Abstract
Phosphatase Src homology region 2 domain-containing phosphatase 1 (SHP-1)-deficient mice display an allergic asthma phenotype that is largely IL-13 and STAT6 dependent. The cell types responsible for the Th2 phenotype have not been identified. We hypothesized that SHP-1 deficiency leads to mast cell dysregulation and increased production and release of mediators and Th2 cytokines, leading to the allergic asthma phenotype. We examined SHP-1 regulation of mast cell differentiation, survival, and functional responses to stimulation using bone marrow-derived mast cells from viable motheaten (mev) mice. We assessed pulmonary phenotypical changes in mev mice on the mast cell-deficient KitW-Sh genetic background. The results showed that SHP-1 deficiency led to increased differentiation and survival, but reduced proliferation, of mast cells. SHP-1–deficient mast cells produced and released increased amounts of mediators and Th2 cytokines IL-4 and -13 spontaneously and in response to H2O2, LPS, and FcεI cross-linking, involving c-Kit–dependent and –independent processes. The FcεRI signaling led to binding of SHP-1 to linker for activation of T cells 2 and enhanced linker for activation of T cells 2 phosphorylation in mev bone marrow-derived mast cells. Furthermore, the number of mast cells in the lung tissue of mev mice was increased and mast cell production and release of Th2 cytokines were distinctly increased upon FcεRI stimulation. When backcrossed to the KitW-Sh background, mev mice had markedly reduced pulmonary inflammation and Th2 cytokine production. These findings demonstrate that SHP-1 is a critical regulator of mast cell development and function and that SHP-1–deficient mast cells are able to produce increased Th2 cytokines and initiate allergic inflammatory responses in the lung.
Asthma is a chronic inflammatory disorder of the airways and the prevalence of asthma in industrialized countries has dramatically increased in the last few decades (1). Allergen-induced Th2 cell activation and Th2 inflammation are believed to be major components of asthma pathogenesis (2, 3). Th2 cytokines IL-4, -5, and -13 are critical in the generation of the allergic asthma phenotype (4–8). However, it is increasingly appreciated that there are different forms of asthma. The allergen–Th2 mechanism is probably one of many in the immunopathogenesis of asthma, and non-T cell Th2 responses may exist (9). We previously reported that mice deficient in phosphatase SHIP-1 or Src homology region 2 domain-containing phosphatase (SHP)-1 develop spontaneous Th2-like inflammatory responses in the lung without obvious allergen exposure, suggesting the involvement of innate immune cells in the phenotype generation (10, 11).
IL-13 is a potent effector cytokine that can directly induce an allergic asthma phenotype in mice when administered into the airway or transgenically expressed in the lung (6–8). Thus, it is conceivable that, regardless of the source, a sufficient quantity of Th2 cytokines, such as IL-13, can be expected to induce a Th2-like inflammatory response in the tissue. Th2 cytokines are produced primarily by activated Th2 cells, but also by other cell types, including mast cells and basophils.
Mast cells are known as effector cells in mediating allergic and anaphylaxis responses through Ag activation of IgE bond to FcεRIand subsequent degranulation and release of inflammatory mediators in the tissues. The role of mast cells in allergic asthma models is less clear. Some studies showed that mast cells play a role in host allergic responses to inhaled allergen without adjuvant, but they are not required in the Th2-biased sensitization with i.p. injection of allergen and adjuvant (12–14). Dysregulated mast cells may play a role in enhancing allergen-induced responses. SHP-1–deficient bone marrow-derived mast cells (BMMCs) produced increased amounts of proinflammatory cytokines TNF-α, IL-6, and -13 after IgE-FcεRI stimulation (15, 16). Also, it has been reported that SHIP-1–deficient mast cells were important in allergen-induced allergic and anaphylactic responses in mice (17). However, whether mast cells are able to produce sufficient cytokines to initiate a particular inflammation in local tissues in response to environmental stimulation is not clear.
The protein tyrosine phosphatase SHP-1 has been recognized as a critical negative regulator in intracellular signaling (18, 19). The motheaten (me) and viable motheaten (mev) mice (20, 21) have mutations that lead to SHP-1 deficiency, resulting in severe and earlyage onset of systemic inflammatory diseases, including pneumonitis (22, 23). When mev mice were backcrossed to RAG-1 null mice, which lack mature T and B cells, the pathology did not change, indicating that T and B cells are not required for the development of the mev phenotype (24). Instead, backcrossing of me mice to strains deficient in myeloid progenitors showed that the me phenotype, including lung pathology, was partially reduced, indicating the involvement of the myeloid cell population (25, 26).
In a previous study, we defined the spontaneous lung inflammation in homozygous mev mice as an allergic inflammatory phenotype, in which the Th2 cytokines and signaling pathway, particularly IL-13, play a major role (11). Because these mice are not exposed to known allergens, it is unclear whether the adaptive immune system is involved in the generation of the phenotype. The cellular source of Th2 cytokines in these mice has not been identified. We hypothesized that SHP-1 is critical in regulating mast cell development and function, especially in the production of Th2 cytokines in response to various stimuli, including those from the environment, and mast cells play a critical role in the generation of allergic inflammatory responses in mev mice. We demonstrate herein that mast cells deficient in SHP-1 have increased cell differentiation, survival, and production and release of Th2 cytokines. Mice deficient in SHP-1 had increased mast cells, as well as increased mediators and Th2 cytokines in the lung tissue. Furthermore, the mev pulmonary phenotype was mast cell dependent, as evidenced by dramatically reduced pulmonary inflammation in mev mice with mast cell deficiency.
Materials and Methods
Animals
mev (Ptpn6me-v) mice and mast cell-deficient (KitW-sh) mice on C57BL/6 genetic background were from The Jackson Laboratory (Bar Harbor, ME). Heterozygous (Ptpn6me-v/+) mice were interbred to generate wild-type (WT), heterozygous, and homozygous mice (Ptpn6me-v, hereafter abbreviated as mev). To generate mev mice with mast cell deficiency, heterozygous mev/+ mice were backcrossed to KitW-sh background and then interbred to obtain SHP-1–deficient and mast cell-deficient mev/KitW-sh mice. The genotype of the mice was determined using PCR primers and protocols provided by The Jackson Laboratory. Mice were used for experiments at 7–9 wk of age, unless indicated otherwise. All mice were housed in cages with microfilters in the specific pathogen-free environment. All procedures performed on mice were in accordance with the National Institutes of Health guidelines for humane treatment of animals and were approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University.
Cell culture
BMMCs were obtained by in vitro differentiation of bone marrow cells obtained from femur and tibia of WT and mev mice by culturing in RPMI 1640 (Invitrogen, Carlsbad, CA) containing 30% WEHI-3B–conditioned media supplemented with 10% heat-inactivated FBS (Invitrogen), 100 µM 2-ME (Sigma-Aldrich, St. Louis, MO), 10 µM MEM nonessential amino acids solution, l-glutamine, sodium pyruvate, HEPES buffer (Sigma-Aldrich), and antibiotics. Bone marrow cells in culture were analyzed at specified time points. By 4 wk in culture, the purity of BMMCs was >98%. Unless otherwise indicated, mature BMMCs between 4–8 wk, with viability >95% by the trypan blue exclusion assay, were used for experiments.
Mast cell maturation
Cultured bone marrow cells were sensitized with IgE (Southern Biotechnology Associates, Birmingham, AL) overnight, stained for cell surface markers c-Kit and FcεRI using APC-conjugated and FITC-conjugated anti-mouse CD117- and FcεRIα-specific Abs, respectively (eBioscience, San Diego, CA), and analyzed by flow cytometry using a FACScan cytometer at different time points.
Proliferation assays for bone marrow cells and BMMCs
Two methods were used to assess cell proliferation. For bone marrow cells, the total number of cells before culture and at specified time points during culture was determined. For mature mast cells, BMMCs were serially diluted in WEHI-3B–conditioned medium and seeded in triplicate in a microplate using 100 µl the cells with indicated densities. Medium alone was used as a background reference. After 3 d, cells were incubated with XTT Working Solution (R&D Systems, Minneapolis, MN) for 4 h at 37°C under 5% CO2. Absorbance values were obtained at 450 nm, with a reference correction at 520 nm, in an ELISA plate reader.
Apoptosis assay
BMMC apoptosis was initiated by growth factor withdrawal. After replacing WEHI-3B–conditioned medium with regular medium for 3 h, BMMCs were incubated in the presence or absence of WEHI-3B (30%), Gleevec (1 µM; Novartis Pharmaceuticals, East Hanover, NJ), LY294002 (10 µM; LC Laboratories, Woburn, MA), or IgE (5 µg/ml; Sigma-Aldrich) for 24 h. The percentage of apoptotic cells in culture was determined by FACS analysis of Annexin V (BD Pharmingen, San Diego, CA), a protein that preferentially binds to negatively charged phosphatidylserine residues that are exposed on the cell surface of early apoptotic cells.
BMMC cytokine production and mediator release in response to stimulation
Before stimulation, BMMCs were rested by removing WEHI-3B–conditioned medium for 3 h. Equal numbers of cells were incubated with appropriate concentrations of H2O2, N-acetylcysteine (NAC; Sigma-Aldrich), LPS, Gleevec, or PMA/ionomycin in IL-3–free media. At the indicated time, the supernatant and cell samples were collected by centrifugation for 5 min at 1200 rpm. The supernatant was tested for cytokines, and cellular RNA was extracted for RT-PCR. The concentrations of cytokines in the supernatant were determined using commercially available ELISA kits (R&D Systems) per the manufacturer’s instructions. Mast cell degranulation was determined by measuring β-hexosaminidase release. BMMCs were sensitized by anti-DNP IgE (1 µg/ml; Sigma-Aldrich) overnight. Then the cells were stimulated with or without DNP-human serum albumin (HSA) (100 ng; Sigma-Aldrich) in the presence or absence of stem cell factor (SCF) or Gleevec. After 15 min, β-hexosaminidase activity in supernatant was measured as follows. Triplicate samples of 10 µl each were added to a 96-well plate followed by 10 µl 1 mM 4-nitrophenyl N-acetyl-β-d-glucosaminide (Sigma-Aldrich N9376). After incubation at 37°C for 1 h, 250 µl 0.1 M Na2CO3/NaHCO3 was added to stop the enzyme reaction. Then the absorbance was measured at 450 nm. The total amount of β-hexosaminidase was also determined after lysing cell samples without any treatment. The values of all samples were relative to the total β-hexosaminidase of WT BMMCs. Single-cell suspensions prepared from lung and spleen were stimulated with PMA/ionomycin, and total and released histamine was determined as described previously (10). Similarly, cell suspensions were stimulated by anti-FcεRIα Ab (0.1 µg/ml) for 21 h, and cytokines secreted into the supernatant were determined by ELISA.
mRNA analysis
Total cellular RNA from BMMCs was obtained using TRIzol reagent (Invitrogen) after appropriate stimulation. The mRNA of specific genes was evaluated by RT-PCR using specific primers with β-actin as an internal control. The primer sequences and PCR conditions for IL-4 and -13 and IFN-γ were described previously (27). The primers for Bcl-2 were sense: 5′-AAG CTG TCA CAG AGG GGC TA-3′ and antisense: 5′-GAC GGT AGC GAC GAG AGA AG-3′, and the annealing temperature was 60°C, with 25 cycles of amplification. RT-PCR products from three independent sets of samples were analyzed by electrophoresis and densitometry.
Immunoprecipitation and Western blot analysis
Protein binding and phosphorylation of signaling molecules were analyzed by immunoprecipitation and Western blot. Specific Abs to SHP-1, linker for activation of T cells 2 (LAT2), phosphotyrosine, and protein A/G agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoprecipitation and Western blot were performed following the manufacturers’ protocols.
Lung and bronchoalveolar lavage samples
Lung tissue and bronchoalveolar lavage (BAL) samples were obtained as previously described by our laboratories (8, 27). Briefly, the mice were anesthetized, and the trachea was isolated by blunt dissection. A small-caliber tubing was inserted and secured in the airway. Three successive volumes of 0.75 ml PBS with 0.1% BSA were instilled, gently aspirated, and pooled. BAL samples were centrifuged, and supernatants were stored at −70°C until assayed. Cells were counted in 100-µl aliquots. One hundred thousand viable BAL cells were centrifuged onto slides by a Cytospin III (Thermo Shandon, Runcorn, U.K.) and stained with the Hema 3 System (Fisher Scientific, Newark, DE). The numbers and types of cells in the pellet were determined. The lung was perfused with cold PBS through the right ventricle with cut vena cava until the pulmonary vasculature was cleared of blood. The whole lung was excised for RNA and protein analyses or for mediator analysis or was inflated with fixatives for histology.
Histology evaluation
H&E stains were performed on lung sections after fixation with Streck solution (Streck Laboratories, La Vista, NE) as described (27). The same microscopic magnification was used for the sample slides from WT and mev mice.
Statistics
Most of the data were assessed by the Student t test and expressed as mean ± SD. One-way ANOVA was used for comparison of multiple groups. Differences between groups with p values ≤ 0.05 were considered statistically significant.
Results
SHP-1 regulates mast cell differentiation, maturation, and proliferation
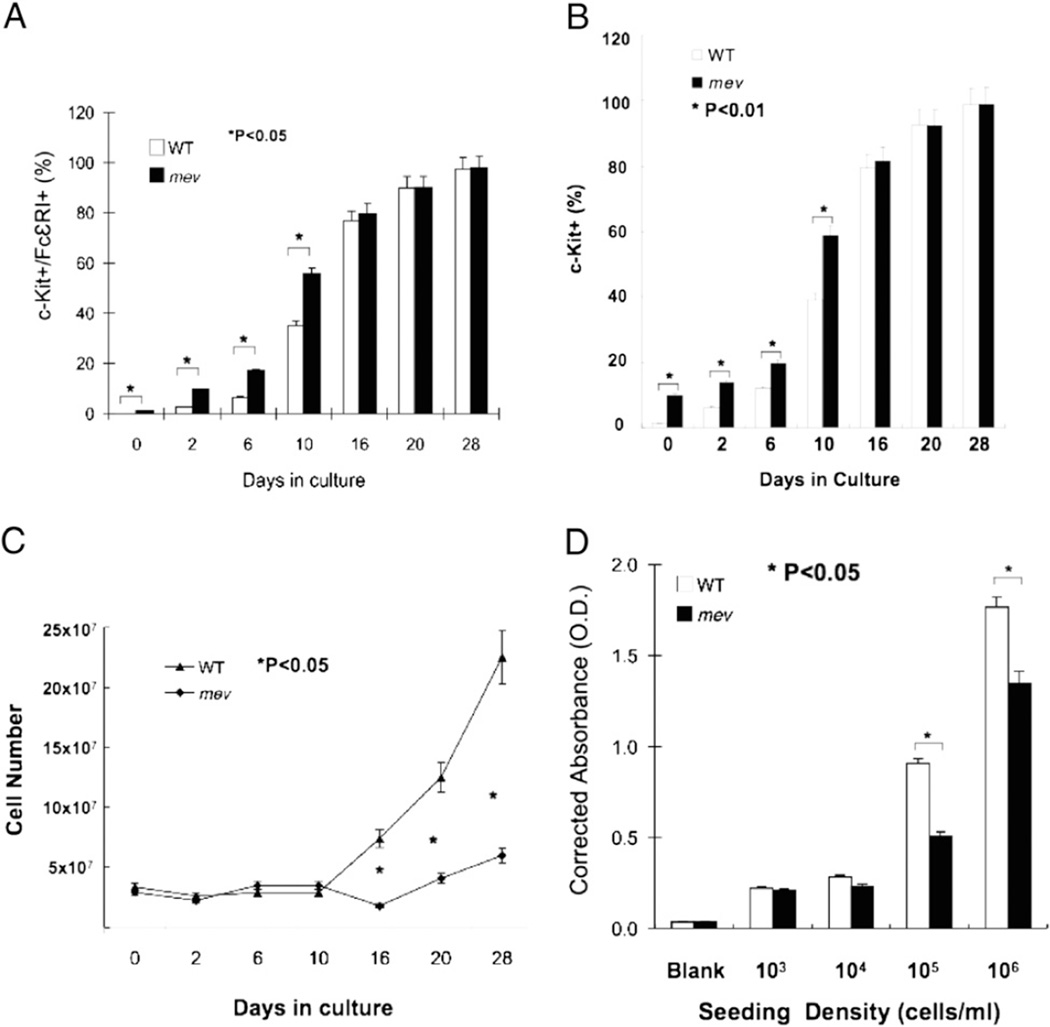
To examine the function of SHP-1 in regulating mast cell differentiation and maturation, we determined the percentage of mature mast cells by FACS analysis of cell surface markers c-Kit (CD117) and FcεRI before culture and at different time points in culture. As shown in Fig. 1A, before culture, bone marrow cells from WT mice contained nearly undetectable levels of c-Kit/FcεRI double-positive cells (0.02%), whereas bone marrow cells from mev mice contained significantly more c-Kit/FcεRI double-positive cells (0.94%), indicating increased mast cell maturation in the bone marrow of mev mice in vivo. Once in culture in conditioned medium, the number of mature mast cells in WT and mev cells increased rapidly. However, the percentage of mature mast cells in mev cells was significantly greater than that of WT cells from days 2–10 in culture (Fig. 1A). After day 16, there was no difference in the percentage of mature mast cells between WT and mev cells. These results suggest that SHP-1 is critical in regulating the rate of mast cell differentiation and maturation in vivo and ex vivo, particularly in the early stage. A similar pattern was seen in the c-Kit single-positive cell population, indicating that a significantly bigger pool of hematopoietic progenitors is present in the bone marrow of mev mice (Fig. 1B).
FIGURE 1.
BMMC differentiation and proliferation. At different time points, aliquots of cultured bone marrow cells from WT and mev mice were incubated with IgE overnight, stained for c-Kit and FcεRI, and analyzed by FACS. For each sample, 10,000 events were counted. Percentage of c-Kit/FcεRI double-positive cells (A) and percentage of c-Kit positive cells (B). Results are mean ± SD of eight pairs of WT and mev mice. C, Total number of bone marrow cells from each sample was determined before culture and at specified time points in culture. Results from three sets of independent experiments. D, After 4 wk in culture, mature BMMCs were serially diluted in conditioned medium and seeded in a microplate using 100 µl of the cells with indicated densities in triplicate. Medium alone was used as a baseline reference. Three days later, cell proliferation was determined by the XTT method. A representative of three experiments with similar results is shown.
We noticed that fewer BMMCs from mev mice were obtained compared with WT mice. To determine whether there was a difference in cell proliferation, bone marrow cells from mev and WT mice were cultured and counted at specified time points. From start to the first 10 d in culture, there was no difference in the numbers of bone marrow cells between mev and WT mice, even with a similar decrease in cell numbers at day 2 (Fig. 1C). However, on day 16, when ~80% of the cells in culture became mature mast cells, the number of mev cells decreased slightly; thereafter, it gradually increased. In contrast, the number of WT cells increased significantly from day 16 (Fig. 1C). To directly assess whether there was a difference in mast cell proliferation without interference from other cell types, we cultured mature mast cells with the same cell densities for 3 d and evaluated cell proliferation using the XTT assay. At a density of 105 and 106 cells/ml, mev BMMCs showed a significantly slower rate of proliferation than WT BMMCs (Fig. 1D). These results demonstrate that mast cells lacking SHP-1 are deficient in cell proliferation in culture.
mev BMMCs are more resistant to apoptosis
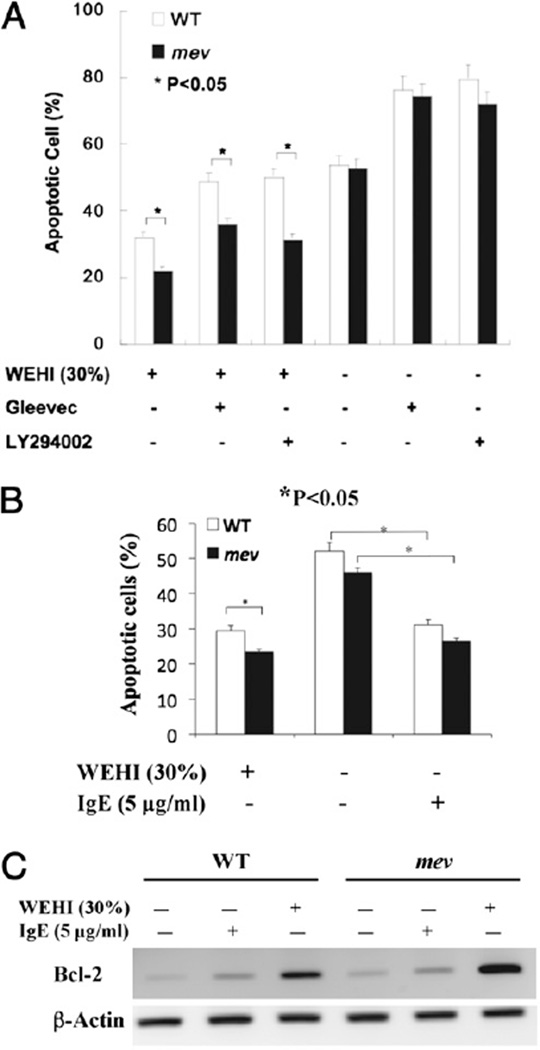
To determine whether mev BMMCs have altered survival capability, we tested the apoptotic response of BMMCs under different conditions. After withdrawing WEHI-3B–conditioned medium, BMMCs were cultured in the presence or absence of IL-3 (WEHI-3B), kinase inhibitor Gleevec, PI3K inhibitor LY294002, or IgE for 24 h, and the percentage of apoptotic cells was determined as the Annexin V-positive population by FACS. In the continued presence of IL-3, ~32% of WT BMMCs were apoptotic, but the Annexin V-positive population in mev BMMCs was significantly smaller. The addition of c-Kit inhibitor Gleevec increased the apoptotic population in WT and mev BMMCs, but the difference between them became even larger, suggesting that mev BMMCs were less sensitive to Gleevec inhibition. The presence of LY294002 showed a similar pattern as Gleevec, indicating that the antiapoptotic effects seen in mev BMMCs were probably through c-Kit and then the PI3K pathway. Interestingly, in the absence of IL-3, the numbers of apoptotic cells in WT and mev BMMCs increased significantly, and Gleevec and LY294002 further enhanced the apoptotic populations. The difference between WT and mev BMMCs disappeared, indicating that SHP-1 deficiency-induced antiapoptotic activity was IL-3 dependent (Fig. 2A). In addition, in the absence of IL-3, incubation with IgE provided some protective effect for BMMCs from apoptosis (Fig. 2B). There was still some difference between WT and mev BMMCs with regard to apoptosis, although it was not statistically significant. We analyzed Bcl-2 mRNA in BMMCs to assess whether the antiapoptosis gene Bcl-2 was involved. In the absence of IL-3 or IgE, Bcl-2 mRNA was seen in WT BMMCs. A clear increase in Bcl-2 mRNA was seen after IgE stimulation, and a significant increase was noted in the presence of IL-3 (Fig. 2C). However, the Bcl-2 mRNA levels were higher in mev BMMCs compared with WT BMMCs under the same conditions (Fig. 2C). These results show that mev BMMCs are more resistant to apoptosis, and the expression of the Bcl-2 gene is highly upregulated in these cells.
FIGURE 2.
Apoptosis of BMMCs under different conditions. After replacing WEHI-3B–conditioned medium, BMMCs were cultured in the presence or absence of WEHI-3B medium (30%), Gleevec (1 µM), or LY294002 (10 µM) for 24 h and analyzed by FACS with Annexin V and PI staining. A, The percentage of apoptotic cells. Three independent experiments were performed with triplicates for each sample. B, The percentage of apoptotic cells in the presence of IgE. C, RT-PCR analysis of expression of antiapoptosis gene Bcl-2 in WT and mev BMMCs. β-Actin mRNA was used as an internal control. A representative of two experiments is shown.
Increased expression of IL-4 and -13 by mev BMMCs in response to reactive oxygen species
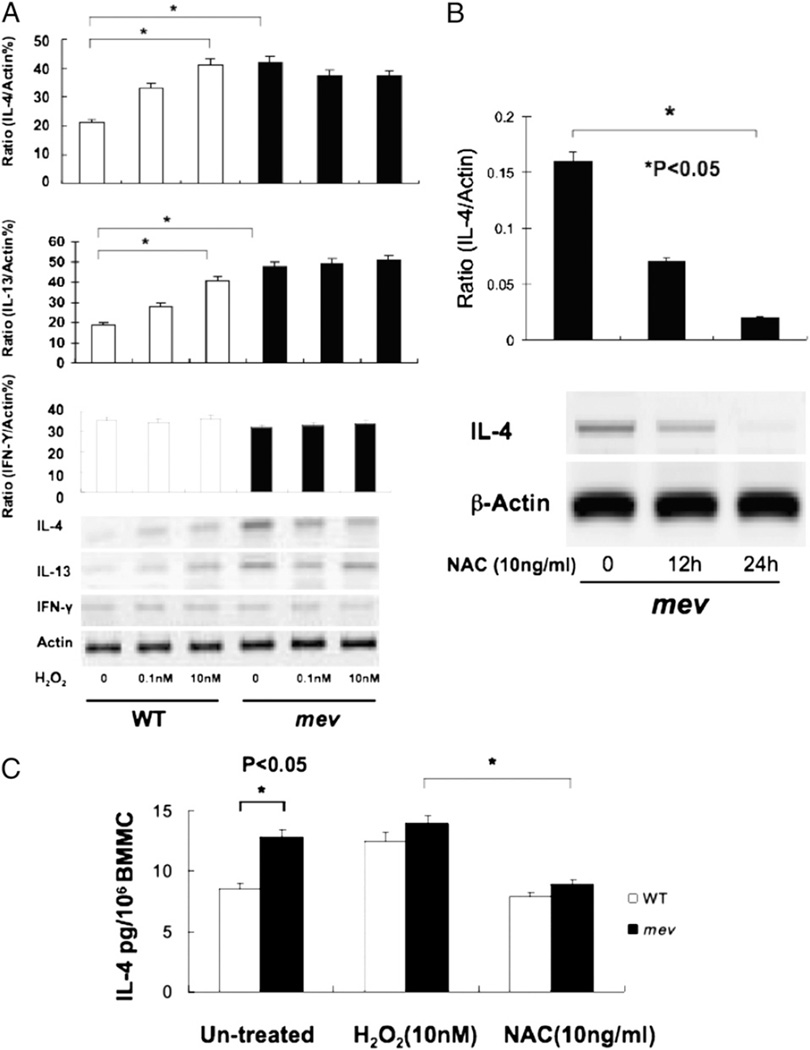
BMMCs can produce inflammatory cytokines in response to H2O2 (28), and SHP-1–deficient mice are more susceptible to oxidant stress in vivo (29). To determine how mev BMMCs would respond to oxidant stress, we examined the expression and production of IL-4 and -13 and IFN-γ by BMMCs after H2O2 stimulation for 24 h. In WT BMMCs, IL-4 mRNA was barely detectable at baseline, but it increased significantly with increasing concentrations of H2O2. Interestingly, the IL-4 mRNA level was significantly higher in mev BMMCs at baseline, but no further increased occurred with H2O2 stimulation. IL-13 expression showed a very similar pattern, whereas IFN-γ expression did not change under these conditions (Fig. 3A). To test whether the expression of IL-4 in mev BMMCs was related to oxidant stress, we incubated the cells with an antioxidant (NAC). Compared with the high-level basal expression in these cells, IL-4 mRNA was significantly reduced in the presence of NAC for 12 h and was barely detectable at 24 h, suggesting that the increased expression of IL-4 in mev BMMCs was mediated by oxidant (Fig. 3B). A similar pattern was seen at the IL-4 protein level. There was a basal level of IL-4 in the culture medium of WT BMMCs and a significantly increased level in mev BMMCs. With H2O2 stimulation, IL-4 was increased in WT BMMCs, but there was no further increase in mev BMMCs. Again, these changes were blocked by NAC (Fig. 3C). These results demonstrate that mev BMMCs have a higher basal level expression of IL-4 and -13 that may be related to oxidant stress.
FIGURE 3.
BMMC expression and secretion of IL-4 and -13 and IFN-γ in response to H2O2 stimulation. A, BMMCs (2 × 106 cells/ml) were stimulated with indicated concentrations of H2O2 overnight, and cDNA from the cells was analyzed for IL-4 and -13 and IFN-γ mRNA expression. Densitometry analysis was performed on three independent experiments and normalized to β-actin (*p < 0.01). B, RT-PCR analysis of IL-4 expression after NAC treatment. cDNA from mev BMMCs treated with NAC (10 ng/ml) was analyzed for IL-4 expression at two time points. Densitometry was performed in three independent experiments and normalized to β-actin. C, IL-4 secretion by BMMCs after H2O2 (10 nM) stimulation or NAC (10 ng/ml) treatment. IL-4 protein in the BMMC culture medium was evaluated by ELISA after stimulation for 24 h (n = 8 for each group).
Increased IL-13 production in response to LPS
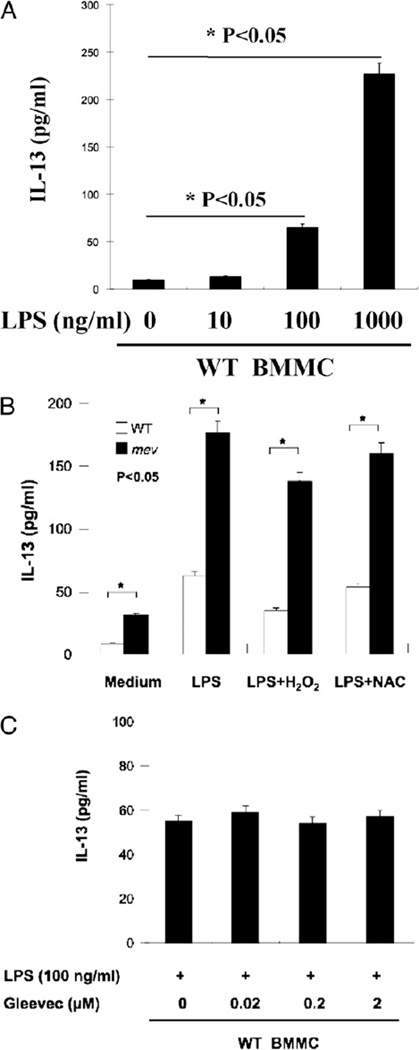
Next, we examined the production of IL-13 by mev BMMCs in response to LPS stimulation. We established that WT BMMCs had very low baseline production of IL-13 and responded well to LPS in a dose-dependent manner (Fig. 4A). However, compared with WT BMMCs, mev BMMCs produced higher levels of IL-13 without any stimulation, and the production of IL-13 was significantly increased when stimulated by LPS. This response was slightly reduced by costimulation with H2O2 without significance (p > 0.05), and it was not affected by NAC, an antioxidant and a potent inhibitor of NF-κB. This suggests that reactive oxygen species (ROS) have little effect on LPS-induced IL-13 production and that the NF-κB pathway is probably not involved in this process (Fig. 4B), which is consistent with the finding in a previous report (30). Interestingly, kinase inhibitor Gleevec had no effect on LPS-induced IL-13 production, indicating that this process is c-Kit independent (Fig. 4C).
FIGURE 4.
BMMC production of IL-13 in response to LPS. A, Dose-response of WT BMMCs to LPS stimulation. WT BMMCs (1 × 107 cells/ml) were incubated with LPS of increasing concentrations for 24 h, and IL-13 in the supernatant was measured by ELISA (n = 3). B, IL-13 production by WT and mev BMMCs (1 × 107 cells/ml) after incubation with LPS (100 ng/ml) with or without H2O2 or NAC (n = 5 for each group). The difference between mev BMMCs stimulated with LPS and LPS+H2O2 was not statistically significant (p > 0.05). C, Effect of Gleevec on LPS-stimulated BMMC production of IL-13. WT BMMCs were incubated with LPS for 24 h in the presence of varying concentrations of Gleevec, and IL-13 in the supernatant was measured by ELISA (n = 3 for each group).
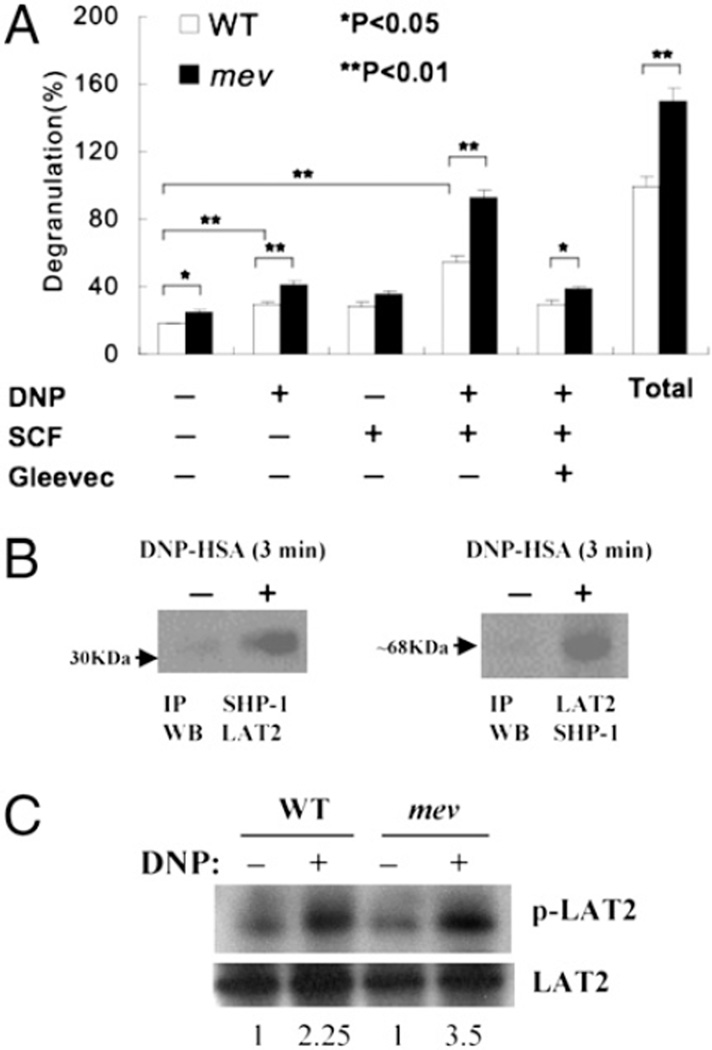
Increased mast cell degranulation in response to FcεRI and SCF signaling
To compare the biological activity of mast cells in degranulation, WT and mev BMMCs were sensitized with anti-DNP IgE and stimulated with DNP-HSA, SCF, and a combination of the two in the presence of kinase inhibitor Gleevec. WT BMMCs had some basal level release of β-hexosaminidase. DNP or SCF stimulation increased the release, but DNP and SCF together had a synergistic effect on the release. This response was significantly reduced by Gleevec. In contrast, mev BMMCs showed a significantly increased release of β-hexosaminidase compared with WT BMMCs in the presence of various stimuli, except SCF. In addition, mev BMMCs had a significantly higher amount of total cellular β-hexosaminidase (Fig. 5A). These results suggest that SHP-1–deficient mast cells may release more mediators into the tissues with or without stimulation, likely reflecting increased degranulation and/or increased mediator production.
FIGURE 5.
BMMC degranulation in response to FcεRI and c-Kit signaling. A, BMMCs (1 × 107 cells/ml) were sensitized with anti-DNP IgE overnight. Then cells were stimulated with DNP-HSA (100 ng), SCF (10 ng/ml), or a combination of DNP-HSA/SCF or DNP-HSA/SCF/Gleevec (1 µM). After 15 min, β-hexosaminidase released in the supernatant was measured by ELISA. Total amount of β-hexosaminidase was also determined after lysing unstimulated control cell samples. Data shown are mean percentage ± SD of triplicates of each sample relative to the total amount of β-hexosaminidase in WT control cells. Three independent experiments were performed with similar results. B, Binding of SHP-1 to LAT2. Protein samples from IgE-sensitized WT BMMCs stimulated with DNP-HSA or control were immunoprecipitated with anti–SHP-1 Ab and subsequently immunoblotted using anti-LAT2 Ab or vice versa. The relative positions of the molecular markers are indicated (arrow). C, LAT2 phosphorylation. Protein samples from WT and mev BMMCs with/without DNP-HSA stimulation for 3 min were immunoprecipitated with antiphosphotyrosine and then immunoblotted with anti-LAT2 or only immunoblotted with anti-LAT2 for total LAT2. The numbers are ratios of p-LAT2/LAT2 of individual samples that were normalized to that of the WT unstimulated sample. A representative of two experiments is shown.
SHP-1 binds and regulates LAT2 in FcεRI signaling
To explore the molecular mechanisms by which SHP-1 regulates mast cell function, we examined the relationship between SHP-1 and the signaling molecule LAT2 (also called NTAL). Sequential immunoprecipitation and immunoblotting of SHP-1 and LAT2 in WT BMMCs after DNP stimulation showed that LAT2 could be coprecipitated with SHP-1 by α-SHP-1 or vice versa (Fig. 5B). Furthermore, immunoprecipitation and immunoblot using anti-LAT2 and α-phosphotyrosine Abs in WT and mev BMMCs showed that phosphorylated LAT2 was increased after DNP stimulation in WT BMMCs, but it had increased even more in mev BMMCs (Fig. 5C). These results demonstrate that SHP-1 can bind and possibly dephosphorylate LAT2 in mast cells upon FcεRI stimulation.
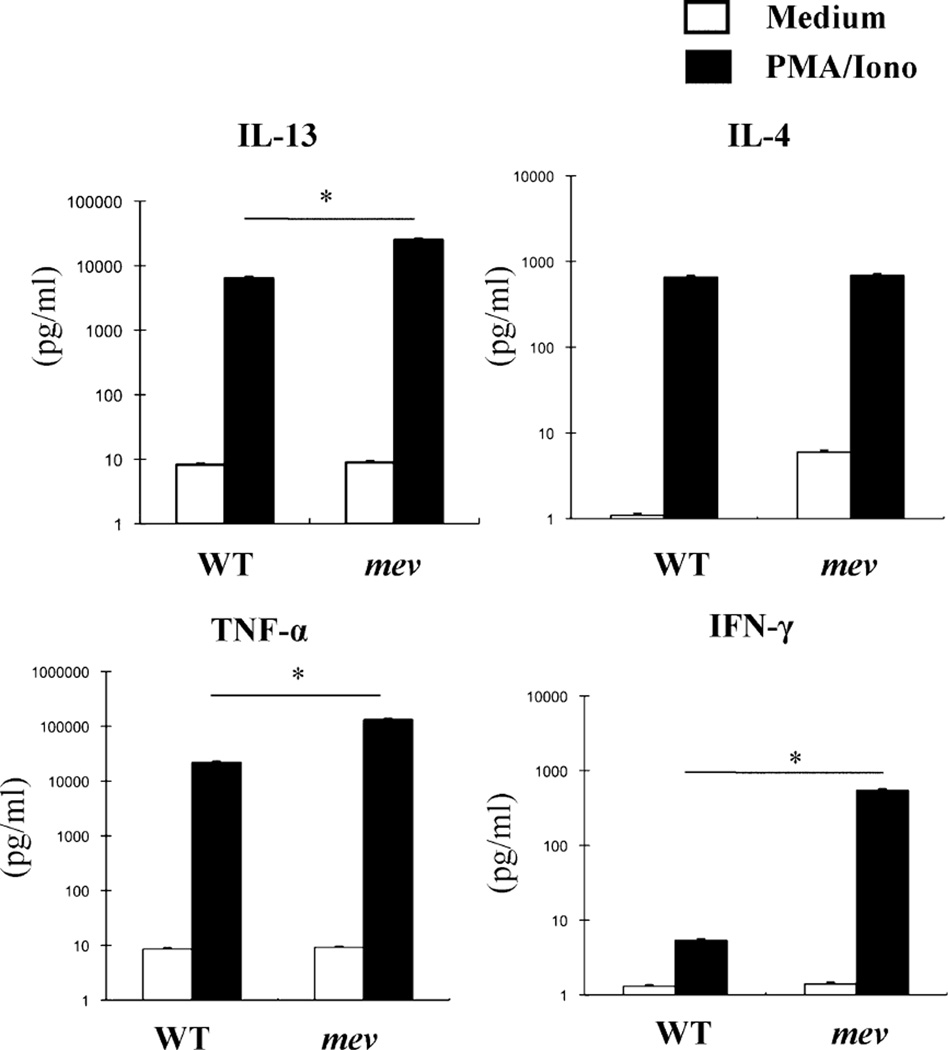
Increased overall cytokine response in mev BMMCs
In the presence of a strong and general stimulation in the form of PMA plus ionomycin, WT and mev BMMCs responded by producing more proinflammatory cytokines. However, mev BMMCs produced significantly more IL-13, TNF-α, and IFN-γ, but similar levels of IL-4 (Fig. 6). These results indicate that SHP-1 regulates mast cell responsiveness to proinflammatory stimulation.
FIGURE 6.
BMMC production of cytokines in response to PMA/ionomycin stimulation. Equal numbers (1 × 106/ml) of BMMCs were incubated with PMA (40 ng/ml)/ionomycin (200 ng/ml) for 24 h, and cytokines in the supernatant were measured by ELISA. Mean ± SD values are in log scale (n = 3 for WT group and n = 6 for mev group). *p < 0.05.
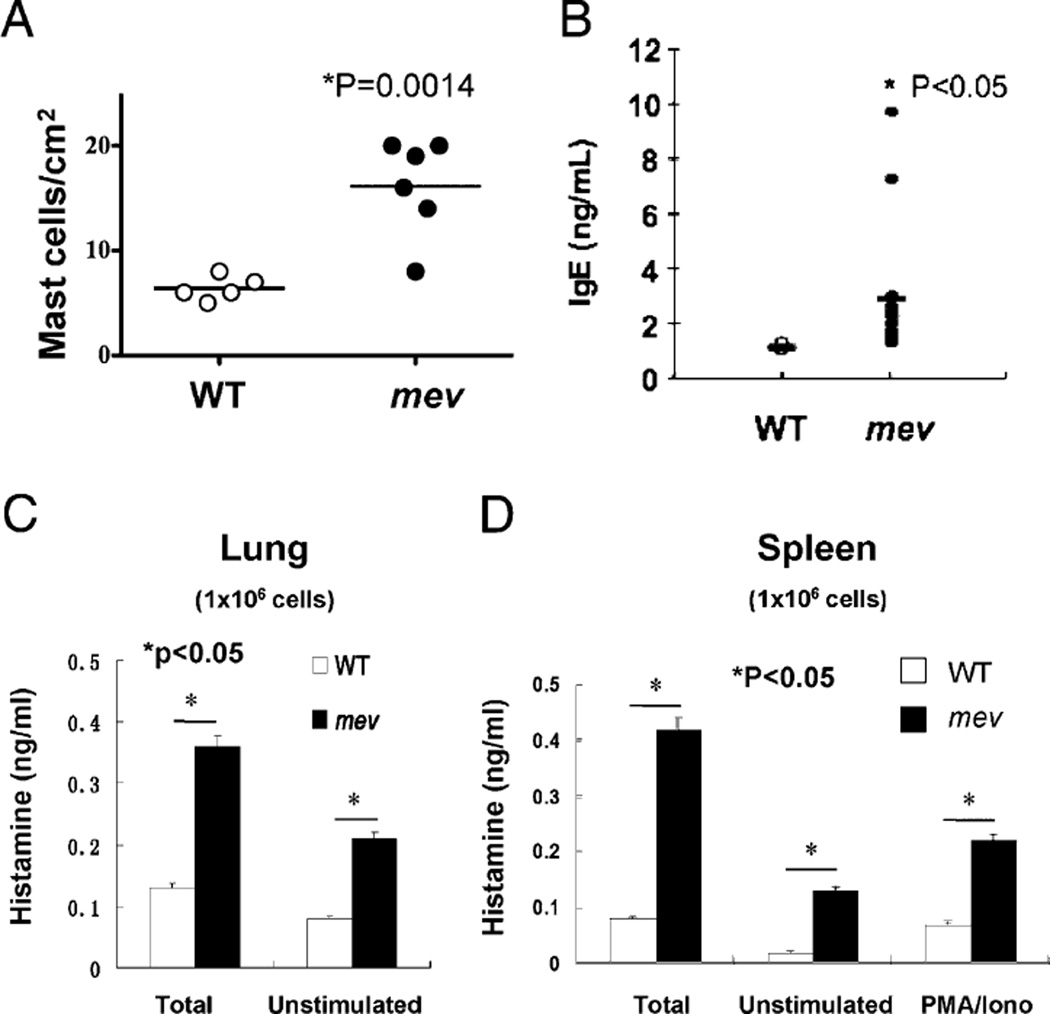
Increased number and activity of mast cells in the lung of mev mice
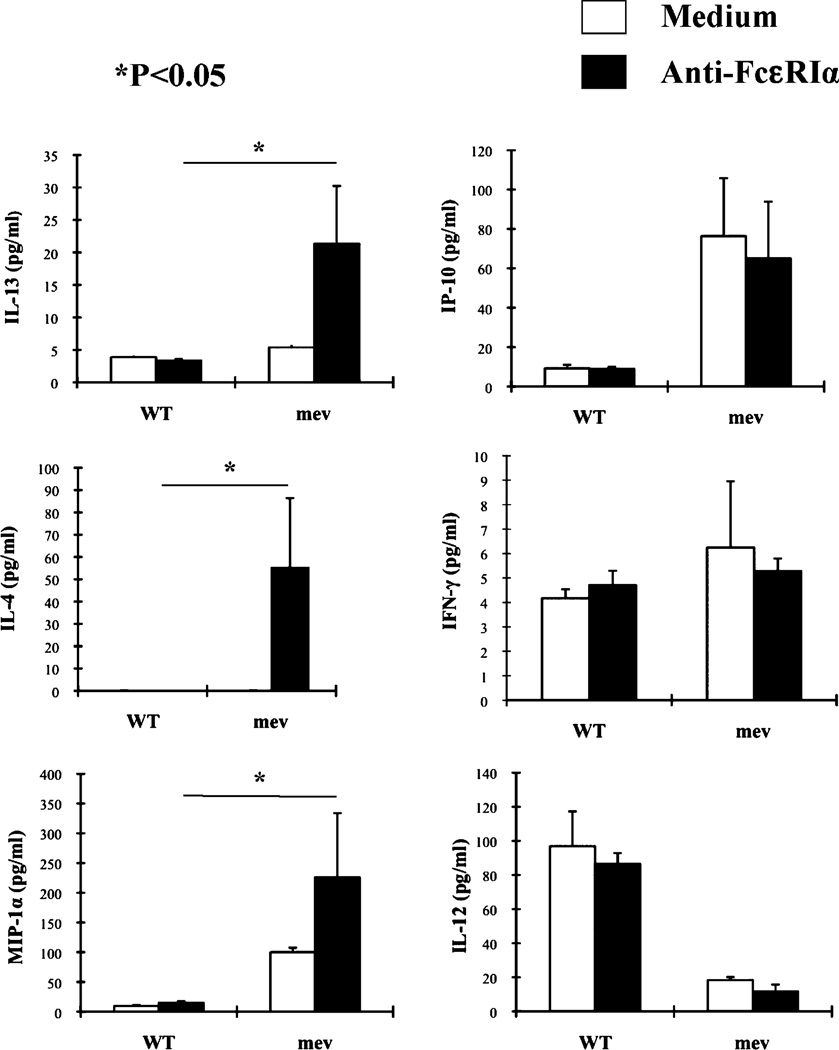
We previously reported that homozygous mev mice develop spontaneous Th2-like allergic inflammation in the lung without known allergen stimulation (11). We examined the inflammatory status of mast cells in mev mice to determine whether mast cells are involved in this process. Toluidine blue staining of lung sections revealed that the number of mast cells in the lung tissue of mev mice was significantly greater than that of WT mice (Fig. 7A). In addition, the levels of total IgE in the BAL fluid of mev mice were significantly higher than those of WT mice, providing a potential basis for mast cell sensitization (Fig. 7B). Total cellular histamine and spontaneously released histamine were detected in isolated lung cells from WT mice. However, total and spontaneously released histamine was significantly increased in mev lung cells, indicating increased mast cell numbers and increased spontaneous release of mediators (Fig. 7C). Similar findings were seen in the spleen of mev mice (Fig. 7D). We next tested a panel of cytokines produced by splenocytes when stimulated by anti-FcεRIα; compared with the cells from WT mice, splenocytes from mev mice produced significantly increased levels of IL-13 and -4 and MIP-1α, suggesting that mast cells, and perhaps basophils, were involved in Th2 cytokine responses. In contrast, IP-10 levels were higher, IFN-γ levels were the same, and IL-12 levels were lower in the spleen of mev mice compared with WT mice, regardless of FcεRI activation. These data indicate that cells other than mast cells or basophils were involved in the Th1 cytokine responses (Fig. 8). These findings indicate that mast cell numbers and activity significantly increased in the lung and spleen of mev mice, and these cells may have been responsible for increased Th2 cytokine production.
FIGURE 7.
Mast cells in the lung tissue. A, Toluidine blue-positive mast cells in the lung sections from WT (n = 5) and mev mice (n = 6) were counted under the microscope. B, BAL IgE levels in WT and mev mice (n = 6). C, Single-cell suspensions of the lung (1 × 106) from WT and mev mice were lysed for total histamine or incubated for 45 min at 37°C without stimulation for spontaneous release of histamine (n = 5 for each group). D, Single-cell suspensions of splenocytes (1 × 106) from WT and mev mice were lysed for total histamine or incubated with/without PMA/ionomycin, and released histamine was determined (n = 5 for each group).
FIGURE 8.
Cytokine production by splenocytes after stimulation. Equal numbers of isolated splenocytes (1 × 106) were incubated with or without anti-FcεRIα Ab (0.1 µg/ml) for 21 h, and cytokines and chemokines in the supernatant were measured by ELISA (n = 3). *p < 0.05 mev versus WT stimulated BMMCs.
Pulmonary Th2 inflammatory phenotype is mast cell dependent
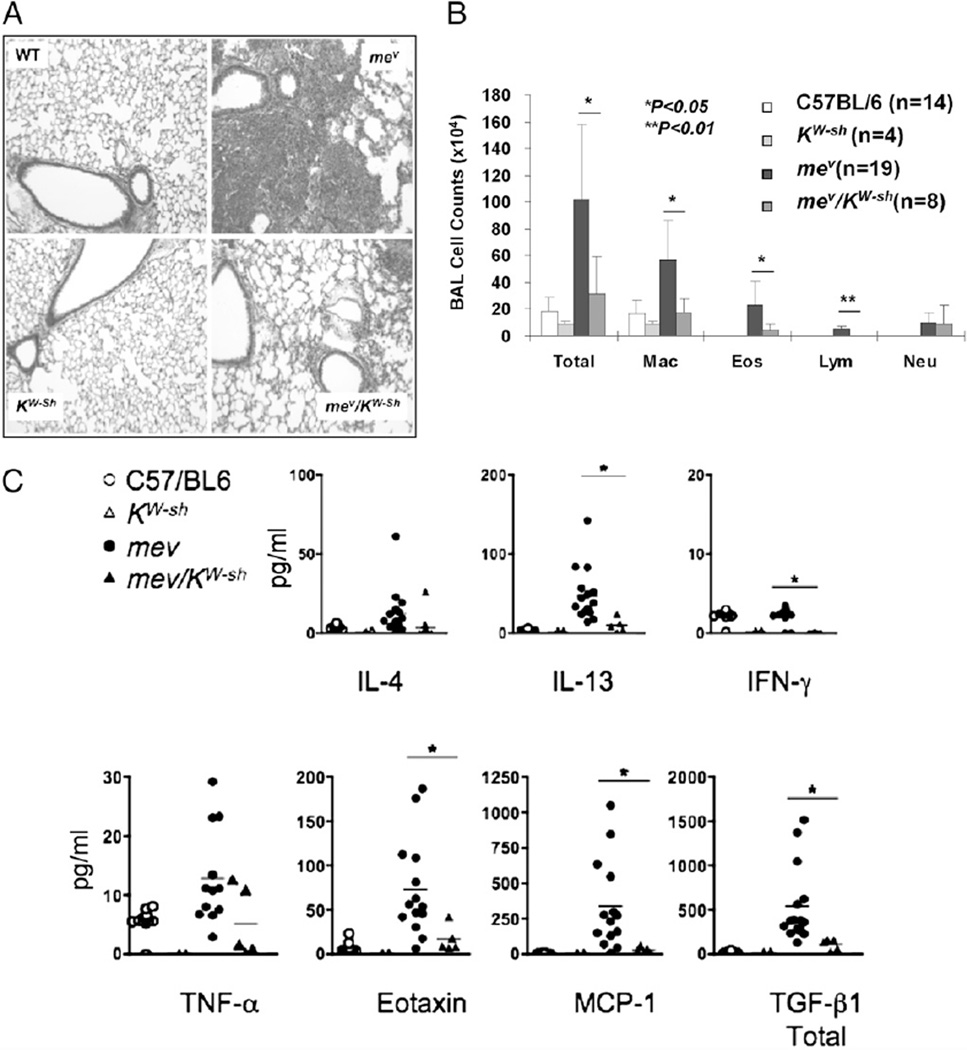
To determine whether the Th2 lung phenotype of mev mice was mast cell dependent, we cross-bred mev mice with the mast cell-deficient KitW-sh mice and examined the changes in lung inflammation. We chose this strain because, unlike Wv mice, the deficiency in KitW-Sh mice is more restricted to mast cells (31). Lung histology revealed that WT mice and KitW-sh mice had no inflammation in the lung, whereas mev mice developed severe lung inflammation, as described before (11). However, mev/KitW-sh mice had significantly reduced inflammation (Fig. 9A). BAL cell counts showed that the total number of cells in the airway was dramatically reduced, although there was some residual inflammation, in mev/KitW-Sh mice compared with mev mice, and the reduction was in macrophages, eosinophils, and lymphocytes (Fig. 9B). An evaluation of the cytokine production in the lungs of these mice showed that with considerable variations among the samples, Th2 cytokines and chemokines had the most dramatic changes in the mev mice and mev/KitW-Sh mice (Fig. 9C). These studies indicate that mast cells deficient in SHP-1 are the main producers of Th2 cytokines in the lung and are largely responsible for the generation of the allergic asthma phenotype of mev mice.
FIGURE 9.
Involvement of mast cells in the spontaneous allergic asthma phenotype of homozygous mev mice. Comparison of WT, mev, KitW-Sh, and mev/KitW-Sh mice for lung histology (A; original magnification ×10), BAL cellularity (B), and BAL cytokines (C) (*p < 0.05).
Discussion
SHP-1 deficiency causes spontaneous inflammation involving multiple organs and tissues in mice (20, 32, 33). We defined the pulmonary pathology in mev mice as a Th2-dominated allergic asthma phenotype (11). Several studies showed that myeloid cells, not T cells or B cells, are important in the development of the inflammatory phenotype in me mice (24–26). More specifically, SHP-1 was shown to interact with the SCF receptor c-Kit (34), and c-Kit deficiency, as in the Wv mice, ameliorates the pathology in me mice (25, 26). In contrast, SHP-1 deficiency partially restored mast cell numbers in the skin of c-Kit–deficient Wv mice (25, 26). SHP-1 was reported to play a regulatory role in BMMCs in vitro (15, 16). However, the mechanisms by which SHP-1 regulates mast cells, the function of mast cells in initiating immune responses to various stimuli, and the role of mast cells in generating allergic inflammatory responses in the lung, particularly in the absence of specific allergen stimulation, have not been defined.
In this study, we presented evidence that SHP-1 is a critical regulator of mast cell differentiation and function, and we identified mast cells as an important cell type in Th2 cytokine production and allergic asthma phenotype generation in SHP-1 deficiency.
The percentage of mature mast cells was significantly greater in the bone marrow of mev mice compared with that of WT mice. This difference was maintained until day 16 in culture (Fig. 1A). The higher seeding density in mev BMMCs is probably an important contributor to this difference. However, in the later stage, the difference between mev and WT mast cell populations disappeared, possibly because the proliferation curves of both genotypes remained flat until day 16, when there was a more obvious acceleration of growth in WT cells.
It was reported that the expression of catalytically inactive SHP-1 mutant constructs in IL-3–dependent transformed mouse pro-B cells or human breast cancer cells enhanced cell proliferation and increased cell survival, suggesting that SHP-1 is a negative regulator in these processes (35, 36). However, our data on mast cell proliferation and survival show that, despite increased differentiation and increased resistance to apoptosis, the proliferation of mev BMMCs was much slower than that of WT BMMCs, indicating a dissociation of these processes. The reason for this is unclear. It was noted that upregulation of Bcl-2 was associated with protection from cell death and, at the same time, a reduced rate of proliferation, possibly through cell accumulation in the G0/G1 phase of the cell cycle (37). Our finding that Bcl-2 was highly upregulated in mev BMMCs suggests that this could be one reasonable explanation for our observations of increased cell survival and decreased proliferation. Other possible explanations for the discrepancy between our study and those mentioned above may include the fact that transfected SHP-1 variant constructs were used in those studies, whereas SHP-1–deficient cells were used in our study, and that the cell types in those studies were different from our primary BMMCs (35, 36).
Of note is our observation that SHP-1 deficiency per se did not offer protection from growth factor withdrawal-induced apoptosis. This suggests that the target of SHP-1 regulation does not generate intrinsic antiapoptotic signals but rather amplifies those initiated by growth factors.
SCF and c-Kit signaling are essential for the development of murine mast cells (38, 39), and SHP-1 negatively regulates c-Kit (34). It is conceivable that SHP-1 deficiency can lead to enhanced c-Kit signaling, but it remains unclear why there is a significant increase in the c-Kit–positive progenitor population in the bone marrow in SHP-1 deficiency, some of which are mast cell progenitors.
In this study, we evaluated the function of SHP-1 in regulating the mast cell cytokine response to oxidant stress and to bacterial product LPS. Our results show that, even at baseline, mev BMMCs had significantly increased expression of IL-4 and -13, but not IFN-γ, and the change was blocked by antioxidant NAC. Mast cells are able to generate intracellular ROS (40), and SHP-1 deficiency led to lower tolerance to oxidant stress in alveolar macrophages and to allergen-induced airway inflammation in vivo (29). Our findings are consistent with the notion that SHP-1 also regulates ROS response in mast cells. Similarly, mev BMMCs had markedly increased production of IL-13 in response to LPS stimulation, which was not affected by H2O2 or NAC, suggesting that ROS and NF-κB were not involved in this activation pathway. A similar finding was reported by Stassen et al. (30). Furthermore, kinase inhibitor Gleevec had no effect on LPS-stimulated production of IL-13 by mast cells, indicating that this process is c-Kit independent. It is likely that signaling molecules in the MAPK pathway, such as ERK, p38, or JNK, are involved (41), and SHP-1 is a regulator of this pathway (42). Studies have shown that SCF–c-Kit signaling cooperates with FcεRI activation in stimulating mast cell degranulation (43), and SHP-1 regulates c-Kit function (34). It was predicted that SHP-1 deficiency would further increase mast cell degranulation in response to FcεRI stimulation. Indeed, mev BMMCs had significantly increased total cellular β-hexosaminidase, increased spontaneous degranulation, and increased degranulation upon FcεRI ligation. This result seems different from what was reported in a recent study by Nataka et al. (16) on SHP-1–deficient me BMMCs. Several explanations may account for the discrepancy. There were some differences in the experimental procedures. For instance, we tested BMMCs derived from mev mice, and the cells were stimulated with 100 ng/ml DNP-HSA for 15 min before mediator measurement; Nataka et al. (16) treated me BMMCs with Tyrode’s buffer for 4 h before stimulation with 5–50 ng/ml DNP-HSA for 30 min. Probably more importantly, Nataka et al. showed the percentage of β-hexosaminidase released from individual samples, which emphasizes the releasability, whereas we presented the release of β-hexosaminidase of all samples relative to the total amount of β-hexosaminidase of WT BMMCs, taking into account that increased release also reflects increased total mediator in mev BMMCs (Fig. 5A).
LAT2, also called NTAL, is an adaptor molecule that can be phosphorylated upon FcεRI cross-linking and c-Kit activation in mast cells; it may function as a positive and negative regulator in human mast cell activation (44). The Y110 of the cytosolic domain of LAT2 is speculated to be a putative site for Src kinase phosphorylation and SHP-1 binding (45). However, the interaction has not been demonstrated. In this study, we demonstrated that upon FcεRI ligation, SHP-1 binds LAT2, and SHP-1 deficiency in mast cells leads to significantly increased phosphorylation of LAT2, which correlates with an overall increase in mast cell activity.
To determine whether SHP-1 dysregulation also affects mast cell behavior in vivo, we examined the number and activity of mast cells in the lung and their potential role in the allergic asthma phenotype in mev mice. These studies revealed that SHP-1 deficiency led to increased mast cell numbers in the lung and, more importantly, markedly increased mast cell activity as evidenced by increased mediator release and Th2 cytokine production in the lung and spleen, both spontaneously and in response to stimulation. This correlates well with our previous finding that the levels of Th2 cytokines in the lung of mev mice were significantly increased (11). In addition, the levels of IgE in the lung tissue were significantly increased. Increased levels of IgE are correlated with increased tissue mast cell numbers and activity (46) and increased mast cell survival in the absence of growth factor in vitro (Fig. 2B). However, the exact role of IgE in the mev phenotype needs to be determined. Finally, a critical role of mast cells in the mev allergic asthma phenotype was demonstrated by dramatically reduced airway inflammation, improved lung pathology, and decreased IL-13 and chemokines in mev mice on the mast cell-deficient KitW-Sh background.
In this study, we demonstrate that mast cells deficient in SHP-1 have increased differentiation, are more resistant to apoptosis, and are hyperresponsive to various stimuli, including oxidant stress, FcεRI cross-linking, and LPS, with enhanced release of mediators and production of proinflammatory cytokines. Furthermore, without SHP-1 regulation in vivo, mast cells are increased in number in the lung, have increased spontaneous activity, and produce more cytokines, favoring a Th2 cytokine milieu in tissues. Our findings support the notion that mast cells are largely responsible for the generation of the Th2-like allergic inflammatory pulmonary phenotype in mev mice. These findings demonstrate an important role of SHP-1 in regulating mast cell functions and a role of mast cells as an initiator, not a mere effector, of tissue inflammation in the lung in response to environmental stimulation.
Acknowledgments
We thank Phyllis Zhu for proofreading the manuscript.
This work was supported by National Institutes of Health Grants HL079349 (to Z.Z.) and AI075025 (to T.Z.) and a grant from the China Scholarship Council (to L.Z).
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BMMC
bone marrow-derived mast cell
- HSA
human serum albumin
- LAT2
linker for activation of T cells 2
- me
motheaten
- mev
viable motheaten
- NAC
N-acetylcysteine
- ROS
reactive oxygen species
- SCF
stem cell factor
- SHP-1
Src homology region 2 domain-containing phosphatase 1
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ Centers for Disease Control and Prevention (CDC) National surveillance for asthma—United States, 1980–2004. MMWR Surveill. Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 2.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am. J. Respir. Cell Mol. Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 3.Garlisi CG, Falcone A, Kung TT, Stelts D, Pennline KJ, Beavis AJ, Smith SR, Egan RW, Umland SP. T cells are necessary for Th2 cytokine production and eosinophil accumulation in airways of antigen-challenged allergic mice. Clin. Immunol. Immunopathol. 1995;75:75–83. doi: 10.1006/clin.1995.1055. [DOI] [PubMed] [Google Scholar]
- 4.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp. Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin. Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 10.Oh SY, Zheng T, Bailey ML, Barber DL, Schroeder JT, Kim YK, Zhu Z. Src homology 2 domain-containing inositol 5-phosphatase 1 deficiency leads to a spontaneous allergic inflammation in the murine lung. J Allergy Clin. Immunol. 2007;119:123–131. doi: 10.1016/j.jaci.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh SY, Zheng T, Kim YK, Cohn L, Homer RJ, McKenzie AN, Zhu Z. A critical role of SHP-1 in regulation of type 2 inflammation in the lung. Am. J. Respir. Cell Mol. Biol. 2009;40:568–574. doi: 10.1165/rcmb.2008-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, Takeda K, Loader J, Miyahara N, Kodama T, et al. Mast cells, Fc ε RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol. 2004;172:6398–6406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp. Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin. Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamata T, Yamashita M, Kimura M, Murata K, Inami M, Shimizu C, Sugaya K, Wang CR, Taniguchi M, Nakayama T. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin. Invest. 2003;111:109–119. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakata K, Yoshimaru T, Suzuki Y, Inoue T, Ra C, Yakura H, Mizuno K. Positive and negative regulation of high affinity IgE receptor signaling by Src homology region 2 domain-containing phosphatase 1. J Immunol. 2008;181:5414–5424. doi: 10.4049/jimmunol.181.8.5414. [DOI] [PubMed] [Google Scholar]
- 17.Haddon DJ, Antignano F, Hughes MR, Blanchet MR, Zbytnuik L, Krystal G, McNagny KM. SHIP1 is a repressor of mast cell hyperplasia, cytokine production, and allergic inflammation in vivo. J Immunol. 2009;183:228–236. doi: 10.4049/jimmunol.0900427. [DOI] [PubMed] [Google Scholar]
- 18.Tsui FW, Martin A, Wang J, Tsui HW. Investigations into the regulation and function of the SH2 domain-containing protein-tyrosine phosphatase, SHP-1. Immunol. Res. 2006;35:127–136. doi: 10.1385/IR:35:1:127. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin. Immunol. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 20.Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered. 1975;66:250–258. doi: 10.1093/oxfordjournals.jhered.a108625. [DOI] [PubMed] [Google Scholar]
- 21.Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. “Viable motheaten,” a new allele at the motheaten locus. I. Pathology. Am. J. Pathol. 1984;116:179–192. [PMC free article] [PubMed] [Google Scholar]
- 22.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 23.Su X, Zhou T, Yang P, Edwards CK, 3rd, Mountz JD. Reduction of arthritis and pneumonitis in motheaten mice by soluble tumor necrosis factor receptor. Arthritis Rheum. 1998;41:139–149. doi: 10.1002/1529-0131(199801)41:1<139::AID-ART17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 24.Yu CC, Tsui HW, Ngan BY, Shulman MJ, Wu GE, Tsui FW. B and T cells are not required for the viable motheaten phenotype. J Exp. Med. 1996;183:371–380. doi: 10.1084/jem.183.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulson RF, Vesely S, Siminovitch KA, Bernstein A. Signalling by the W/Kit receptor tyrosine kinase is negatively regulated in vivo by the protein tyrosine phosphatase Shp1. Nat. Genet. 1996;13:309–315. doi: 10.1038/ng0796-309. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz U, Bergemann AD, Steinberg HN, Flanagan JG, Li X, Galli SJ, Neel BG. Genetic analysis reveals cell type-specific regulation of receptor tyrosine kinase c-Kit by the protein tyrosine phosphatase SHP1. J Exp. Med. 1996;184:1111–1126. doi: 10.1084/jem.184.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Jr, Chapman HA, Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin. Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frossi B, Rivera J, Hirsch E, Pucillo C. Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J Immunol. 2007;178:2549–2555. doi: 10.4049/jimmunol.178.4.2549. [DOI] [PubMed] [Google Scholar]
- 29.Cho YS, Oh SY, Zhu Z. Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2008;39:412–419. doi: 10.1165/rcmb.2007-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stassen M, Müller C, Arnold M, Hültner L, Klein-Hessling S, Neudörfl C, Reineke T, Serfling E, Schmitt E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-k B is decisively involved in the expression of IL-9. J Immunol. 2001;166:4391–4398. doi: 10.4049/jimmunol.166.7.4391. [DOI] [PubMed] [Google Scholar]
- 31.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shultz LD, Green MC. Motheaten, an immunodeficient mutant of the mouse. II. Depressed immune competence and elevated serum immunoglobulins. J Immunol. 1976;116:936–943. [PubMed] [Google Scholar]
- 33.Clark EA, Shultz LD, Pollack SB. Mutations in mice that influence natural killer (NK) cell activity. Immunogenetics. 1981;12:601–613. doi: 10.1007/BF01561700. [DOI] [PubMed] [Google Scholar]
- 34.Kozlowski M, Larose L, Lee F, Le DM, Rottapel R, Siminovitch KA. SHP-1 binds and negatively modulates the c-Kit receptor by interaction with tyrosine 569 in the c-Kit juxtamembrane domain. Mol. Cell. Biol. 1998;18:2089–2099. doi: 10.1128/mcb.18.4.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paling NR, Welham MJ. Role of the protein tyrosine phosphatase SHP-1 (Src homology phosphatase-1) in the regulation of interleukin-3-induced survival, proliferation and signalling. Biochem. J. 2002;368:885–894. doi: 10.1042/BJ20021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Q, Siminovitch KA, Fialkow L, Fukushima T, Downey GP. Negative regulation of myeloid cell proliferation and function by the SH2 domain-containing tyrosine phosphatase-1. J Immunol. 1999;162:3220–3230. [PubMed] [Google Scholar]
- 37.Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol. Chem. 1996;271:12695–12698. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- 38.Copeland NG, Gilbert DJ, Cho BC, Donovan PJ, Jenkins NA, Cosman D, Anderson D, Lyman SD, Williams DE. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- 39.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 40.Swindle EJ, Metcalfe DD, Coleman JW. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J Biol. Chem. 2004;279:48751–48759. doi: 10.1074/jbc.M409738200. [DOI] [PubMed] [Google Scholar]
- 41.Masuda A, Yoshikai Y, Aiba K, Matsuguchi T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunol. 2002;169:3801–3810. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 42.Krautwald S, Büscher D, Kummer V, Buder S, Baccarini M. Involvement of the protein tyrosine phosphatase SHP-1 in Ras-mediated activation of the mitogen-activated protein kinase pathway. Mol. Cell. Biol. 1996;16:5955–5963. doi: 10.1128/mcb.16.11.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwaki S, Spicka J, Tkaczyk C, Jensen BM, Furumoto Y, Charles N, Kovarova M, Rivera J, Horejsi V, Metcalfe DD, Gilfillan AM. Kit- and Fc epsilonRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell. Signal. 2008;20:195–205. doi: 10.1016/j.cellsig.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and Fc ε RI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 45.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 46.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp. Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]