Abstract
Background
Early prediction of disease progression in men with very low risk (VLR) prostate cancer who selected active surveillance (AS) rather than immediate treatment could reduce morbidity associated with overtreatment.
Methods
We evaluated the association of six biomarkers [Periostin, (−5,−7) proPSA, CACNA1D, HER2/neu, EZH2, and Ki67] with different Gleason scores and biochemical recurrence (BCR) on prostate cancer tissue microarrays (TMAs) of 80 radical prostatectomy (RP) cases. Multiplex Tissue Immunoblotting (MTI) was used to assess these biomarkers in cancer and adjacent benign areas of 5μm sections. Multivariate logistic regression (MLR) was applied to model our results.
Results
In the RP cases, CACNA1D, HER2/neu and Periostin expression were significantly correlated with aggressive phenotype in cancer areas. MLR model in cancer area yielded a ROC-AUC = 0.98, while in cancer adjacent benign areas, yielding a ROC-AUC = 0.94. CACNA1D and HER2/neu expression combined with Gleason score in a MLR model yielded a ROC-AUC = 0.79 for BCR prediction. In the small biopsies from an AS cohort of 61 VLR cases, a MLR model for prediction of progressors at diagnosis retained (−5,−7) proPSA and CACNA1D, yielding a ROC-AUC of 0.78, which was improved to 0.82 after adding tPSA into the model.
Conclusions
Molecular profile of biomarkers is capable of accurately predicting aggressive prostate cancer on retrospective RP cases and identifying potential aggressive prostate cancer requiring immediate treatment on the AS diagnostic biopsy but limited in BCR prediction.
Impact
Comprehensive profiling of biomarkers using MTI predicts prostate cancer aggressive phenotype in RP and AS biopsies.
Keywords: prostate cancer, aggression, biochemical recurrence, biomarkers, multiplex tissue immunoblotting (MTI)
INTRODUCTION
Prostate cancer is the most common cancer in men in the US and is the second most common cause of cancer death in men. Prostate cancer usually occurs after age 50 and the incidence increases with age. There are 233,000 estimated new cases and 29,480 estimated prostate cancer deaths in 2014 in the US (1). Radical prostatectomy (RP) is an effective treatment for patients with organ-confined disease and has been demonstrated to reduce the risk of death from prostate cancer (2). Nearly 40% of prostate cancer patients who choose definitive therapy will undergo RP. In 38-52% of cases, advanced disease with potentially bad prognosis are found in surgical specimens (3). Each category of extraprostatic disease is associated with significantly increased risk of cancer recurrence and progression, measured at the earliest time with a detectable prostate specific antigen (PSA) (>0.20 ng/ml), or biochemical recurrence (BCR) (4). The natural history of prostate cancer progression after BCR following surgery can be highly variable (i.e. 3-13 years); however, at least two thirds of BCR patients develop disease spread if left untreated and many will die because of distant progression (5).
In most cases, BCR is used as a surrogate measure for more clinically meaningful critical endpoints such as distant progression or cancer-specific mortality. Inaccurate risk classifications could result in inappropriate or unnecessary treatments. Unfortunately, existing tools [e.g. nomograms like Shariat (6), Swanson(7) and Capra(8)] that rely solely on clinical variables such as PSA velocity, grade, and stage are unable to predict which men will go on to metastasis and ultimately prostate cancer specific death. These tools may be unable to identify those men that have already demonstrated BCR. Therefore, a better method to predict whether a prostate cancer patient has a more aggressive phenotype at surgery will help to standardize treatments of the more aggressive cancer patients early and efficiently and spare patients of less aggressive cancer from the morbidity associated with adjuvant therapy.
PSA screening and prostate biopsies have resulted in over-diagnosis and over-treatment of prostate cancer (9-11). In fact, In fact, low grade, low stage prostate cancer of up to ~56% men remained undetected during their lifetime (9-12). In the present era, 30 men require treatment for prostate cancer in order to save one man (13). Such overtreatment can always create a chance of a decreased quality of life once sexual function and urinary function are compromised (14). The accurate identification of men with prostate cancer that are destined to progress to life threatening disease and who will benefit from curative intervention must be a high research priority, with the goal of reducing unnecessary treatments.
In 1995, Dr. Ballentine Carter started an active surveillance (AS) program with the delayed surgical intervention as a treatment option in Johns Hopkins Medical Institutions (JHMI). Patients are enrolled if they meet Epstein inclusion very low risk (VLR) criteria (15-17). These criteria include PSA density (PSAD) <0.15 ng/ml/g and favorable diagnostic needle biopsy characteristics (i.e. Gleason score ≤6, ≤2 cores involved with cancer, ≤50% of any core involved with cancer). Applications of these criteria for selection have been validated to identify men with indolent prostate cancer (15, 18-20). The current JHMI AS patients are followed-up semi-annually with assessment of serum total PSA (tPSA), free PSA (fPSA), digital rectal examination (DRE) and annually with 12 core surveillance biopsy to decide requirement for immediate prostate cancer intervention under AS program (17). Furthermore, according to the published experience with AS from different groups, about 1/3 of AS patients will require definitive treatment due primarily to upgrading overtime(21) and we cannot determine at diagnosis which patients will eventually fail from the program. Recently, there is a paradigm shift that stems from recent discoveries identifying important clinically and pathological predictive pretreatment parameters that includes an expanded AS criteria including from very low to low risk prostate cancer (22, 23). In fact, the National Comprehensive Cancer Network (NCCN) now recognizes four separate risk groups that include very low risk, low risk, intermediate risk and high risk of newly diagnosed patients with prostate cancer (24).
This study evaluated six tissue molecular biomarkers (MBs) to predict at entry into the AS program the likelihood of failure. The biomarkers evaluated include stromal protein (Periostin), membranous channels and transmembrane proteins (CACNA1D, HER2/neu), nuclear proliferating factor Ki67, epigenetic regulators (EZH2), and prostate specific protein (−5,−7) proPSA. They were chosen based on their location at cellular and tissue level, function and their importance in previous biomarker research (see discussion). We hypothesized that a combination of these molecular biomarkers could predict an aggressive prostate cancer phenotype in RP specimens. To test this hypothesis, we evaluated our biomarker panel using two JHMI tissue microarrays (TMAs) made up of 80 cases of prostate cancer stratified by Gleason score, 22 of which have BCR. A novel multiplex tissue immunoblotting (MTI) technology was applied to quantify the expression of these 6 biomarkers on single 5 μm TMA tissue sections of RP or biopsy specimens. Also, the biomarker research panel was applied to an AS cohort with VLR prostate cancer to see if we could predict those men who failed quickly with unexpected aggressive outcomes (progressors). The overall objective of the study was to develop an integrated, quantitative molecular biomarker-based predictor for the early detection of a clinically aggressive prostate cancer based on biopsy specimens in men being considered for AS for VLR or LR prostate cancer.
MATERIALS AND METHODS
Construction of TMA681 & TMA682
The two TMAs (TMA681 and TMA682) were prepared using a Beecher MT1 manual arrayer (Beecher Instruments, Silver Spring, MD) under the supervision of the Prostate Cancer Biorepository Network (PCBN). The formalin fixed, paraffin embedded (FFPE) RP prostate cancer tissues and normal (benign) cancer-adjacent controls to be included in the two tissue TMAs were selected and reviewed by a JHMI pathologist (JIE). Hematoxylin and eosin (H&E) stained slides from all selected cases were reviewed and “mapped” by the pathologist: the adjacent normal-appearing along with staged and/or graded index tumor areas were identified and marked on the H&E slide for each case. Using these marked “template slides”, the tissue blocks were coordinately marked, and 0.60mm diameter cores were punched from the normal-appearing and prostate tumor areas and then transferred to recipient blocks. 5 μm thick sections of these TMAs were cut and used for downstream MTI studies.
The two TMAs included 80 unique prostate cancer patients representing different Gleason scores (3+3, 3+4, 4+3, and ≥8) with quadruplicates of cancer and cancer-adjacent benign areas. Among these 80 cases, 22 had BCR based on PSA exceeding 0.2 ng/ml on follow-up. The average follow-up time after BCR is 6.6 years (ranging from 1 ~ 14 years). Among the 22 BCR cases, 12 cases had PSA increases only, 1 case had local recurrence, 4 cases had distant metastasis, 3 cases had both local recurrence and distant metastasis and 2 cases received adjuvant radiation treatment and the PSA level decreased to < 0.2 ng/ml after radiation treatment. The detailed demographics of the total 80 cases stratified by Gleason scores of which 22 cases had BCR are shown in Table 1 and further sub-grouped based on clinical features. In the 80 cases, one patient in TMA 682 died from non-cancer cause; there is no follow-up data in 5 cases of TMA 681 and 5 cases of TMA 682.
Table 1.
Prostate Cancer Patients Demographics in TMA681 & 682 (N=80)
| Variable | Subgroups | Recurrence |
Total | |
|---|---|---|---|---|
| No | Yes | |||
| Age | 59.04 ± 6.57 | 58.82 ± 5.80 | ||
| Gleason score | 6 | 7 | 1 | 8 |
| 7 (3+4) | 14 | 3 | 17 | |
| 7 (4+3) | 15 | 3 | 18 | |
| >=8 | 11 | 15 | 26 | |
| TNM stage | T2 | 26 | 2 | 28 |
| T3A | 16 | 15 | 31 | |
| T3B | 3 | 5 | 8 | |
| Gland weight (g) | 51.94 ± 14.90 | 56.19 ± 21.38 | ||
| PSA (ng/mL) | 8.29 ± 5.29 | 13.17 ± 10.15 | ||
| PSAD (ng/mL/g) | 0.17 ± 0.11 | 0.25 ± 0.20 | ||
| exposure (year) | 5.06 ± 4.50 | 3.82 ± 2.36 | ||
| Race | A | 6 | 3 | 9 |
| H | 1 | 0 | 1 | |
| O | 3 | 1 | 4 | |
| C | 37 | 18 | 55 | |
| Surgical margin | Negative | 41 | 16 | 57 |
| Positive | 6 | 6 | 12 | |
| Seminal vesicle status | Negative | 46 | 16 | 62 |
| Positive | 1 | 6 | 7 | |
| Lymph node status | Negative | 46 | 22 | 68 |
| Positive | 1 | 0 | 1 | |
| capsular penetration (fcp) | Negative | 38 | 16 | 54 |
| Positive | 8 | 6 | 14 | |
| capsular penetration (ecp) | Negative | 34 | 8 | 42 |
| Positive | 13 | 14 | 27 | |
| Organ confinement status | Nonconfined | 21 | 20 | 41 |
| Confined | 26 | 2 | 28 | |
Note:
a. Age, Gland weight, PSA, PSAD, exposure data were shown as men ± SD.
b. For race data, A: African American; H: Hispanic; O: Others; C: Caucasian
c. PSAD (ng/mL/g) =PSA (ng/mL)/Gland weight (g)
Test TMAs for IHC Optimization
A separate TMA (TMA 475), which included prostate cancer and normal prostate tissue, was kindly provided by PCBN and was used to optimize dilutions of antibodies of our biomarkers before applying the antibodies to TMA 681 & TMA 682.
Active Surveillance Biopsies
FFPE biopsies from a total of 61 patients (29 progressors and 32 non-progressors) enrolled in the AS program at JHMI were used for MTI study. Progressors were defined as men that met all the AS criteria at entry; however, during monitoring were found to have a tumor increased in volume and/or Gleason score >6) result on biopsy follow-up or retropubic radical prostatectomy (i.e. more aggressive prostate cancer than entry criteria). For each patient, continuous 5μm tissue sections were cut and used either for H&E staining and pathologic assessment or for profiling biomarkers using MTI. The H&E slides were first reviewed by a pathologist and the cancer area were marked for reference of data analysis later on. Only slides with cancer and adjacent to the corresponding read H&E slides were used for MTI study.
All cases used for TMAs and AS biopsies were consented under an Institutional Review Board (IRB) approved protocol at Johns Hopkins University School of Medicine.
Multiplex tissue immunoblotting (MTI)
MTI is a method that can be used to detect multiple molecular targets in FFPE tissues, while retaining both quantitative and histomorphologic diagnostic and prognostic features (25-27). Starting with a 5μm FFPE tissue section on a standard glass slide, proteins were transferred from the tissue sections (TMA or biopsy) onto a series of overlapped thin membranes (P-Film, 20/20 GeneSystems, Inc., Rockville, MD). Each membrane was probed with one of the 6 biomarkers [CACNA1D, Periostin, HER2/neu, EZH2, Ki67 and (−5,−7 proPSA)] followed by incubation with FITC-conjugated secondary antibodies. Total proteins collected on the blotted membranes were biotinylated, and followed by incubation with Streptavidin-linked Cy5. The fluorescence signals of biomarkers and total proteins were acquired using a Typhoon 9410 imager (GE Healthcare, Piscataway, NJ) and quantified with ImageQuant5.2 software (GE Healthcare). The quantified signals of biomarkers were divided by that of total protein for normalization. The log transformed ratio was used for downstream data analysis. Supplementary Table S1 provides the detailed information about the primary antibodies and dilutions used in the study. The quality of the antibodies was checked using Western blot of prostate cancer cell lines (data not shown). This MTI method helps to preserve valuable tissue and while multiplexing prognostic biomarkers for prostate cancer.
Statistical Analysis
GraphPad Prism 6 software (La Jolla, CA) was used to generate scatterplots of the biomarker expressions within the different Gleason score groups (3+3, 3+4, 4+3, and ≥8) as well as to generate the predictive probability plots. One-way ANOVA analysis followed by Dunnet's multiple comparisons test was used to evaluate the biomarker expressions in the four Gleason score groups. STATA 13.0 (STATA™, StataCorp LP, College Station, TX) was used for all statistical modeling. Statistical significance was defined as a p < 0.05. Statistical modeling included standard and/or backwards stepwise multivariate logistic regression (MLR) to discriminate less aggressive from more aggressive prostate cancer. Decision curve analysis (DCA) (28-30) was used to evaluate different MLR models for the prediction of more aggressive prostate cancer, patient that experienced BCR, and progressors. DCA is a method for evaluating and comparing different prediction models (28-30), which gives an expected net benefit per patient relative to the assumption that all patients are treated or not treated. For these evaluations, treatment was defined as the significant/interested changes in the patients (aggressiveness, BCR, progressors, etc.). The interpretation of net benefit is made by comparing a model curve to a baseline curve where all patients are considered as interested changes, and if the model curve is above the standard curve at a certain probability, then the model would be considered a better predictor of the outcome.
RESULTS
Differential expression of CACNA1D, HER2/neu and Periostin in prostate cancer with different Gleason scores
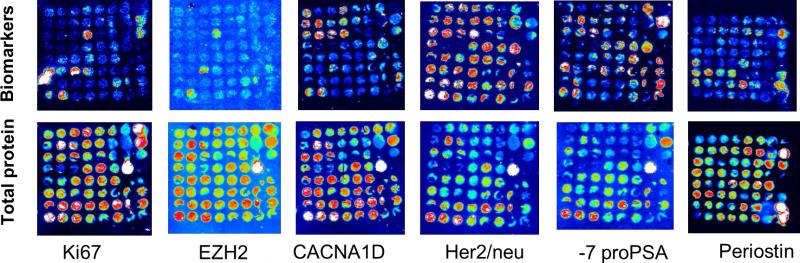
We optimized all MTI primary antibodies for 6 of our interested biomarkers on a test TMA (TMA 475) containing normal control and prostate cancer tissue samples. Our results showed that proteins on a single 5μm FFPE tissue slide could be successfully transferred onto a series of 6 membranes and probed with 6 biomarkers simultaneously (Fig. 1).
Fig. 1.
Test of antibodies on Multiplex Tissue Immunoblotting (MTI) using TMA 475.
Representative results of MTI using the 6 biomarkers. Signal intensity: white-red-yellow-green-blue-black from maximum to minimum.
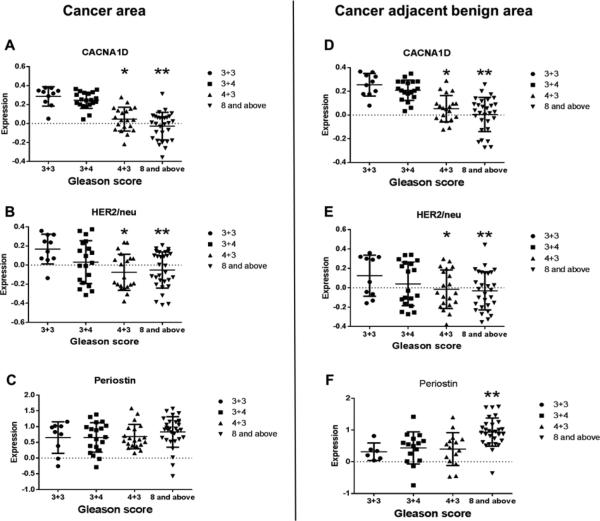
After optimization of the 6 biomarkers, we performed MTI with these 6 biomarkers on our study TMAs sections containing cancer and caner adjacent benign tissue from 80 different prostate cancer patients and determined the relative expression of the biomarkers. These sections include 4 groups with different Gleason scores (3+3, 3+4, 4+3, and ≥ 8). Among the 6 biomarkers, CACNA1D, HER2/neu and Periostin were differentially regulated among the four Gleason score groups in cancer area and cancer adjacent benign areas. In the cancer areas, CACNA1D and HER2/neu relative expression were significantly lower in the Gleason score groups of 4+3 and ≥ 8 compared with the Gleason score group of 3+3 (p < 0.05). There was a trend of gradually decreased relative expression of both markers with increased Gleason scores (Fig. 2A & 2B). Pearson's correlation analysis showed that CACNA1D had a strong negative correlation with Gleason score (correlation coefficient = −0.73, p < 0.001), while HER2/neu showed a moderate negative correlation (correlation coefficient = −0.39, p = 0.001). Interestingly, CACNA1D and HER2/neu expression behaved similarly in the cancer adjacent benign areas (Fig. 2A & 2B vs 2D & 2E), suggesting a possible field effect for these markers. Periostin expression was significantly higher in prostate cancer cases with Gleason score ≥ 8 compared with that of Gleason score 3+3 in cancer adjacent benign areas (p < 0.05, Fig. 2F). Although Periostin expression was not statistically significantly different in cancer areas, there was a trend for the expression to increase with increasing Gleason score.
Fig. 2.
Expression of biomarkers in cancer areas and cancer adjacent benign areas.
(A~C) prostate cancer areas. (D~F) Cancer-adjacent benign areas. Data are shown as mean ±SD. * and ** represents statistical significance (p < 0.05) between Gleason score groups of 4+3 and ≥8 vs. the Gleason score group of 3+3, respectively.
Biomarker expression in TMAs distinguishes less aggressive from more aggressive prostate cancer
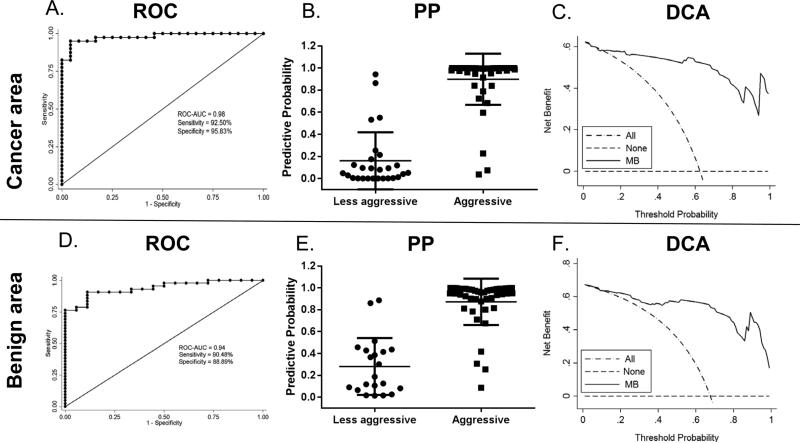
Although the intermediate risk group of clinically localized prostate has a Gleason score of 7, it includes both Gleason 3+4 and Gleason 4+3, patients with the two pathological statuses have significantly different treatment: most Gleason 3+4, if it is organ-confined, could be managed conservatively while all Gleason 4+3 necessitates immediate intervention with surgery and/or adjuvant therapy. Therefore, we grouped Gleason score 3+3 & 3+4 cases as a less aggressive phenotype and Gleason score 4+3 & ≥ 8 cases as a more aggressive phenotype. Using multivariate logistic regression (MLR), our results show that a model retaining CACNA1D, HER2/neu and Ki67 expression distinguishes less aggressive and more aggressive prostate cancer in cancer areas (Fig. 3A, Table S2 & S3). Using this model, the predictive probability was close to 100% for most cases in the aggressive groups (Gleason 4+3 & ≥ 8) (Fig. 3B). The odds ratios (ORs) of Ki67, CACNA1D, HER2/neu were 4.41e7, <0.01, and 0.02, respectively (Table S2). Interestingly, a MLR model that retained CACNA1D and Periostin expression was able to distinguish less aggressive from more aggressive prostate cancer in cancer adjacent benign areas (Fig. 3D, Table S2 & S3). The predictive probability was also close to 100% for most cases in the aggressive groups (Fig. 3E).The ORs for Periostin and CACNA1D were 1.14 and <0.01, respectively (Table S2). The DCA curves showed that prediction of aggressiveness using molecular biomarker (MB) model in both cancer areas (Fig. 3C) and cancer adjacent benign areas (Fig. 3F) had superior advantage over that of treating all patients as more aggressive.
Fig. 3.
Distinguish less aggressive from more aggressive prostate cancer with molecular biomarkers.
Aggressiveness prediction of multivariate logistic regression model in cancer areas (A, B) and cancer adjacent benign areas (D, E). Decision curve analysis of MB model in cancer areas (C) and cancer adjacent benign areas (F). ROC: receiver operating characteristics; PP: predictive probability; DCA: decision curve analysis.
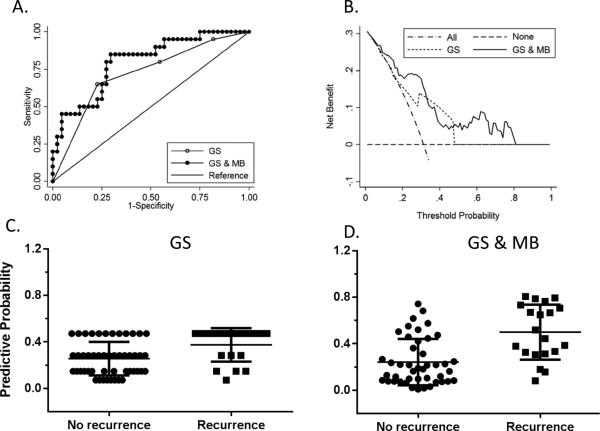
Biomarker expression in TMAs predicts BCR in prostate cancer
In the 80 TMA cases, standard logistic regression using Gleason score alone was able to predict BCR with a ROC-AUC of 0.71 while a step-wised MLR model that combines Gleason score with CACNA1D and HER2/neu yields a better ROC-AUC of 0.79 for the prediction of BCR (Fig. 4A, Table S2 & S3). However, there was no significant difference between these two models of BCR prediction (p = 0.15). The combination model could be used to predict recurrence with ORs of 6.34, 3.83, and 6.77 for Gleason score, CACNA1D and HER2/neu, respectively. DCA curve showed only a limited power for prediction of BCR with Gleason score alone within the probability range of 0.10~0.50 while combination of Gleason score with molecular biomarkers showed improved prediction capability within a wider probability range (0.10~0.80) (Fig. 4B). The predictive probability of BCR using Gleason score alone or combination of Gleason score with MBs is shown in Fig. 4C & 4D respectively.
Fig. 4.
Prediction of BCR using Gleason score alone and Gleason score combined with biomarkers.
Prediction BCR with Gleason score only (A) vs Gleason score and biomarkers (B). Decision curve analysis of BCR prediction using GS or GS & MB model in cancer areas (C & D). Predictive probability of BCR using GS or GS & MB model in cancer areas.
Biomarkers and tPSA separate progressors and non-progressors in the active surveillance cohort
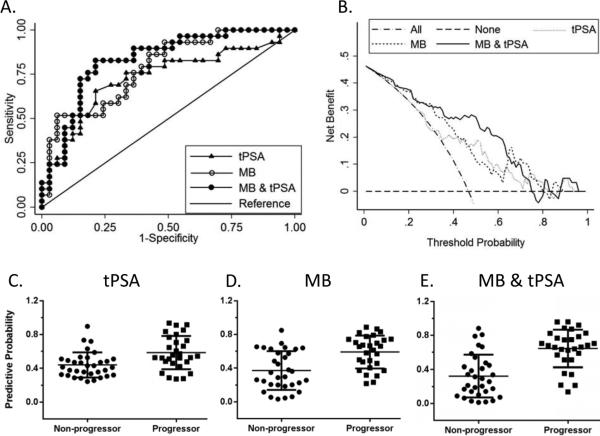
Using the biomarkers evaluated in the TMAs, we also evaluated the predictive ability of the biomarker molecular profiles in a total of 61 AS biopsy cases (32 non-progressors and 29 progressors). In the AS program, progressors are defined as men that met all the above VLR criteria at entry (tumor increased in volume and/or Gleason score > 6); however, during monitoring, they were found to have a more aggressive prostate cancer than entry criteria on biopsy follow-up (i.e. AS event; or clinically aggressive prostate cancer). A MLR model which retained (−5,−7) proPSA and CACNA1D was able to distinguish progressors from nonprogressors in the AS cohort (Fig.5A). This model had an accuracy of 72.13% in the prediction of progressors. The ORs for (−5,−7) proPSA and CACNA1D are 0.20 and 7.93, respectively. The predictive probability of this model is shown in Fig. 5D.
Fig. 5.
Prediction of active surveillance progressor status with tPSA, biomarkers alone, biomarkers & tPSA.
(A) ROC comparison of models using tPSA, molecular biomarkers alone and biomarkers combined with tPSA. (B) Decision curve analysis of the three models. Progressor predictive probability of tPSA only(C), biomarker only (D) and biomarkers & tPSA combined model (E).
We further tried to separate progressors and non-progressors in the AS cohort by using pre-biopsy tPSA alone or adding the tPSA result to the biomarker model. We found that tPSA alone has limited prediction capability with an ROC-AUC of 0.73, while combining molecular biomarkers and tPSA improved the ROC-AUC of the model to 0.83, but without significant difference compared to the biomarker only model (p = 0.26). The combination model (tPSA-MB) had a sensitivity of 79.31%, specificity of 78.13%, with the tPSA had an odds ratio of 1.24 in this model. The combined model could correctly classify the two groups with an accuracy of 78.69% (Fig. 5A, Table S4 & S5). A plot of the predictive probabilities is shown in Fig. 5C & 5E. DCA curve showed that the tPSA only and the molecular biomarker only model improved the net benefit within the probability range of 0.35~0.80 and 0~0.80 respectively, while the combined tPSA-MB model improved the net benefit within the probability range of 0~0.75. DCA curve comparing the three models to each other showed that the combined model was superior to the biomarker only model within the probability range of 0.30~0.65 (Fig. 5B). This suggests that a subset of the biomarkers evaluated have the potential to differentiate progressors from non-progressors patients that can remain annual monitoring in our preliminary data of 61 biopsy cases with high specificity. Adding tPSA improves the model, especially within the probability range of 0.30~0.65.
DISCUSSION
Development and validation of sensitive and more accurate methods for early detection is pivotal in the management of prostate cancer, the over-diagnosis and over-treatment of which is a significant healthcare problem (13). AS has become the preferred method for management of VLR and many LR patients. Currently, the new NCCN guidelines have included VLR and LR group of prostate cancer for AS (24). For VLR or LR cases, abnormal morphological changes are minimal and the future tumor progress could not be effectively predicted by pathological grading. Meanwhile molecular level changes do occur and may signal the potential disease progression. Characterization of prostate cancer necessitates a comprehensive panel of biomarkers considering the heterogeneity of prostate cancer biology (31). Therefore, we reason that a comprehensive panel of biomarkers involving the glandular acini and the stromal areas could be useful in the prediction of VLR and LR prostate cancer progression in an AS monitoring program.
To make full use of the valuable biopsy samples with limited size of cancer area, we adopted MTI as a method to profile the expression of 6 biomarkers on a single 5μm section in TMAs of 80 RP cases. Using a backwards stepwise MLR model, we found CACNA1D, HER2/neu and Periostin expression could significantly distinguish less aggressive from more aggressive prostate cancer with high sensitivity and specificity in the cancer area. Interestingly, in the cancer adjacent benign area, a different MLR model retains CACNA1D and Periostin could also distinguish less aggressive from more aggressive prostate cancer, implying the importance of these biomarkers in the prediction of aggressiveness in RP cases. Moreover, our data of cancer adjacent benign area support the concept of a possible field effect that involves changes of tumor progression at molecular level prior to changes in glandular morphology. Our model also showed an improved prediction of BCR compared with the prediction using Gleason score alone. Furthermore, in a total of 61 AS cases, we successfully separated progressors and non-progressors in the AS cohort with (−5,−7) proPSA and CACNA1D among these verified biomarkers, which are retained in a MLR model for molecular biomarkers to predict the AS cases that require definitive treatment.
It's worthwhile to note that our RP long term follow-up MLR biomarker model differs from the cancer area and the cancer adjacent benign area, suggests that biology of molecular alterations in progression and changes in the field may differ in the cancer areas. This is supported by a systemic genomic analysis of prostate cancer and morphologically normal tissue (31). Notably, the changes of biomarkers in cancer adjacent benign area show a slightly different pattern compared with the cancer area, with changes of stromal protein Periostin expression being more significant. Further characterization of the molecular features of prostate cancer with VLR or LR biomarker expression patterns in these two histologic areas could provide an additional means to stratify the AS and RP cases to more accurately assess the progression of prostate cancer and effectively personalize the management of treatment.
Interestingly, CACNA1D stands out as a new useful marker in prostate cancer area, cancer adjacent benign area, and also biopsies in the prediction of cancer progression (upgrading). CACNA1D is an ERG target gene upregulated in TMPRSS2-ERG positive prostate cancer cells (32). Overexpression of CACNA1D in prostate cancer cells was reported to regulate the ligand independent activation of AR. ERG induced expression of CACNA1D was reported to promote entry of calcium ions into cytosol (33). Here we found that the expression of CACNA1D showed a trend of decrease with increasing Gleason score in RP specimens. However, the progressor AS biopsies have relatively higher expression of CACNA1D as an early event which consists with results in our RP model for 3+3 and 3+4 Gleason scores. It has been reported that blocking L-type calcium channel-mediated Ca2+ influx suppressed castration-induced apoptotic cell death in prostate epithelial cells (34, 35). We presume that the reduced expression of CACNA1D in more aggressive prostate cancer and progressors may contribute to decreased Ca2+ influx and results in the escape of apoptosis, thus suggesting that decreased CACNA1D expression could be a more aggressive phenotype in higher grade prostate cancer or progressors in AS.
The role of HER2 in prostate cancer remains controversial (36, 37). HER2/neu can often be overexpressed in prostate cancer (38, 39), but may depend on the antibody reagents used and 2 differentially expressed forms of HER2/neu transcripts were reported (40), which may needs to be considered during interpretation of results. Antibodies detecting variable epitopes of HER2/neu may be one reason for the controversy. In the current study, we showed that in cancer area, along with CACNA1D and Gleason score, HER2/neu could be used to efficiently predict aggressiveness and recurrence of prostate cancer in RP or biopsy specimens.
Moreover, prostate cancer progression involves both epithelial changes and stromal changes. Periostin was identified as an important biomarker well correlating with the aggressive phenotype (41-43). Both stromal and epithelial Periostin expression were reported to be significantly increased in tumor tissues: stromal expression was significantly higher than epithelial expression as compared to normal tissue. While high stromal expression was significantly associated with shorter survival, a low epithelial score significantly correlated with shorter PSA-free survival, suggesting that Periostin apparently plays an opposing biological role depending on its tissue localization (44). Here we included Periostin in our biomarker panel quantification in both epithelia and stroma since our MTI method could not separate the expression of these two areas. Our results suggest that along with CACNA1D, Periostin could predict the malignancy of prostate cancer based on the cancer adjacent area (Fig. 3B).
Our MLR modeling system showed its power in the prediction of prostate cancer progression in biopsies, and RP cases with high sensitivity and specificity. Our early data was validated on 80 RP cases of our TMAs and 61 AS biopsies at diagnosis (i.e. entry to AS). In the latter case, the AS study was compromised by the fact that the total cancer surface area in the AS biopsies is often limited. Hence, due to this minimal cancer area in VLR prostate cancer, the expansion of the AS biopsy sample size is needed for obtaining additional results for the verification of the statistical stability of our models. Alternatively, the AS program can be altered to expand criteria for entry into the program, which is already being done at JHMI and other institutions (21, 45). Additionally, MTI shows its advantage in multiplexing up to 6 biomarkers using the same 5μm tissue section, it does have technical disadvantages: it relies on the relatively high expression of protein and the use of good quality antibodies. The detection of lowly expressed protein levels is limited, such as Ki67 in AS biopsies. Finally, even if super-thin P-film was used in MTI, the protein transferred onto the membranes show a gradual decrease from the one at the bottom closest to the tissue to the one on top. We normalized the expression of each biomarker to the total protein to balance these differences in total protein content on the same membrane.
Notably, we have developed a panel of 6 biomarkers profiled on the same 5μm biopsy tissue slide using MTI, an integrated, high-throughput quantitative molecular biomarker-based method for predicting prostate cancer progression. Our model involving these biomarkers show robust strength in the prediction of aggressiveness in the RP cases and more importantly, this method and biomarkers could be used to predict the progress of VLR and LR prostate cancer at diagnosis, which will reduce the over-diagnosis and over-treatment and greatly facilitate the effective management of prostate cancer patients. Future studies will significantly expand the number of biopsy cases studied and also include nuclear morphometry of Feulgen (DNA) stained or H&E stained biopsies of AS patients as a variable to more accurately predict the prostate cancer aggressive phenotype of progressors. Given the new problems of managing AS cohorts, new criteria for selection and new technology for multiplexing tissue biomarkers are needed to support the advancement of this concept to improve healthcare in the future for prostate cancer.
Supplementary Material
ACKNOWLEDGEMENT
We appreciate the help of Department of Pathology in Johns Hopkins Medical Institutions and the PCBN for the construction of TMA; The P-Films used in MTI were kindly provided by Vladimir Knezevic in 20/20 GeneSystems, Inc. We appreciate the help of Nan Hu and Chaoyu Wang in Dr. Philip Taylor's lab at NCI for assisting in the conduction of experiments.
Financial support:
This project is supported by two grants: EDRN/NCI U01CA152813 grant (Hui Zhang P.I.) with an administrative supplement to Dr. Robert W. Veltri, Prostate Cancer Foundation and Patana Fund of The Brady Urological Institute at Johns Hopkins University.
Footnotes
Conflict of Interest:
A patent entitled “Biomarkers for assessing cancer patients for treatment” including biomarkers used here was filed in the United States on February 9, 2015 and currently under processing. The filing number is: PCT/US2015/015074.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Loeb S, Schaeffer EM, Trock BJ, Epstein JI, Humphreys EB, Walsh PC. What are the outcomes of radical prostatectomy for high-risk prostate cancer? Urology. 2010;76:710–4. doi: 10.1016/j.urology.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Myers RP, Blute ML. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004;172:1328–32. doi: 10.1097/01.ju.0000138681.64035.dc. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr., Dotan ZA, DiBlasio CJ, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7005–12. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA : the journal of the American Medical Association. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 6.Shariat SF, Kattan MW, Vickers AJ, Karakiewicz PI, Scardino PT. Critical review of prostate cancer predictive tools. Future oncology. 2009;5:1555–84. doi: 10.2217/fon.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson GP, Yu C, Kattan MW, Hermans MR. Validation of postoperative nomograms in prostate cancer patients with long-term follow-up. Urology. 2011;78:105–9. doi: 10.1016/j.urology.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039–46. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schroder FH, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute. 2003;95:868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 10.Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. Journal of the National Cancer Institute. 2002;94:981–90. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 11.Walsh PC. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Urol. 2003;170:313–4. [PubMed] [Google Scholar]
- 12.Tornblom M, Eriksson H, Franzen S, Gustafsson O, Lilja H, Norming U, et al. Lead time associated with screening for prostate cancer. Int J Cancer. 2004;108:122–9. doi: 10.1002/ijc.11554. [DOI] [PubMed] [Google Scholar]
- 13.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. The New England journal of medicine. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:2141–9. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA : the journal of the American Medical Association. 1994;271:368–74. [PubMed] [Google Scholar]
- 16.Bastian PJ, Mangold LA, Epstein JI, Partin AW. Characteristics of insignificant clinical T1c prostate tumors. A contemporary analysis. Cancer. 2004;101:2001–5. doi: 10.1002/cncr.20586. [DOI] [PubMed] [Google Scholar]
- 17.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 18.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA : the journal of the American Medical Association. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 19.Walsh PC. Radical prostatectomy versus watchful waiting in early prostate cancer. J Urol. 2005;174:1291–2. [PubMed] [Google Scholar]
- 20.Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. The New England journal of medicine. 2005;352:1977–84. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 21.Klotz L. Active surveillance for prostate cancer: a review. Current urology reports. 2010;11:165–71. doi: 10.1007/s11934-010-0110-z. [DOI] [PubMed] [Google Scholar]
- 22.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 29:2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 23.Veltri R, Isharwal S. Reply to Indolent Prostate Cancer. Urology. 2011;77:e2. [Google Scholar]
- 24.Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 25.Chung JY, Braunschweig T, Baibakov G, Galperin M, Ramesh A, Skacel M, et al. Transfer and multiplex immunoblotting of a paraffin embedded tissue. Proteomics. 2006;6:767–74. doi: 10.1002/pmic.200401343. [DOI] [PubMed] [Google Scholar]
- 26.Chung JY, Hong SM, Choi BY, Cho H, Yu E, Hewitt SM. The Expression of Phospho-AKT, Phospho mTOR, and PTEN in Extrahepatic Cholangiocarcinoma. Clinical Cancer Research. 2009;15:660–7. doi: 10.1158/1078-0432.CCR-08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung JY, Braunschweig T, Hu N, Roth M, Traicoff JL, Wang QH, et al. A multiplex tissue immunoblotting assay for proteomic profiling: a pilot study of the normal to tumor transition of esophageal squamous cell carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1403–8. doi: 10.1158/1055-9965.EPI-05-0651. [DOI] [PubMed] [Google Scholar]
- 28.Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC medical informatics and decision making. 2008;8:53. doi: 10.1186/1472-6947-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Medical decision making : an international journal of the Society for Medical Decision Making. 2006;26:565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steyerberg EW, Vickers AJ. Decision curve analysis: a discussion. Medical decision making : an international journal of the Society for Medical Decision Making. 2008;28:146–9. doi: 10.1177/0272989X07312725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper CS, Eeles R, Wedge DC, Van Loo P, Gundem G, Alexandrov LB, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nature genetics. 2015 doi: 10.1038/ng.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulo P, Ribeiro FR, Santos J, Mesquita D, Almeida M, Barros-Silva JD, et al. Molecular subtyping of primary prostate cancer reveals specific and shared target genes of different ETS rearrangements. Neoplasia. 2012;14:600–11. doi: 10.1593/neo.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R, Zeng X, Zhang R, Huang J, Kuang X, Yang J, et al. Ca1.3 channel alpha protein is overexpressed and modulates androgen receptor transactivation in prostate cancers. Urologic oncology. 2013 doi: 10.1016/j.urolonc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Martikainen P, Isaacs J. Role of calcium in the programmed death of rat prostatic glandular cells. Prostate. 1990;17:175–87. doi: 10.1002/pros.2990170302. [DOI] [PubMed] [Google Scholar]
- 35.Connor J, Sawczuk IS, Benson MC, Tomashefsky P, O'Toole KM, Olsson CA, et al. Calcium channel antagonists delay regression of androgen-dependent tissues and suppress gene activity associated with cell death. Prostate. 1988;13:119–30. doi: 10.1002/pros.2990130204. [DOI] [PubMed] [Google Scholar]
- 36.Carles J, Lloreta J, Salido M, Font A, Suarez M, Baena V, et al. Her-2/neu expression in prostate cancer: a dynamic process? Clin Cancer Res. 2004;10:4742–5. doi: 10.1158/1078-0432.CCR-04-0115. [DOI] [PubMed] [Google Scholar]
- 37.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Critical reviews in oncology/hematology. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 38.Neto AS, Tobias-Machado M, Wroclawski ML, Fonseca FL, Teixeira GK, Amarante RD, et al. Her- 2/neu expression in prostate adenocarcinoma: a systematic review and meta-analysis. J Urol. 2010;184:842–50. doi: 10.1016/j.juro.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 39.Isharwal S, Miller MC, Epstein JI, Mangold LA, Humphreys E, Partin AW, et al. Prognostic value of Her-2/neu and DNA index for progression, metastasis and prostate cancer-specific death in men with long-term follow-up after radical prostatectomy. Int J Cancer. 2008;123:2636–43. doi: 10.1002/ijc.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gang A. Differential expression of full-length and a truncated Her-2/neu oncogene receptor in prostate cancer assessed using relative quantitative RT-PCR. Molecular Urology. 1998;2:305–10. [Google Scholar]
- 41.Chen J, Xi J, Tian Y, Bova GS, Zhang H. Identification, prioritization, and evaluation of glycoproteins for aggressive prostate cancer using quantitative glycoproteomics and antibody- based assays on tissue specimens. Proteomics. 2013;13:2268–77. doi: 10.1002/pmic.201200541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian Y, Bova GS, Zhang H. Quantitative glycoproteomic analysis of optimal cutting temperature- embedded frozen tissues identifying glycoproteins associated with aggressive prostate cancer. Analytical chemistry. 2011;83:7013–9. doi: 10.1021/ac200815q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian Y, Choi CH, Li QK, Rahmatpanah FB, Chen X, Kim SR, et al. Overexpression of periostin in stroma positively associated with aggressive prostate cancer. PLoS One. 2015;10:e0121502. doi: 10.1371/journal.pone.0121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuzzo PV, Rubagotti A, Zinoli L, Ricci F, Salvi S, Boccardo S, et al. Prognostic value of stromal and epithelial periostin expression in human prostate cancer: correlation with clinical pathological features and the risk of biochemical relapse or death. BMC cancer. 2012;12:625. doi: 10.1186/1471-2407-12-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suardi N, Briganti A, Gallina A, Salonia A, Karakiewicz PI, Capitanio U, et al. Testing the most stringent criteria for selection of candidates for active surveillance in patients with low-risk prostate cancer. BJU international. 2010;105:1548–52. doi: 10.1111/j.1464-410X.2009.09057.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.