol. 1973 Apr;59(4):531–541. doi: 10.1093/ajcp/59.4.531. [DOI] [PubMed] [Google Scholar]
- Smith I., Lightstone P. J., Perry J. D. Separation of human tissue alkaline phosphatases by electrophoresis on acrylamide disc gels. Clin Chim Acta. 1968 Mar;19(3):499–505. doi: 10.1016/0009-8981(68)90278-7. [DOI] [PubMed] [Google Scholar]
- Stevens J., Thomas F. Alkaline phosphatase: reaction rate analysis at 340 nm. Clin Chim Acta. 1972 Mar;37:541–543. doi: 10.1016/0009-8981(72)90484-6. [DOI] [PubMed] [Google Scholar]
- TASWELL H. F., JEFFERS D. M. ISOENZYMES OF SERUM ALKALINE PHOSPHATASE IN HEPATOBILIARY AND SKELETAL DISEASE. Am J Clin Pathol. 1963 Oct;40:349–356. doi: 10.1093/ajcp/40.4.349. [DOI] [PubMed] [Google Scholar]
- Walker B. A., Eze L. C., Tweedie M. C., Evans D. A. The influence of ABO blood groups, secretor status and fat ingestion on serum alkaline phosphatase. Clin Chim Acta. 1971 Dec;35(2):433–444. doi: 10.1016/0009-8981(71)90218-x. [DOI] [PubMed] [Google Scholar]
- Whitfield J. B., Pounder R. E., Neale G., Moss D. W. Serum -glytamyl transpeptidase activity in liver disease. Gut. 1972 Sep;13(9):702–708. doi: 10.1136/gut.13.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]


