Abstract
The nucleus accumbens (NAcc) is often implicated in schizophrenia (SZ) pathology, but with little evidence to support its role. This study examined postmortem human tissue to determine if abnormalities are present in the dopaminergic or glutamatergic systems in the NAcc in SZ. We compared the protein levels of tyrosine hydroxylase (TH) and vesicular glutamate transporters vGLUT1 and vGLUT2 in control (n = 7) and schizophrenia (n = 13) subjects using western blot analysis. The SZ subjects were further divided by treatment status: SZ on-drug (SZ-ON, n = 6) and SZ off-drug (SZ-OFF, n = 7), to assess the effects of antipsychotic treatment. TH protein levels were similar between control and SZ subjects, and there was no difference between SZ-ON and SZ-OFF subjects. Protein levels of vGLUT1 were similar in control and SZ subjects, and there was no difference in vGLUT1 protein levels between SZ-ON and SZ-OFF subjects. In contrast, vGLUT2 protein levels were significantly elevated in the SZ group (25% increase). Protein levels of vGLUT2 did not differ between SZ-ON and SZ-OFF subjects. Similar levels of TH suggest the presynaptic DA pathway may be normal in the NAcc in SZ. The elevated vGLUT2 protein levels, but not vGLUT1, suggest the NAcc receives increased glutamatergic input in SZ, possibly from thalamic or other subcortical origins. The similarity between SZ-ON and SZ-OFF subjects suggests that the results are not caused by APD treatment. These findings provide further insight into the role of the NAcc in SZ.
Keywords: striatum, dopamine, antipsychotic, western blot, human
1. Introduction
The cause of schizophrenia (SZ) remains elusive, however decades of research have revealed common pathologies, some of which are now hallmarks of the disorder. One of these hallmarks is abnormalities in the dopamine (DA) system in the striatum of patients with SZ (Miyake et al, 2011). Of special interest is a subregion of the ventral striatum, the nucleus accumbens (NAcc), which has been assumed to be a prime location for the elevated DA levels in SZ, based on its functional properties and evidence of antipsychotic drug (APD) action here (Deutch and Cameron, 1992; Deutch et al, 1992; Roberston and Fibiger, 1992; Merchant and Dorsa, 1993). Until recently, imaging studies have had to analyze the striatum as a single region, without the ability to distinguish between key functional and anatomical areas. Thus, not only has the role of the NAcc has never been confirmed, but recent studies with improved imaging techniques suggest that this subregion may not be implicated as previously thought (Howes et al, 2009, 2011; Kegeles et al, 2010). Additionally, conclusions surrounding the DA abnormalities in the striatum lack solid support from postmortem studies, which offer the ability to study these subregions individually. Previous studies in postmortem NAcc report conflicting results, typically finding no change (Crow et al, 1979; Farley et al, 1977; Owen et al, 1978; Toru et al, 1982) or increases (Bird et al, 1979; Mackay et al, 1982) in DA measures.
Another, more recent hypothesis of SZ is a causal role of glutamate abnormalities. Within the NAcc, a hypothesis emerged that pathology of the glutamate system could drive DA dysfunction (Lodge and Grace, 2007, 2011). NMDA antagonists have been shown to enhance DA efflux in the striatum in both preclinical studies (Miller and Abercrombie, 1996) and imaging studies in healthy volunteers (Kegeles et al, 2000). Further, stimulation of glutamatergic input to the NAcc in rats causes elevated DA release in the region (Blaha et al, 1997; Legault and Wise, 1999). It is not known however, if glutamatergic abnormalities are actually present in the NAcc in SZ.
The purpose of this study was to determine if neurochemical abnormalities are present in the NAcc of postmortem SZ. To study the dopaminergic system, we analyzed tyrosine hydroxylase (TH), the rate-limiting synthesizing enzyme of DA. To study the glutamatergic system, we analyzed the vesicular glutamate transporters vGLUT1 and vGLUT2, which are essential for the uptake and storage of glutamate into synaptic vesicles (Bellocchio et al, 2000; Takamori et al, 2000). They have complementary localization patterns in cortical and subcortical structures, respectively (Hisano et al, 2000; Fremeau et al, 2001; Herzog et al, 2001) thus studying both markers provides a comprehensive analysis of glutamatergic input to the NAcc. The western blot analyses were performed both in SZ subjects that were on medication at the time of death, and in SZ subjects that were off medication at the time of death to assess APD effects.
2. Methods
2.1. Postmortem Tissue
Postmortem human brain tissue was obtained from the Maryland Brain Collection. DSM-IV diagnosis of SZ was confirmed by two psychiatrists based on patient medical records, family interviews, autopsy reports, and neuropathologic assessments. Schizophrenia subjects were considered off-drug if they had been untreated with APDs for at least 6 months prior to death. Control cases had no history of psychiatric or neurological disease. Cases were chosen based on the best match of age, race, sex, postmortem interval (PMI), and tissue pH level.
Striatal tissue was fresh frozen on dry ice and stored at −80°C until used for this study. The NAcc was dissected from tissue blocks of 7 control subjects, 6 SZ subjects on medication (SZ-ON), and 7 SZ subjects off medication (SZ-OFF). Thionin staining was used to assess the quality of the morphology for each case. Each group started with 10 cases but several were excluded due to poor tissue quality. The final numbers for each group listed above were those with good preservation that were included in the study. The tissue was sectioned on a cryostat at −16°C in 4 series of 16 μm thick sections. It was not feasible to separate the core and shell of the NAcc since the boundary between the subregions is not visible in fresh tissue, so the NAcc was analyzed as a whole.
2.2. Western Blotting
2.2.1. Protein studies
Samples from human tissue were processed as described previously (Rice et al, 2014). Briefly, one series of tissue was sonicated in a lysis buffer (diluted 1:5) containing Tris-HCL (pH 8.0), EDTA, sodium dodecyl sulfate, and a protease inhibitor cocktail (Sigma; P8340). Tissue homogenate was centrifuged at 13,500 rpm for 15 min at 4°C. Total protein concentration of the resulting supernatant was measured using a modified Lowry technique (Bio-Rad, Hercules, CA, USA; 500-0113, 500-0114).
2.2.2. Gel electrophoresis and western blotting
Western blot analysis was used to measure protein levels of TH, vGLUT1, and vGLUT2 (Table 2). The gel electrophoresis and western blot assays were performed as described previously (Rice et al, 2014) with one variation; samples used for vGLUT assays were not heated to 95°C since this causes aggregation of the protein. Samples of 60 μg of total protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 4–20% polyacrylamide gradient gels (Lonza, Basel, Switzerland; 58505), then transferred to polyvinylidene fluoride (PVDF) membranes for 21 h at 4°C. One set of membranes was blotted for TH. A separate set of membranes was blotted for vGLUT2, stripped and reblotted for vGLUT1. Final reblots for actin were performed for all experiments as a control for tissue preservation and protein loading. All three groups were run together in the same experiment which required simultaneous incubation and development of two western blot membranes. The experiments were performed in duplicate. Preincubation with the respective control peptide completely eliminates vGLUT1 (Barksdale et al, 2014) and vGLUT2 (Zeng et al, 2012) signal.
Table 2.
Primary antibodies used in this study
| Antibody | Manufacturer | Dilution | Incubation |
|---|---|---|---|
| Anti-TH | Sigma; T2928 | 1:10000 | 21 h @ 4°C |
| Anti-vGLUT1 | MAb Technologies; VGT1-3 | 1:9000 | 1 h @ RT |
| Anti-vGLUT2 | Synaptic Systems; 135 403 | 1:8000 | 21 h @ 4°C |
| Anti-Actin | Millipore; MAB1501 | 1:40000 | 21 h @ 4°C |
2.2.3. Analysis
Films were scanned using a flatbed scanner. Using NIH ImageJ, a box of consistent area was placed around each band at the expected molecular weight to measure optical density. An optical density standard curve was created using a step calibration tablet (Stouffer Industries Inc.; Mishawaka, IN, USA; T2120, series #130501), and each measurement was calibrated to the standard curve. A background subtraction in ImageJ was performed for each film. TH and vGLUT protein measures were normalized to actin. The normalized values for each duplicate were then averaged for the analysis.
All data sets were first assessed for normality with the Kolmogorov–Smirnov test. This was followed by an unpaired t-test or Wilcoxon test for CTRL vs SZ analyses. Welch's correction was used in cases of unequal variance. For CTRL vs SZ-ON vs SZ-OFF, a One-Way ANOVA or Kruskal-Wallis test was used. A Grubb's outlier analysis was used to identify potential outliers in each data set. Outliers were excluded only in situations where methodological considerations warranted exclusion. Presence of outliers, their inclusion/exclusion, and the impact on the results is noted in the Results section. All statistical tests were two-tailed with significance of p < 0.05.
3. Results
3.1. Actin Protein Levels in Human NAcc
Protein levels of actin were measured in SZ and control subjects. Western blotting yielded an intense band at the correct molecular weight of ~42 kDa. Un-normalized actin levels did not differ between the NC and SZ groups. The mean un-normalized actin levels for the blots used in the TH analysis were 0.250±0.007 (NC) and 0.247±0.012 (SZ); p = 0.563. The mean un-normalized actin levels for the blots used in the vGLUT analysis were 0.251±0.034 (NC) and 0.292±0.046 (SZ); p = 0.053.
3.2. Tyrosine Hydroxylase Protein Levels in Human NAcc
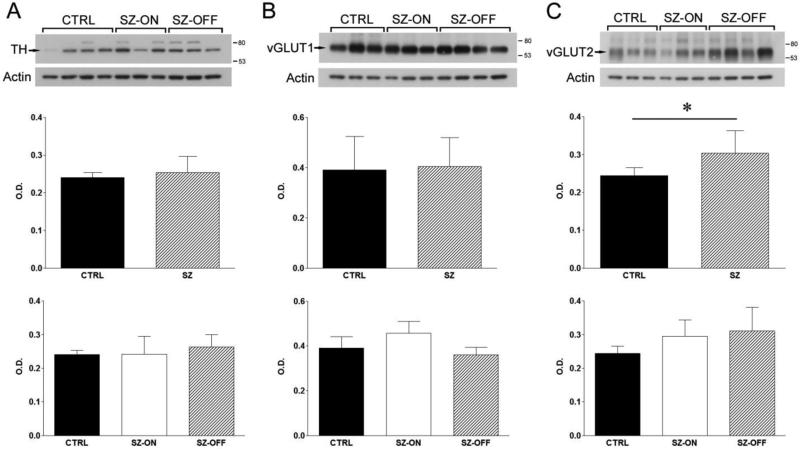
Protein levels of TH were measured in SZ-ON, SZ-OFF, and control subjects. Western blotting yielded an intense band in the correct molecular weight range, 62-68 kDa and a less intense upper band present only in some samples (Figure 1A). One control case was detected as an outlier. This case was noted to have poor protein concentration during protein extraction, so it was removed from the analysis. Removal of the data point resulted in normal distribution for the control group and had no effect on the significance of the results. TH protein levels were similar between control and SZ groups (p = 0.35, Figure 1A). When the SZ cases were analyzed separately based on APD status, there was no difference in TH protein levels between the three groups (p = 0.49, Figure 1A).
Figure 1.
Western blot analysis of TH, vGLUT1, and vGLUT2. Experiments were done in control (CTRL), schizophrenia on-drug (SZ-ON), and schizophrenia off-drug (SZ-OFF) subjects. Western blots were quantified by measuring calibrated optical density (relative units) of each band. Arrows point to the primary band at the expected molecular weight of each respective protein that was analyzed. Standards of 80 and 53 kDa markers are indicated on the right. Actin was a loading control. (A) Representative blots of TH for each group. Middle panel, TH protein levels of CTRL and all SZ cases. Lower panel, TH protein levels of CTRL, SZ-ON, and SZ-OFF groups. (B) Representative blots of vGLUT1 for each group. Middle panel, vGLUT1 protein levels of CTRL and all SZ cases. Lower panel, vGLUT1 protein levels of CTRL, SZ-ON, and SZ-OFF groups. (C) Representative blots of vGLUT2 for each group. Middle panel, vGLUT2 protein levels of CTRL and all SZ cases. Lower panel, vGLUT2 protein levels of CTRL, SZ-ON, and SZ-OFF groups. Bars represent standard deviation. *p < 0.05.
3.3. Vesicular Glutamate Transporter Protein Levels in Human NAcc
Protein levels of vGLUT1 and vGLUT2 were measured in SZ-ON, SZ-OFF, and control subjects. Western blotting for vGLUT1 detected a light smear and one major broad band at the expected molecular weight, ~63kDa (Figure 1B). Protein levels of vGLUT1 were similar between control and SZ groups (p = 0.81, Figure 1B). When the SZ cases were separated into SZ-ON and SZ-OFF groups, there were no significant differences in vGLUT1 protein levels between the three groups (p = 0.36, Figure 1B). In the vGLUT1 data, one SZ-OFF case was detected as an outlier. There were no methodological issues that indicated the outlier should be excluded so it was left in the analysis. Inclusion of the outlier had no effect on the non-normal distribution of the data, or on the significance of the results.
Western blotting for vGLUT2 detected one major broad band at the expected molecular weight, 65kDa, with multiple less intense bands (Figure 1C). There was a significant increase in vGLUT2 protein levels in the SZ group compared to controls (p = 0.006, Figure 1C). When the SZ cases were separated into SZ-ON and SZ-OFF groups, there were no differences in vGLUT2 protein levels between the three groups (p = 0.09, Figure 1C). The same control case that was detected as an outlier in the TH analysis was also an outlier in the vGLUT2 data. It was also excluded from this data set for the same reason. Removal of the outlier resulted in normal distribution of the control group and statistical significance in the CTRL vs SZ analysis.
4. Discussion
In this study we found that TH protein levels did not differ between control and SZ groups in the NAcc. Protein levels of vGLUT2 were significantly increased in SZ subjects compared to controls, whereas levels of vGLUT1 were similar. There were no differences in TH, vGLUT1, or vGLUT2 protein measures between the SZ-ON and SZ-OFF subjects. To our knowledge, this is the first study to analyze TH and vGLUT protein levels in postmortem NAcc in on-drug and off-drug SZ subjects.
4.1. TH Levels in NAcc
The results from the western blot experiments suggest that TH levels do not differ in the NAcc of SZ subjects compared to controls. Since TH is the rate-limiting enzyme in DA synthesis and correlates well with DA in the striatum (Bacopoulos and Bhatnagar, 1977; Hefti et al, 1980), these findings suggest that DA synthesis is normal in the NAcc in SZ. Previous studies of DA in postmortem SZ tissue primarily reported similar findings in the NAcc (Farley et al, 1977; Owen et al, 1978; Crow et al, 1979; Toru et al, 1982), though not all (Bird et al, 1979; Mackay et al, 1982). Our finding is also consistent with neuroimaging studies in patients that do not detect differences in presynaptic DA synthesis capacity in the ventral striatum (Howes et al, 2009, 2011; Kegeles et al, 2010). Additionally, our recent ultrastructural study in postmortem human tissue reported similar densities of symmetric synapses, the type dopaminergic input forms in the region, in the NAcc core and shell of SZ and control subjects (McCollum et al, 2015).
It does not appear that APD treatment confounded or masked our results since the SZ-ON and SZ-OFF groups had similar TH protein levels. Further, a study in rats found no difference in TH immunolabeling in the core or shell after 4 weeks of haloperidol treatment (Marchese et al, 2002).
4.2. Vesicular Glutamate Transporter Levels in NAcc
Protein levels of vGLUT1 were similar between groups, consistent with the immunohistochemical results of Nudmamud-Thanoi et al, (2007). mRNA levels of vGLUT1 in cortical areas that project to the NAcc are unchanged (dorsolateral prefrontal cortex), or elevated (anterior cingulate cortex) in SZ (Oni-Orisan et al, 2008). It is puzzling that increased production of vGLUT1 in the anterior cingulate does not translate to increased protein levels in the NAcc, but perhaps other targets of the anterior cingulate are the recipient of increased vGLUT protein levels. In contrast, protein levels of vGLUT2 were elevated in the NAcc of the SZ group compared to controls. Increased vGLUT2 suggests the NAcc receives increased glutamatergic input in SZ. vGLUT1 and vGLUT2 comprise largely complementary localization patterns in the brain. vGLUT1 mRNA is found at highest concentrations in the cortex and hippocampus, while vGLUT2 is most concentrated in subcortical structures such as the thalamus, amygdala, and brainstem (Hisano et al, 2000). Within some regions however, both forms are expressed; vGLUT2 is also present in some layers of the neocortex and regions of the hippocampus (Fremeau et al, 2001). It has been hypothesized that the NAcc receives elevated hippocampal input in a model of SZ (Lodge and Grace, 2007, 2011). However, a study of vGLUT2 mRNA in the hippocampus in SZ was unable to compare levels due to low detection (Uezato et al, 2009). Another potential source of elevated input is the thalamus which contains high levels of vGLUT2. A study in postmortem SZ found increased vGLUT2 mRNA in the thalamus (Smith et al, 2001). The synaptic inputs from both the hippocampus and thalamus make a morphologically similar type of synapse (French and Totterdell, 2004), which we recently reported to be increased in density the NAcc in SZ (McCollum et al, 2015). The functional implications of excessive glutamatergic input are discussed below.
Previous studies have not investigated the effects of APD treatment on protein levels of these transporters in the human NAcc. Our observations, showing similar levels of the vGLUTs between the SZ-ON and SZ-OFF groups indicate that APDs are not impacting our results. Research on APD treated animals largely supports this conclusion. APDs have no effects on vGLUT1 density in the NAcc as determined with immunohistochemistry (Nudmamud-Thanoi et al., 2007). In cortex, APD treatment had no impact on vGLUT1 levels, but vGLUT2 levels were decreased in some but not all areas (Oni-Orisan et al, 2008). This suggests regional specificity of APDs within brain regions, making it difficult to draw conclusions on how haloperidol might affect vGLUT2 levels in the NAcc.
4.3. Understanding the Role of the NAcc in SZ
Though the findings reported here are static in nature, it is worth considering their potential functional implications in SZ. Complex processing of cortico-striato-thalamo-cortical signals occurs within the NAcc (Haber et al, 2003). Glutamatergic input from the amygdala and hippocampus provide affective valence and contextual gating, respectively, by facilitating the probability of cortical input to evoke an action potential in the NAcc (Grace, 2000; O'Donnell and Grace, 1995). Excessive glutamatergic input from either of these regions would result in a loss of the gating mechanism, allowing otherwise weak cortical input to elicit a response in the NAcc (O'Donnell and Grace, 1995). Loss of gating due to enhanced input to NAcc neurons could result in dysregulation of DA release in the NAcc (Boley et al, 2014; Lodge and Grace, 2007, 2011), and in hyperexcitability of NAcc projection neurons, making them more likely to respond to otherwise weak stimuli (O'Donnell and Grace, 1995). The increase in markers of glutamatergic input in the NAcc reported here supports hypotheses suggesting that disruption of the brain's ability to effectively gate information flow through the NAcc could lead to some of the symptoms of SZ (Lodge and Grace, 2007, 2011).
The similar levels of TH in control and SZ cases in the current study suggest that the DA synthesis pathway is not abnormal in the NAcc in SZ, however it remains possible that DA release is dysregulated due to elevated glutamatergic input as discussed above. Additionally, elevated glutamatergic input to the NAcc could enhance GABAergic drive to the midbrain. This enhanced drive could disinhibit midbrain DA neurons (Bocklisch et al, 2013; Grace and Bunney, 1985). Due to the feedforward organization of striatonigrostriatal pathways in primates (Haber et al, 2000), increased excitation of NAcc neurons could drive enhanced DA release in the dorsal striatum. This could be a source of elevated DA levels in the dorsal striatum in SZ (Howes et al, 2009, 2011; Kegeles et al, 2010).
4.4. Methodological Considerations
The results of this study should be interpreted in the context of their limitations. First, the NAcc is a complex and heterogeneous region; subregions referred to as the core and shell have been identified across species (Brauer et al, 2000; Meredith et al, 1996; Záborszky et al, 1985) and further heterogeneity exists within each subregion. Our recent ultrastructural study in postmortem SZ found an increased density of excitatory type synapses only in the core of the NAcc, not the shell (McCollum et al, 2015). Since the western blot experiments in this study were performed on the NAcc as a whole, it is possible that unique variations within the NAcc territories have been missed.
Second, the small sample sizes of these groups should be taken into consideration. The number of off-drug SZ subjects was the limiting factor in the sample size and drove the selection of matching controls and on-drug SZ cases. Importantly, the power analysis from our recent paper shows that we need an n = 6 per group for TH (Perez-Costas et al, 2012). Further, our findings are consistent with imaging and postmortem studies in the NAcc, as well as hypotheses of SZ discussed above. Postmortem off-drug SZ subjects are rare, so the cohort collected for this study, although small, provides a unique analysis of postmortem SZ.
4.5. Conclusions
Our findings suggest that the presynaptic DA pathway is normal in the NAcc in SZ, but that the region receives increased glutamatergic input, most likely from subcortical regions. They further indicate that our results are not due to antipsychotic medication. This study provides insight into the role of the NAcc in SZ, highlighting potential abnormalities in the interaction between the dopaminergic and glutamatergic systems in the region.
Table 1.
Demographic information for all cases used in this study
| CTRL | SZ | SZ-ON | SZ-OFF | |
|---|---|---|---|---|
| N | 7 | 13 | 6 | 7 |
| Age, years | 42 ± 15 | 46 ± 12 | 44 ± 9 | 48 ± 14 |
| Race | 4C, 3AA | 10C, 3AA | 5C, 1AA | 5C, 2AA |
| Sex | 4M, 3F | 9M, 4F | 3M, 3F | 6M, 1F |
| PMI, hours | 16.14 ± 6.09 | 15.38 ± 7.54 | 15.67 ± 9.69 | 15.14 ± 6.95 |
| pH | 6.71 ± 0.20 | 6.59 ± 0.29 | 6.58 ± 0.14 | 6.60 ± 0.39 |
| Freezer time, years | 14.14 ± 5.73 | 18.0 ± 4.47 | 16.33 ± 4.76 | 19.42 ± 3.99 |
Mean ± standard deviation. CTRL, control; SZ, schizophrenia; C, Caucasian; AA, African American; M, male; F, female; PMI, postmortem interval. SZ subjects were analyzed together, and when divided into two groups based on medication status, SZ-ON and SZ-OFF, for each analysis.
Acknowledgements
We would like to thank the staff of the Maryland Brain Collection for their assistance with collection of the human tissue used in this study, and Courtney Walker for her technical assistance.
Role of Funding Sources
This research was supported by the National Institute of Mental Health F31MH098566 (LAM) and RO1MH066123 (RCR). The funding source had no role in study design, data collection, analysis and interpretation of data, writing the manuscript, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Roberts and McCollum conceived the idea and methodology of this study. Dr. McCollum conducted the data collection and statistical analyses. Both authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Bacopoulos N, Bhatnagar R. Correlation between tyrosine hydroxylase activity and catecholamine concentration or turnover in brain regions. J. Neurochem. 1977;29(4):639–643. doi: 10.1111/j.1471-4159.1977.tb07780.x. [DOI] [PubMed] [Google Scholar]
- Barksdale K, Lahti A, Roberts R. Synaptic proteins in the postmortem anterior cingulate cortex in schizophrenia: Relationship to treatment and treatment response. Neuropsychopharmacology. 2014;39(9):2095–2103. doi: 10.1038/npp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr., Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289(5481):957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Bird ED, Spokes EG, Iversen LL. Increased dopamine concentration in limbic areas of brain from patients dying with schizophrenia. Brain. 1979;102(2):347–360. doi: 10.1093/brain/102.2.347. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur. J. Neurosci. 1997;9(5):902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong J, House D, Yvon C, Roo M, Tan KR, Lüscher C. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341(6153):1521–1525. doi: 10.1126/science.1237059. [DOI] [PubMed] [Google Scholar]
- Boley AM, Perez SM, Lodge DJ. A fundamental role for hippocampal parvalbumin in the dopamine hyperfunction associated with schizophrenia. Schizophr. Res. 2014;157(1-3):238–243. doi: 10.1016/j.schres.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer K, Häußer M, Härtig W, Arendt T. The core–shell dichotomy of nucleus accumbens in the rhesus monkey as revealed by double-immunofluorescence and morphology of cholinergic interneurons. Brain Res. 2000;858(1):151–162. doi: 10.1016/s0006-8993(00)01938-7. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Baker HF, Cross AJ, Joseph MH, Lofthouse R, Longden A, Owen F, Riley GJ, Glover V, Killpack WS. Monoamine mechanisms in chronic schizophrenia: post-mortem neurochemical findings. Br. J. Psychiatry. 1979;134:249–256. doi: 10.1192/bjp.134.3.249. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46(1):49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Iadarola MJ. Regionally specific effects of atypical antipsychotic drugs on striatal Fos expression: The nucleus accumbens shell as a locus of antipsychotic action. Mol. Cell. Neurosci. 1992;3(4):332–341. doi: 10.1016/1044-7431(92)90030-6. [DOI] [PubMed] [Google Scholar]
- Farley IJ, Price KS, Hornykiewicz O. Dopamine in the limbic regions of the human brain: normal and abnormal. Adv. Biochem. Psychopharmacol. 1977;16:57–64. [PubMed] [Google Scholar]
- Fremeau R, Troyer M, Pahner I, Nygaard G, Tran C, Reimer R, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- French S, Totterdell S. Quantification of morphological differences in boutons from different afferent populations to the nucleus accumbens. Brain Res. 2004;1007(1-2):167–177.. doi: 10.1016/j.brainres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Grace A. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res. Brain Res. Rev. 2000;31(2-3):330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333(2):271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber S, Fudge J, McFarland N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195(1):123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi G, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci. 2001;21(22):RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano S, Hoshi K, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Nogami H. Regional expression of a gene encoding a neuron-specific Na(+)-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Brain Res. Mol. Brain Res. 2000;83(1-2):34–43. doi: 10.1016/s0169-328x(00)00194-7. [DOI] [PubMed] [Google Scholar]
- Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Valmaggia L, Murray RM, McGuire P. Dopamine synthesis capacity before onset of psychosis: A prospective [18 F]-DOPA PET imaging study. Am. J. Psychiatry. 2011;168(12):1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin M-CC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Pychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Kegeles L, Abi-Dargham A, Frankle W, Gil R, Cooper T, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kegeles L, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann J, Heertum R, Cooper TB, Carlsson A, Laruelle M. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol. Psychiatry. 2000;48(7):627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31(4):241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lodge D, Grace A. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007;27(42):11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D, Grace A. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol. Sci. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AV, Iversen LL, Rossor M, Spokes E, Bird E, Arregui A, Creese I, Synder SH. Increased brain dopamine and dopamine receptors in schizophrenia. Arch. Gen. Psychiatry. 1982;39(9):991–997. doi: 10.1001/archpsyc.1982.04290090001001. [DOI] [PubMed] [Google Scholar]
- Marchese G, Casu M, Bartholini F, Ruiu S, Saba P, Gessa G, Pani L. Sub-chronic treatment with classical but not atypical antipsychotics produces morphological changes in rat nigro-striatal dopaminergic neurons directly related to “early onset” vacuous chewing. Eur. J. Neurosci. 2002;15(7):1187–1196. doi: 10.1046/j.1460-9568.2002.01944.x. [DOI] [PubMed] [Google Scholar]
- McCollum LA, Walker CK, Roche JK, Roberts RC. Elevated excitatory input to the nucleus accumbens in schizophrenia: A postmortem ultrastructural study. Schizophr. Bull. 2015 doi: 10.1093/schbul/sbv030. e-pub ahead of print 27 March 2015. doi: 10.1093/schbul/sbv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant K, Dorsa D. Differential induction of neurotensin and c-fos gene expression by typical versus atypical antipsychotics. Proc. Nat. Acad. Sci. 1993;90(8):3447–3451. doi: 10.1073/pnas.90.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Pattiselanno A, Groenewegen HJ, Haber SN. Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J. Comp. Neurol. 1996;365(4):628–639. doi: 10.1002/(SICI)1096-9861(19960219)365:4<628::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Miller DW, Abercrombie ED. Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res. Bull. 1996;40(1):57–62. doi: 10.1016/0361-9230(95)02144-2. [DOI] [PubMed] [Google Scholar]
- Miyake N, Thompson J, Skinbjerg M, Abi-Dargham A. Presynaptic dopamine in schizophrenia. CNS Neurosci. Ther. 2011;17(2):104–109. doi: 10.1111/j.1755-5949.2010.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudmamud-Thanoi S, Piyabhan P, Harte MK, Cahir M, Reynolds GP. Deficits of neuronal glutamatergic markers in the caudate nucleus in schizophrenia. J Neural Transm Suppl. 2007;72:281–5. doi: 10.1007/978-3-211-73574-9_34. [DOI] [PubMed] [Google Scholar]
- Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered vesicular glutamate transporter expression in the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2008;63(8):766–75. doi: 10.1016/j.biopsych.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F, Cross AJ, Crow TJ, Longden A, Poulter M, Riley GJ. Increased dopamine-receptor sensitivity in schizophrenia. Lancet. 1978;2(8083):223–226. doi: 10.1016/s0140-6736(78)91740-3. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J. Neurosci. 1995;15(5 Pt 1):3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front. Psychiatry. 2012;3:31. doi: 10.3389/fpsyt.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M, Smith K, Roberts R, Perez-Costas E, Melendez-Ferro M. Assessment of cytochrome C oxidase dysfunction in the substantia nigra/ventral tegmental area in schizophrenia. PLoS ONE. 2014;9:e100054. doi: 10.1371/journal.pone.0100054. doi: 10.1371/journal.pone.0100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Vesicular glutamate transporter transcript expression in the thalamus in schizophrenia. Neuroreport. 2001;12(13):2885–2887. doi: 10.1097/00001756-200109170-00026. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407(6901):189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Toru M, Nishikawa T, Mataga N, Takashima M. Dopamine metabolism increases in post-mortem schizophrenic basal ganglia. J. Neural. Transm. 1982;54(3-4):181–191. doi: 10.1007/BF01254928. [DOI] [PubMed] [Google Scholar]
- Uezato A, Meador-Woodruff JH, McCullumsmith RE. Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord. 2009;11(7):711–725. doi: 10.1111/j.1399-5618.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J. Neurosci. 2012;32(45):15791–15801. doi: 10.1523/JNEUROSCI.2598-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L, Alheid G, Beinfeld M, Eiden L, Heimer L, Palkovits M. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14(2):427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]