Abstract
Epidemiological studies have established significant associations between ambient pollutants, including fine particulate matter (PM2.5) and ozone (O3), and cardiopulmonary morbidity and mortality. One mechanism that has been proposed is a pulmonary/systemic inflammatory response. Although controlled human exposure studies have examined the independent inflammatory responses of PM2.5 and O3, no studies have previously examined their joint effects. The study objective was to examine the independent and combined associations between ambient PM2.5 and O3 and acute respiratory/inflammatory responses. Using our concentrated ambient particle (CAP) facility for PM2.5, we studied 10 mild asthmatic and 13 non-asthmatic individuals. The 2-hr exposures included CAP (range 48–199 µg/m3) and filtered air (FA), with/without O3 (120 ppb), in a randomized block design. Response measures included pulmonary function and inflammatory indices in induced sputum (IL-6, cytology) and blood (IL-6, TNF-α) measured before and after exposures. Three hrs post exposure there was an increase in blood levels of IL-6, but only after CAP alone exposures; the IL-6 increase was associated with increasing PM2.5 mass concentration (p=0.005). Some individuals switched to shallow breathing during CAP+O3, possibly accounting for an attenuation of the resultant blood IL-6 response. Asthmatic and non-asthmatic responses were similar. There were no adverse changes in pulmonary function or other inflammatory measures. The study demonstrated an acute IL-6 response to PM2.5, providing evidence to support the epidemiological findings of associations between ambient levels of particles and cardiopulmonary morbidity and mortality.
INTRODUCTION
Epidemiological studies have demonstrated that increases in air pollutant levels are associated with adverse health effects (Burnett et al., 1998; Dockery and Pope, 1994; Dockery, 2001; Samet and Krewski, 2007). Both pulmonary and systemic effects have been reported. Two of the major components in urban smog, fine particles with an aerodynamic diameter less than 2.5 µm (PM2.5) and ozone (O3), are key contributors to these adverse health effects (Laden et al., 2006; Samet and Krewski, 2007; Schwartz et al., 1996). Although the mechanism of pollutant induced health effects is not clearly understood, it has been suggested that inhaled pollutants can initiate local airway inflammation, leading to a systemic inflammatory state (Brook et al., 2004). The process linking the initial pulmonary response to the subsequent or concomitant systemic inflammatory response has not yet been fully elucidated.
In epidemiological studies of the health effects of air pollution, mortality and hospital admissions have routinely been examined as health outcomes. Increasingly, there is also evidence of associations between ambient PM levels and intermediary inflammatory outcomes, such as white blood cells, interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), C-reactive protein and fibrinogen (Delfino et al., 2008; Dubowsky et al., 2006; Rückerl et al., 2007; Samet and Krewski, 2007; Schwartz, 2001). A representative example is illustrated by a study of 30 young soldiers exposed to high levels of PM with an aerodynamic diameter less than 10 µm (PM10, 125±7 µg/m3) during a five-week long air pollution episode caused by forest fires in Southeast Asia. Systemic levels of IL-6, IL-1β and granulocyte-macrophage colony stimulating factor were increased over 75% during the forest fire “haze” compared to the post fire period when PM10 levels were reduced by 68% or 85 µg/m3 (Tan et al., 2000; van Eeden et al., 2001). In a panel study of 44 non-smokers over 60 yrs of age, positive associations were observed between ambient levels of PM2.5 (1–7 day moving average) and acute increases in IL-6, CRP and white blood cells (Dubowsky et al., 2006). In summary, epidemiological studies have shown positive associations between high as well as ambient levels of particles and acute health effects, in both healthy and susceptible populations.
Controlled human exposure studies have examined both respiratory and systemic acute effects of concentrated ambient particles (CAP) alone (Ghio et al., 2004), as well as the combined effects of CAP+O3 on arterial diameter (Brook et al., 2002; Urch et al., 2004) and on blood pressure (Urch et al., 2005), but no previous study has compared the independent and combined effects of PM2.5 and O3 on inflammatory outcomes. The combined short-term effects of PM and O3 have also been examined in time-series studies, with both mortality (Bell et al., 2004; Bell et al., 2005; Bell et al., 2007) and hospital admissions (Dominici et al., 2006) as health outcomes. The challenge of population studies is separating out individual pollutant effects from the pollution mixture (Sarnat et al., 2001; Sarnat et al., 2005). Controlled human exposure studies, on the other hand, provide a controlled exposure atmosphere with the ability to compare acute responses to single or multiple pollutants and offer the opportunity to explore mechanisms.
We undertook a controlled human exposure study to examine and compare pulmonary and systemic inflammatory markers after 2-hr controlled exposures to Toronto fine CAP with or without O3. Measures of response included pulmonary function, induced sputum cytology and the inflammatory cytokines IL-6 and TNF-α. Responses of mild asthmatics, a potentially more susceptible group, were compared to those of non-asthmatic individuals.
MATERIALS AND METHODS
Study Participants
Participants were recruited from the University of Toronto and the Greater Toronto Area. Controlled exposures were carried out between July, 1999 and February, 2003 at the Gage Occupational and Environmental Health Unit. The study was approved by the Human Subjects Research Ethics Review Committees of the University of Toronto and St. Michael’s Hospital. Participants gave written informed consent prior to enrolment. Thirteen non-asthmatic and 10 mild asthmatic (11 male, 12 female) non-smokers of 18–40 years of age were enrolled. An initial screening visit ensured that participants met the inclusion criteria before they continued with the exposure phase of the study. Subjects were excluded if they had cardiovascular disease, diabetes or baseline spirometry <75% of the predicted normal values for forced vital capacity (FVC) and forced expiratory 1-sec volume (FEV1) (Hankinson et al., 1999). All participants were free of respiratory tract infections for at least three weeks before exposure testing. Subjects underwent skin prick testing with 12 common inhalant allergen to test for atopy (Western Allergy Services, Canada). Asthmatics had physician-diagnosed asthma (subject-reported) that was confirmed by a positive methacholine challenge (PC20 ≤ 8 mg/ml) and were untreated with inhaled or systemic corticosteroids for at least one month prior to and during their study participation. Short-acting inhaled bronchodilators were not taken after midnight prior to each visit. Non-asthmatics were excluded from the study if their PC20 was ≤ 8 mg/ml.
Exposure Groups
Participants were randomized to one of two groups: 1) no added O3; or 2) added O3 of 120 ppb (Table 1). Within each group, exposures were assigned in random order and included filtered air (FA) and target levels of both 60 and 150 µg/m3 CAP. Not all subjects received the lower CAP exposure. After completing exposures within their O3 group, subjects were asked if they were willing to carry on with exposures for the other O3 group. Due to the lengthy time commitment involved, only eight subjects consented to continue, and completed exposures from both O3 groups. There were six different exposure types and a total of 13–16 participants within each. Overall there were 88 exposures, 43 without and 45 with O3. Exposures were separated by at least two weeks, to minimize possible carryover effects from the previous exposure.
Table 1.
Participant Exposure Order Groupings.
| No Ozone Group | Ozone Group | |||||
|---|---|---|---|---|---|---|
| Participants | FA | CAP60 | CAP150 | FA+O3 | CAP60+O3 | CAP150+O3 |
| 4 N, 3 A | X | X | X | |||
| 4 N, 4 A† | X | X | X | |||
| 2 N | X | X | X | X | ||
| 3 N, 3 A | X | X | X | X | X | X |
| Exposure totals: | 15 | 13 | 15 | 16 | 13 | 16 |
Abbreviations: FA, filtered air; CAP60, concentrated ambient particle, target 60 µg/m3; CAP150, concentrated ambient particle, target 150 µg/m3; O3, ozone; N, non-asthmatic; A, asthmatic.
One participant did not complete the CAP60+O3 exposure.
CAP and O3 Exposures
Subjects arrived at the lab around 0900 hrs for each exposure, to reduce the impact of diurnal variation. CAP exposures were carried out using a Harvard sequential, high-flow (1,000 L/min) two-stage virtual impactor designed to concentrate ambient particles between 0.15 and 2.5 µm aerodynamic diameter (Sioutas et al., 1997). The CAP facility also included a clean air/inlet dilution system to maintain CAP levels at the target concentrations, and a participant enclosure with face mask delivery. The CAP facility and exposure characterization have been described in detail previously (Petrovic et al., 2000; Urch et al., 2004; Urch et al., 2005). Briefly, the 2-hr average CAP and O3 levels were determined using the gravimetric exposure PM2.5 mass concentrations and the 15-sec O3 analyzer concentrations (Dasibi, model 1008RS, Dasibi Environmental Corp., Glendale, CA). During exposures a sample was collected on a 47 mm Gelman Teflon 2 µm pore size filter. After exposures, temperature/humidity conditioned filters were analyzed for total mass followed by analysis of the major inorganic ions by ion chromatography (Dionex, model DX-300, Dionex Corp., Sunnyvale, CA).
Exposure Protocol
For each exposure a 3-day testing protocol was followed, as detailed with timelines for each test in Table 2. The exposure was carried out on day-2, with tests before (pre) and after (post). The following three sections describe the pulmonary function measures and exposure breathing parameters, induced sputum and blood cytokines.
Table 2.
Timeline of Outcome Measurements for the 3-day Exposure Protocol.
| Time | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| 9 am | flow-volume | blood (pre) | blood (20-hr post) |
| PC20† | DlCO/lung volumes | flow-volume | |
| sputum (pre) | flow-volume | PC20† | |
| sputum (20-hr post) | |||
| 11 am – 1 pm | 2-hr exposure†† | ||
| 1:10 pm | blood (10-min post) | ||
| flow-volume | |||
| DlCO/lung volumes | |||
| 3 pm | 30-min exercise test | ||
| flow-volume | |||
| 4 pm | sputum (3-hr post) | ||
| blood (3-hr post) | |||
Abbreviations: PC20, methacholine challenge provocation concentration causing a 20% decrease in forced expiratory 1-sec volume from control; DlCO, lung diffusion capacity for carbon monoxide.
The post exposure time in brackets is the time the test was carried out with reference to the end of the 2-hr exposure.
Asthmatics only.
Respiratory minute ventilation and frequency measured at start and every 30 minutes thereafter.
Pulmonary Function and Exposure Breathing Parameters
Standardized testing procedures were followed (ATS, 1995) using equipment/methodology described previously (Liu et al., 1997). Measures included spirometry, lung volumes, airway resistance, and a single-breath diffusion test of carbon monoxide uptake. A methacholine challenge was carried out in asthmatics only, to determine the PC20, the day before and after exposures. Respiratory minute ventilation (MV, L/min) and frequency (fr, breaths/min) were measured at the start of the exposure and every 30 min thereafter with a precision turbine-type flow meter (VMM-401, Interface Assoc., Aliso Viejo, CA). The tidal volume (VT, L/breath) was calculated as the ratio of MV to fr.
Induced Sputum Cytology and Cytokines
Sputum was induced through inhalation of hypertonic saline (3, 4, then 5%) nebulized with an ultrasonic nebulizer (Liu et al., 1999). Sputum plugs were extracted and processed as per Pizzichini et al. (1996). A technician blinded to the exposure type performed differential cell counts by identifying 400 cells as neutrophils, macrophages, eosinophils, lymphocytes, basophils or bronchial epithelial cells. Two non-asthmatics and one asthmatic were unable to produce sputum samples. In only half of the 88 exposures were subjects able to produce sputum samples for pre and post exposures. Sputum supernatants were analyzed for IL-6, IL-8, IL-10, TNF-α and leukotriene-B4 using ELISA kits (Amersham Pharmacia Biotech, NJ). Some sputum samples yielded low supernatant volumes, insufficient for cytokine analyses, which resulted in IL-6 data available for only 30/88 (34%) of exposures (pre and post) and less for the other mediators. Since data was even less sparse within each of the six exposure types, we do not report results for any sputum mediators.
Blood IL-6 and TNF-α
Venous blood was collected in EDTA tubes and plasma frozen at −70°C. The plasma was later analyzed for IL-6 and TNF-α using ELISA kits.
Statistical Analysis
The study design was a randomized block with subjects randomly assigned to no O3 or added O3 groups (see Exposure Groups). Thus, each person acted as their own control. In order to examine the exposure-induced change, the post minus pre-exposure difference (Δ) was calculated for each response variable, separately for the three post exposure time points (10 min, 3 and 20 hrs). For breathing parameters measured during exposure (e.g., tidal volume) and pulmonary function, the percent change (%Δ) was calculated. Analysis of variance and multiple regression was used to assess the association between these measures of change (Δ, %Δ) and CAP, O3 and their interaction using the SAS MIXED procedure (SAS Institute, Inc., Cary, NC, version 9).
We initially hypothesized that we would observe dose response associations between the 2-hr exposure mass concentrations and study outcome measures. We thus pre-selected exposure CAP target levels (60 and 150 µg/m3) that we hoped would be distinct from each other. However, the CAP exposure level control was not as precise as that for O3 levels, and when gravimetric PM determinations were completed there was some overlap in mass concentrations between the 60 and 150 µg/m3 CAP groups. This overlap in mass concentrations would have made interpretation of the results difficult for inter-group comparisons when treating CAP as categorical. In order to obtain a distinct dichotomy of CAP levels, we redefined the two CAP groups as <100 and ≥100 µg/m3, or roughly mid-point between the target levels of 60 and 150 µg/m3. As a result, three individuals that had CAP targets of 60 µg/m3, but in fact received higher gravimetric mass concentrations, were now in the ≥100 µg/m3 CAP group, and one individual that had a CAP target of 150 µg/m3, was now in the <100 µg/m3 CAP group.
With the re-defined CAP groups, an initial analysis was carried out using O3 as a binary variable and CAP as a 3-level categorical variable, considering the effects of CAP with a mass concentration <100 and ≥100 µg/m3 compared to exposures without CAP (FA and FA+O3). Each model included a random subject effect. In a second approach, CAP was considered as a continuous fixed effect and O3 as a binary variable, since O3 levels were relatively constant during O3 exposures, whereas CAP levels varied during CAP exposures. We also examined the possible modification of CAP effects by O3 (CAP-O3 interaction) and by asthma status. In order to examine associations within a given exposure (e.g., for CAP+O3 exposure, association between tidal volume 2-hr %Δ and 3-hr post IL-6 Δ), the Pearson correlation coefficient was calculated. Differences in physical characteristics, and baseline spirometry and mediators between asthmatics and non-asthmatics were tested using an unpaired t test. Data are reported as mean values ± standard errors.
RESULTS
Participant Characteristics
The 23 participants had a mean age of 27±1 years, ranging from 21 to 40, and normal spirometry— FEV1 and FVC were >90% of the predicted normal values (Table 3). Asthmatics had a lower mean FEV1/FVC ratio than non-asthmatics (79±2% vs. 85±2%, p=0.03) and higher sputum eosinophils (3.6±1.2% vs. 0.4±0.2%, p=0.03, data not shown). The methacholine PC20, which defined the two groups, ranged from 0.08 to 7.6 mg/ml for asthmatics. Baseline blood IL-6 and TNF-α were not significantly different between asthmatics and non-asthmatics.
Table 3.
Participant Physical Characteristics, Baseline Spirometry and Mediators.
| Asthmatics | Non-Asthmatics | |
|---|---|---|
| Gender (male/female) | 4/6 | 7/6 |
| Age (years) | 27.5 ± 1.9 | 26.5 ± 1.5 |
| BMI (kg/m2) | 21.6 ± 0.9 | 22.9 ± 0.7 |
| Skin prick (# positive) | 2.5 | 1.8 |
| PC20 (mg/ml) | 2.2 | > 8 |
| FEV1 (%pred) | 90.4 ± 2.5 | 99.3 ± 3.4 |
| FVC (%pred) | 96.7 ± 2.6 | 98.2 ± 2.9 |
| FEV1/FVC (%)† | 78.9 ± 2.2 | 84.9 ± 1.5 |
| Blood TNF-α (pg/ml) | 0.10 ± 0.02 | 0.16 ± 0.04 |
| IL-6 (pg/ml) | 0.42 ± 0.06 | 0.66 ± 0.17 |
Values reported as means ± SEs.
Skin prick test is # allergens with wheal diameter ≥ 3 mm.
Abbreviations: BMI, body mass index; PC20, see Table 2; FEV1, forced expiratory 1-sec volume; FVC, forced vital capacity; %pred, percent of predicted normal values (according to Hankinson et al. 1999).
Unpaired t-test, p=0.03, asthmatics versus non-asthmatics.
Exposure PM, Gaseous and Environmental Measurements
The mean PM2.5 concentrations for CAP≥100 and CAP≥100+ O3 were quite similar (140 and 142 µg/m3, respectively) and approximately double those for the lower CAP groups (64 and 68 µg/m3) (Table 4). The mean O3 concentrations were close to the 120 ppb target (117–119 ppb) and showed less variability than PM2.5. The other gaseous pollutant levels, including O3 for the no O3 group, were below ambient levels. For exposures with added O3, nitric oxide (NO) levels were lower and nitrogen dioxide (NO2) levels higher compared to the no O3 group. This was an experimental artefact, as the ambient NO in the inlet air reacted with the added O3 to form NO2, thus increasing NO2 and decreasing NO levels; even so, the sum of NO+NO2 was still below the ambient NO+NO2 level.
Table 4.
Pollutant Levels and Environmental Conditions during Exposures.
| No Ozone Group | Ozone Group | |||||
|---|---|---|---|---|---|---|
| FA | CAP<100 | CAP≥100 | FA+O3 | CAP<100+O3 | CAP≥100 +O3 | |
| Observations | 15 | 12 | 16 | 16 | 12 | 17 |
| Pollutants | ||||||
| PM2.5 (µg/m3) | 8±2 | 64±3 | 140±6 | 2±1 | 68±5 | 142±7 |
| O3 (ppb) | 2±1 | 6±2 | 5±1 | 117±1 | 119±1 | 119±1 |
| NO (ppb) | 31±9 | 20±3 | 29±6 | 9±2 | 6±1 | 9±2 |
| NO2 (ppb) | 21±2 | 18±1 | 22±2 | 52±9 | 34±3 | 50±6 |
| Inorganic Ions | ||||||
| NH4+ (µg/m3) | 0.0±0.1 | 2.4±2.0 | 6.2±4.7 | 0.0±0.1 | 6.0±9.3 | 6.4±4.1 |
| SO42− (µg/m3) | 0.0±0.1 | 7.2±5.6 | 17.6±12.0 | 0.1±0.2 | 11.5±9.4 | 19.3±12.4 |
| NO3− (µg/m3) | 0.0±0.2 | 2.4±1.6 | 14.2±11.7 | 0.1±0.2 | 7.8±14.6 | 14.9±11.2 |
| Environmental | ||||||
| Temp (°C) | 23.6±0.3 | 24.4±0.3 | 24.1±0.3 | 23.7±0.2 | 23.5±0.3 | 23.6±0.3 |
| RH (%) | 22±2 | 28±4 | 26±3 | 25±3 | 36±4 | 24±4 |
Abbreviations: FA, filtered air; CAP<100 and CAP≥100, concentrated ambient particle exposures with a gravimetric 2-hr integrated mass concentration <100 and ≥100 µg/m3; PM2.5, particulate matter with an aerodynamic diameter <2.5 µm; O3, ozone; NO, nitric oxide; NO2, nitrogen dioxide; NH4+; ammonium; SO42−, sulphate; NO3−, nitrate; temp/RH, temperature and relative humidity.
Note: Filter sample (PM2.5/inorganic ions), gaseous pollutants and temp/RH were taken from the FA/CAP airstream delivered to the subject.
For all exposures, SO2 and CO levels were below 5.1 ppb & 0.6 ppm, respectively.
Exposure Breathing Parameters and Pulmonary Function
There were no significant effects of CAP or O3 for the 2-hr percent change in exposure breathing parameters (MV, fr, VT) or for the percent change in any pulmonary function measures including the PC20 of the asthmatics (data not shown).
Sputum Cytology
No significant CAP- or O3-induced changes were observed in sputum total cell counts. Furthermore, the two main cell types, macrophages and neutrophils, also showed no significant changes in the absolute number or percentage of cells (data not shown).
Blood IL-6
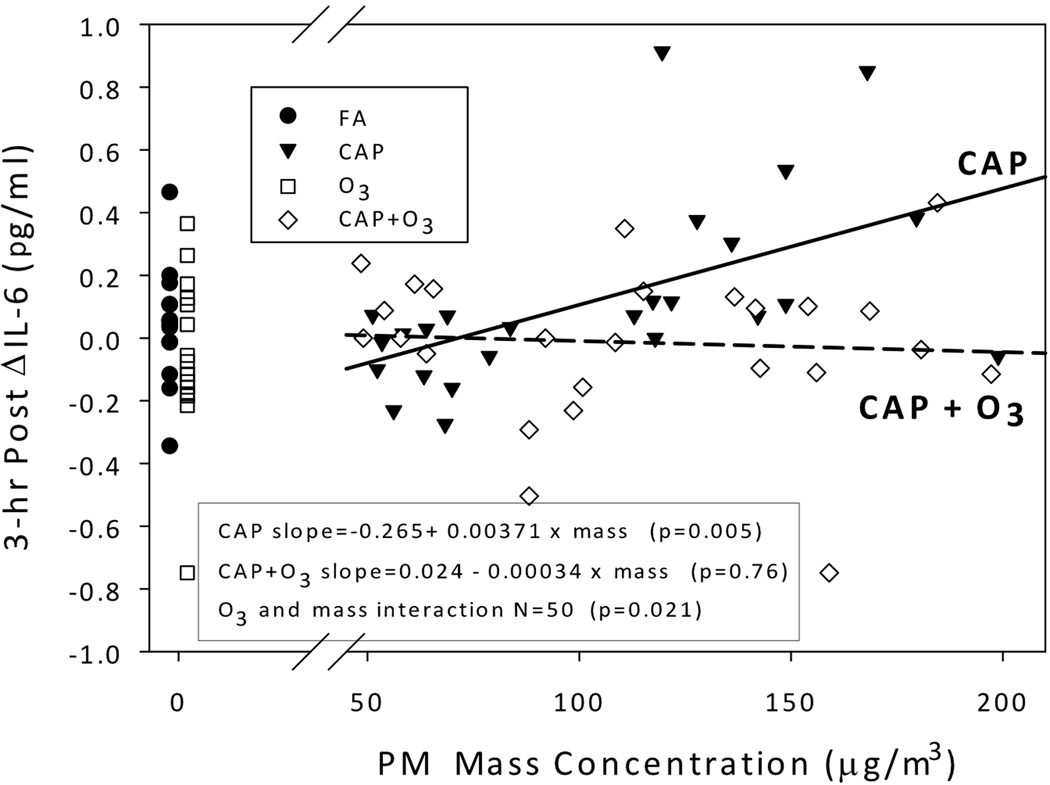
There were no significant effects of CAP or O3 on Δ IL-6 levels immediately after or the day after the 2-hr chamber study in the 3-level CAP models. At 3 hrs after exposure, however, for CAP alone exposures with a mass concentration ≥100 µg/m3, blood IL-6 levels were increased above pre-exposure; the least square mean increase was 0.29 pg/ml (95% CI: 0.16, 0.42, p<0.0001). The 3-hr post Δ IL-6 for CAP <100 µg/m3, with or without O3, did not increase, with responses similar to those of FA and FA+O3. Since we only observed differences in Δ IL-6 between the two levels of CAP, we further examined the Δ IL-6 association for CAP-containing exposures only, treating CAP as a continuous variable (Figure 1). For CAP alone exposures we observed a significant positive association of mass concentration with the 3-hr post Δ IL-6 levels (slope= 0.00371 × mass, p=0.005), compared to no association for CAP+O3 exposures (slope= −0.00034 × mass, p=0.76). Thus there was significant modification of CAP effects by O3 (CAP and O3 interaction, p=0.021). Ozone, as a main effect, and having asthma (covariate) were not significantly associated with the 3-hr post Δ IL-6 (p=0.14 and 0.66, respectively).
FIG 1.
Association between CAP mass concentration and blood IL-6 change 3-hrs post for CAP and CAP+O3 exposures. Individual data are shown for 80 exposures. Data for FA (●) and O3 (□) are shown separately to the left. A solid regression line is shown for CAP observations (▼) and dashed line for CAP+O3 observations (◊). There was a significant positive association between PM mass concentration and IL-6 change 3-hrs after for CAP but not for CAP+O3 exposures, with significant effect modification by ozone. The 3-hr post ΔIL-6= 3-hr IL-6 minus pre-exposure IL-6. Abbreviations: FA, filtered air; CAP, concentrated ambient particle; O3, ozone; PM, particulate matter.
Exposure Tidal Volume versus Blood IL-6
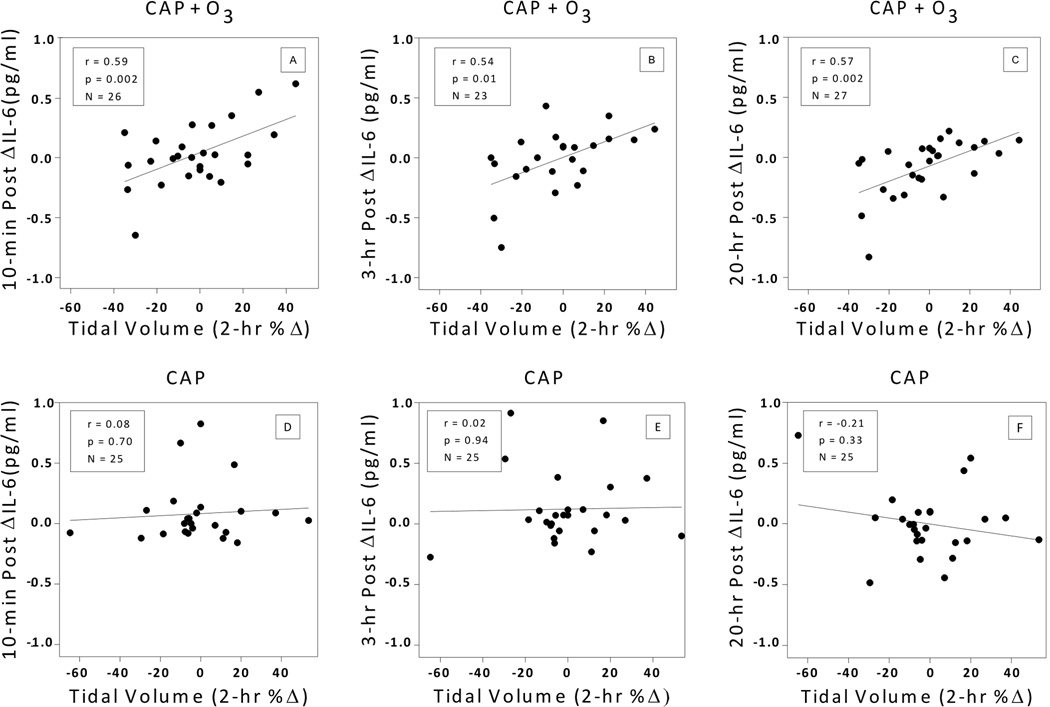
The latter finding of no increase in IL-6 after CAP+O3 compared to CAP alone led us to examine whether the pattern of breathing in individuals differed between the CAP+O3 and CAP exposures, potentially modifying the IL-6 responses. The result of this further investigation was, for CAP+O3 exposures, we observed a significant positive association between the 2-hr percent change in VT during exposure and the post exposure change in blood IL-6, at all three post exposure time points (Figures 2a–c). The correlation coefficients ranged from r=0.54 to 0.59 (p≤0.01). In stark contrast, for CAP exposures without O3, there were no significant associations (p>0.3) between VT and IL-6 (Figures 2d–f). Respiratory MV was weakly positively associated with the CAP+O3 IL-6 change (r=0.32 to 0.38, p≥0.06), while fr was not associated with IL-6 (p>0.2) (data not shown).
FIG 2.
Association between tidal volume 2-hr percent change and blood IL-6 change post exposure. Individual data are shown. Regression lines are shown by solid lines. Pearson partial correlations coefficients are reported, controlling for repeated measures of CAP<100 and CAP≥100. (A–C) CAP+O3 exposures: 10-min post; 3-hrs post; and 20-hrs post. (D–F) CAP exposures: 10-min post; 3-hrs post; and 20-hr post. There were significant positive associations between tidal volume percent change during CAP+O3 exposures and IL-6 changes at all three time points after but not for CAP alone exposures. Tidal volume 2-hr %Δ= 100 × [(2-hr minus 0-hr) / 0-hr]. Post ΔIL-6= post IL-6 minus pre-exposure IL-6. Abbreviations: CAP, concentrated ambient particle; O3, ozone.
In Figure 1, it is apparent that IL-6 did not increase after CAP+O3 exposures (slope= −0.00034 × mass), as well as after O3 alone exposures (mean decrease of 0.06±0.06). Furthermore, for CAP+O3 we observe that as VT decreases so does IL-6 after exposure, but not after CAP alone. Thus, the presence or absence of O3 with CAP may be an important factor. To better visualize this association, we created a 2×2 table of the binary 2-hr %Δ in VT and the binary 3-hr post Δ IL-6, separately for O3 and no O3 exposures; both variables were dichotomized as a decrease, or an increase ≥0 (Table 5). Results for the 39 exposures with O3 showed that 70% of the 20 responding with a decrease in VT, had a corresponding decrease in IL-6, compared to only 32% among the 19 who responded with no decrease in VT (p=0.026). No significant differences were seen for the corresponding changes among those not exposed to O3.
Table 5.
Tidal Volume 2-hr Percent Change by 3-hr Post Exposure Blood IL-6 Changes, for Ozone and no Ozone Exposures.
| Ozone Exposures† | No Ozone Exposures††, ## | |||
|---|---|---|---|---|
| Tidal Volume | IL-6 decrease | IL-6 decrease ≥0 | IL-6 increase | IL-6 decrease ≥0 |
| VT decrease | 14 (70.0%) | 6 (30.0%) | 9 (40.9%) | 13 (59.1%) |
| VT increase ≥0 | 6 (31.6%) | 13 (68.4%) | 4 (23.5%) | 13 (76.5%) |
Abbreviations: VT, tidal volume.
Values are number of exposures (percent of row total).
Tidal volume 2-hr percent change= 100 × [(2-hr VT minus 0-hr VT)/0-hr VT].
IL-6 3-hr post exposure change= 3-hr IL-6 minus pre-exposure IL-6.
Fisher’s Exact test: Odds Ratio= 5.1, p=0.026 (n=39).
Fisher’s Exact test: Odds Ratio= 2.3, p=0.32 (n=39).
Breslow-Day comparison of two odds ratios p=0.42.
Blood TNF-α
There were no significant CAP or O3 induced changes in blood TNF-α at any of the post exposure time points.
DISCUSSION
In a controlled human exposure study, we demonstrated a PM2.5-associated increase in systemic blood IL-6, but only for CAP exposures without added O3. This finding was contrary to our a priori hypothesis of a more adverse response to CAP+O3. We explored three possibilities to explain this. First, although the overall mean PM mass concentrations were similar for the CAP and CAP+O3 exposure groups (107±8 versus 111±8 µg/m3, p=0.72), we could not control individual PM constituent levels, which might have differed enough between CAP and CAP+O3 exposures to explain the different responses. The study design was a randomized block, so this should have helped to reduce any constituent level exposure bias. A comparison of CAP versus CAP+O3 constituent concentrations of ammonium, sulphate and nitrate showed similar levels across the CAP<100 and CAP≥100 exposures (Table 4). We did not measure elemental carbon, organic carbon, or trace elemental constituents of CAP. It is possible that they differed between CAP and CAP+O3 exposures, but unlikely given the randomized sequence and the similarity of other constituents. Thus, differences in CAP with and without ozone are unlikely to explain differences in response. Second, 15/23 subjects were different between the CAP and CAP+O3 exposure groups. We thus examined group differences that could have accounted for the heterogeneity in blood IL-6 responses. Baseline characteristics and other exposure measures such as minute ventilation and pulmonary function changes revealed no significant differences between the two groups. However, individuals exposed to CAP alone had a significantly lower pre-exposure IL-6 compared to those individuals exposed to CAP+O3 (0.46±0.07 vs. 0.81±0.14 pg/ml, p=0.04); yet for CAP-containing exposures the pre-exposure IL-6 was not significantly correlated with the 3-hr post Δ IL-6 (r= −0.19, p=0.18). Thus, differences among individual subjects are unlikely to explain differences in IL-6 response by the CAP and CAP+O3 groups.
A third possible explanation is that breathing patterns differed between CAP and CAP+O3 exposures, causing differences in inhaled particle deposition and the resulting IL-6 response. Indeed, for CAP+O3 exposures we observed a significant positive association between the 2-hr tidal volume percent change and the post exposure IL-6 change, at all three time points (Figures 2a–c). However, in dramatic contrast, we saw no associations of any breathing parameters with IL-6 for CAP alone exposures (Figures 2d–f). Thus, for CAP+O3 exposures, we can infer that individuals who had a decrease in their tidal volume over the course of the exposure had a corresponding decrease in IL-6 after. The plausibility of this inference is strengthened by the fact that all three time points showed a similar strength of the association. Of note, among the O3 exposures 70% of the individuals who exhibited shallow breathing had a decrease in IL-6, compared to only 32% among those without shallow breathing (p=0.026). This difference was attenuated for exposures without O3, 41% versus 24%, (p=0.32). This weakly suggests that O3 may have been the stimulus of this response.
The latter finding of breathing pattern changes during exposures provides insight into the mechanism of PM-induced inflammation, specifically when PM is combined with O3 as an ambient co-pollutant mixture. The shallow breathing response that we observed has been reported previously for O3 exposures (2 hrs at 500 ppb, with intermittent exercise) by Hazucha et al. (1989). The mechanism the authors proposed was reflex inhibition of respiratory effort, likely originating from airway neural C-fibers. The effects of different breathing patterns on airway particle deposition (Finlay and Martin, 2008; Heyder et al., 1975; Kim and Hu, 2006) and O3 uptake (Ultman et al., 2004) have been examined, showing an association between lower tidal volumes and less PM deposition/O3 uptake. Our finding that some individuals switch to shallow breathing during CAP+O3 exposures and that this in turn may attenuate the systemic IL-6 response, suggests a protective mechanism. It was not apparent that there were any exposure cues to identify the CAP+O3 exposures. When subjects were asked if they thought they had been exposed to a pollutant, only 52% (15/29) of the CAP+O3 exposures were correctly identified. Furthermore, there was no significant difference in tidal volume changes between those who correctly identified the pollutant exposures vs. those who didn’t (data not shown). The latter evidence supports the hypothesis that the altered breathing pattern was most likely an involuntary reflex, possibly mediated through airway C-fiber stimulation by O3, or a combined effect of particles and O3. An irritant reflex mechanism has been proposed as a response to inhalation of O3 (Hazucha et al., 1989; Lee and Widdicombe, 2001) and particles (Passannante et al., 1998).
In other human CAP studies, Ghio et al. (2003) reported no significant changes in blood TNF-α or IL-6 immediately and 20 hrs after 2-hr exposures with intermittent exercise (IE) to fine CAP (121±14 µg/m3) compared to FA. Gong et al. (2003) observed a 36% increase in blood IL-6 among asthmatics 4 hrs after a 2-hr exposure with IE to fine CAP (174±8 µg/m3), which was similar to our mean 56% increase in IL-6 after CAP≥100 (59% for asthmatics, 55% for non-asthmatics). Gong’s reported 18% increase in IL-6 for asthmatics after FA was similar to our observed increase of 8%. In contrast though, Gong and authors observed a 26% increase in blood IL-6 among non-asthmatics and this response was not greater than that of FA (39% increase). Similar to Ghio et al. (2003), we also did not observe any significant CAP-induced changes in TNF-α. Delfino et al. (2008) measured blood levels of mediators including CRP, IL-6, TNF-α and the TNF-α soluble receptor II in a panel of 29 non-smokers over 65 yrs of age that had a history of coronary artery disease. The authors showed significant positive associations between markers of primary combustion products (PM2.5 elemental and black carbon and primary organic carbon) and IL-6, CRP and TNF-α soluble receptor II. Associations were positive but largely non-significant with the cytokine TNF- α. They suggested that the differences in significance for TNF-α may have been due to the shorter half-lives and lower levels for TNF-α compared to its soluble receptor. Thus our finding of no significant changes in TNF-α may not necessarily indicate lack of response.
The association between CAP exposure and IL-6 response was not modified by having asthma. Although one might have expected a greater response in the individuals with asthma, the asthmatics who participated in our study were well-controlled and mild, as evidenced by a mean FEV1/FVC ratio of 79% compared to 85% for non-asthmatics, and baseline sputum eosinophils of 3.6%, although significantly elevated when compared to the 0.4% for non-asthmatics, also indicative of very mild inflammation. The fact that our mild asthmatic subjects were not more susceptible should, however, not be generalized to represent responses for more severe asthma or for other respiratory diseases.
The strength of our study was that we compared CAP+O3 responses to the individual pollutant responses, whereas previous studies have only examined CAP alone. The CAP target PM2.5 concentration of 150 of µg/m3 (maximum, 199 µg/m3) is higher than that typically observed in urban environments over 24 hrs. However, levels exceeding 150 µg/m3 can occur for one to two hr periods in many North American locations and are commonly encountered over even longer durations throughout developing nations (Michaels and Kleinman, 2000; Streets et al., 2007). In a panel study of asthmatic children living in Mexico City, high levels of daily PM10 > 200 µg/m3 (~100 µg/m3 PM2.5) and hourly O3 > 100 ppb were observed simultaneously about 25% of the time over an 8 month period (Romieu et al., 1996). Not all subjects received the six exposures due to the lengthy time commitment; even so, the dependent variables in statistical analyses were the change over time within a subject, thus each person acted as their own control. Many variables were measured: pulmonary function/ breathing parameters (15); sputum cytology (5); and two blood measurements including TNF-α and IL-6, the latter being significant. No multiple comparison adjustment was applied because our lack of fine CAP-induced pulmonary function changes is supported by other studies (Ghio et al., 2000; Gong et al., 2003) and the sample size for the five sputum cytology variables was too small. Strengthening the plausibility of our main finding of an increase in IL-6 with CAP exposure was the significant association between IL-6 and exposure mass concentration.
In conclusion, for healthy individuals and mild asthmatics we have demonstrated a transient increase in systemic IL-6, 3 hrs after CAP exposure. Furthermore, the blood IL-6 increase was significantly associated with the PM2.5 mass concentration and not O3. We hypothesize that modification of the CAP response by concomitant O3 exposure may be modulated through the pulmonary autonomic response to CAP+O3 in some of our subjects, resulting in reduced tidal volume and reduced IL-6 response. Acute effects of this magnitude in healthy individuals are accommodated by normal defence mechanisms, thus are transient and sub-clinical. However, more prolonged exposures at elevated PM levels and episodic exposures in susceptible populations provide an increased risk. Our data provide evidence to support the association between ambient levels of particles and cardiopulmonary morbidity and mortality. We did not show a greater effect with the concomitant CAP+O3 exposure, but did provide some insight that may help to explain equivocal epidemiological findings when comparing health effects of PM and O3, alone and combined.
Acknowledgments
This work was supported by Natural Resources Canada, Health Canada (Toxic Substances Research Initiative), Air Quality Health Effects Research Section Government of Canada, Environment Canada, Ontario Thoracic Society, AllerGen NCE Inc., NIH/NIEHS (P01 ES09825) and U.S. EPA (R832416). We thank the staff at GOEHU that contributed and Vladimir Lukic for the Figure illustrations. Although the research described in this article was funded in part by the U.S. EPA through grant R832416 to Harvard University, it has not been subjected to the Agency's required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
Footnotes
DECLARATION OF INTEREST
The authors declare they have no competing financial interests.
REFERENCES
- American Thoracic Society. Standardization of Spirometry. 1994 Update. Am. J. Respir. Crit. Care. Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. J. Am. Med. Assoc. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Kim JY, Dominici F. Potential confounding of particulate matter on the short-term association between ozone and mortality in multisite time-series studies. Environ. Health Perspect. 2007;115:1591–1595. doi: 10.1289/ehp.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Cakmak S, Brook JR. The effect of the urban ambient air pollution mix on daily mortality rates in 11 Canadian cities. Can. J. Public Health. 1998;89:152–156. doi: 10.1007/BF03404464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ. Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am. J. Physiol. 1994;266:L612–L619. doi: 10.1152/ajplung.1994.266.6.L612. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA., III Acute respiratory effects of particulate air pollution. Annu. Rev. Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- Dockery DW. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ. Health Perspect. 2001;109(Suppl 4):483–486. doi: 10.1289/ehp.01109s4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. J. Am. Med. Assoc. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ. Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay WH, Martin AR. Recent advances in predictive understanding of respiratory tract deposition. J. Aerosol Med. Pulm. Drug Deliv. 2008;21:189–205. doi: 10.1089/jamp.2007.0645. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhal. Toxicol. 2003;15:1465–1478. doi: 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Huang YC. Exposure to concentrated ambient particles (CAPs): a review. Inhal. Toxicol. 2004;16:53–59. doi: 10.1080/08958370490258390. [DOI] [PubMed] [Google Scholar]
- Gong H, Jr, Sioutas C, Linn WS. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient particles in metropolitan Los Angeles. Res. Rep. Health Eff. Inst. 2003:1–36. [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Hazucha MJ, Bates DV, Bromberg PA. Mechanism of action of ozone on the human lung. J. Appl. Physiol. 1989;67:1535–1541. doi: 10.1152/jappl.1989.67.4.1535. [DOI] [PubMed] [Google Scholar]
- Heyder J, Armbruster L, Gebhart J, Grein E, Stahlhofen W. Total deposition of aerosol particles in the human respiratory tract for nose and mouth breathing. J. Aerosol Sci. 1975;6:311–328. [Google Scholar]
- Kim CS, Hu SC. Total respiratory tract deposition of fine micrometer-sized particles in healthy adults: empirical equations for sex and breathing pattern. J. Appl. Physiol. 2006;101:401–412. doi: 10.1152/japplphysiol.00026.2006. [DOI] [PubMed] [Google Scholar]
- Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am. J. Respir. Crit. Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Widdicombe JG. Modulation of airway sensitivity to inhaled irritants: Role of inflammatory mediators. Environ. Health Perspect. 2001;109(Suppl. 4):585–589. doi: 10.1289/ehp.01109s4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Leech JA, Urch RB, Silverman FS. In vivo salicylate hydroxylation: a potential biomarker for assessing acute ozone exposure and effects in humans. Am. J. Respir. Crit. Care Med. 1997;156:1405–1412. doi: 10.1164/ajrccm.156.5.9610105. [DOI] [PubMed] [Google Scholar]
- Liu L, Leech JA, Urch RB, Poon R, Zimmerman B, Kubay JM, Silverman F. A comparison of biomarkers of ozone exposure in human plasma, nasal lavage, and sputum. Inhal. Toxicol. 1999;11:657–674. doi: 10.1080/089583799196790. [DOI] [PubMed] [Google Scholar]
- Michaels RA, Kleinman MT. Incidence and apparent health significance of brief airborne particle excursions. Aerosol Sci. Technol. 2000;32:93–105. [Google Scholar]
- Passannante AN, Hazucha MJ, Bromberg PA, Seal E, Folinsbee L, Koch G. Nociceptive mechanisms modulate ozone-induced human lung function decrements. J. Appl. Physiol. 1998;85:1863–1870. doi: 10.1152/jappl.1998.85.5.1863. [DOI] [PubMed] [Google Scholar]
- Petrovic S, Urch B, Brook J, Datema J, Purdham J, Liu L, Lukic Z, Zimmerman B, Tofler G, Downar E. Cardiorespiratory effects of concentrated ambient PM2.5: A pilot study using controlled human exposures. Inhal. Toxicol. 2000;12(Suppl 1):173–188. [Google Scholar]
- Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE, Dolovich J. Measurement of inflammatory indices in induced sputum: effects of selection of sputum to minimize salivary contamination. Eur. Respir. J. 1996;9:1174–1180. doi: 10.1183/09031936.96.09061174. [DOI] [PubMed] [Google Scholar]
- Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, Whited MC, Etzel RA. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. Am. J. Respir. Crit Care Med. 1996;154(2 Pt 1):300–307. doi: 10.1164/ajrccm.154.2.8756798. [DOI] [PubMed] [Google Scholar]
- Rückerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Küchenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J, Peters A. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ. Health Perspect. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet J, Krewski D. Health effects associated with exposure to ambient air pollution. J. Toxicol. Environ. Health A. 2007;70:227–242. doi: 10.1080/15287390600884644. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Schwartz J, Catalano PJ, Suh HH. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ. Health Perspect. 2001;109:1053–1061. doi: 10.1289/ehp.011091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J. Air Waste Manag. Assoc. 1996;46:927–939. [PubMed] [Google Scholar]
- Schwartz J. Air pollution and blood markers of cardiovascular risk. Environ. Health Perspect. 2001;109(Suppl 3):405–409. doi: 10.1289/ehp.01109s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioutas C, Koutrakis P, Godleski JJ, Ferguson ST, Kim CS, Burton RM. Fine particle concentrators for inhalation exposures—Effect of particle size and composition. J. Aerosol Sci. 1997;28:1057–1071. [Google Scholar]
- Streets DG, Fu JS, Jang CJ, Hao J, He K, Tang X, Zhang Y, Wang Z, Li Z, Zhang Q, Wang L, Wang B, Yu C. Air quality during the 2008 Beijing Olympic Games. Atmosph. Environ. 2007;41:480–492. [Google Scholar]
- Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, D'Yachkova Y, Hogg JC. The human bone marrow response to acute air pollution caused by forest fires. Am. J. Respir. Crit. Care Med. 2000;161:1213–1217. doi: 10.1164/ajrccm.161.4.9904084. [DOI] [PubMed] [Google Scholar]
- Ultman JS, Ben-Jebria A, Arnold SF. Uptake distribution of ozone in human lungs: intersubject variability in physiologic response. Res. Rep. Health Eff. Inst. 2004:1–23. [PubMed] [Google Scholar]
- Urch B, Brook JR, Wasserstein D, Brook RD, Rajagopalan S, Corey P, Silverman F. Relative contributions of PM2.5 chemical constituents to acute arterial vasoconstriction in humans. Inhal. Toxicol. 2004;16:345–352. doi: 10.1080/08958370490439489. [DOI] [PubMed] [Google Scholar]
- Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ. Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10) Am. J. Respir. Crit. Care. Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]