Abstract
Hyperpolarization-activated cyclic nucleotide gated (HCN) channels function in the brain to limit neuronal excitability. Limiting the activity of these channels has been proposed as a therapy for Major Depressive Disorder, but the critical role of HCN channels in cardiac pacemaking has limited efforts to develop therapies directed at the channel. Previous studies indicated that the function of HCN is tightly regulated by its auxiliary subunit, TRIP8b (tetratricopeptide repeat-containing Rab8b interacting protein), which is not expressed in the heart. To target the function of the HCN channel in the brain without affecting channels’ function in the heart, we propose disrupting the interaction between HCN and TRIP8b. We developed a high throughput fluorescence polarization (FP) assay to identify small molecules capable of disrupting this interaction. We used this FP assay to screen a 20,000-compound library and identified a number of active compounds. The active compounds were validated using an orthogonal AlphaScreen assay to identify one compound (0.005%) as the first confirmed hit for inhibiting the HCN-TRIP8b interaction. Identifying small molecules capable of disrupting the interaction between HCN and TRIP8b should enable the development of new research tools and small molecule therapies that could benefit patients with depression.
Keywords: High throughput screening, protein-protein interactions, fluorescence methods, HCN, TRIP8b
Introduction
Major Depressive Disorder (MDD) is a highly prevalent public health problem, affecting 1 in 5 people worldwide. Despite the enormous burden of MDD, antidepressant treatment options – primarily drugs targeting monoaminergic neurotransmitters – have not changed over the past half century1. Thus, development of new therapies targeting mechanisms other than monoaminergic pathways could address a major unmet clinical need.
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are encoded by the HCN1–4 gene family and mediate a hyperpolarization-activated nonspecific cationic current2. HCN channels play an important role in governing the excitability of neurons in the brain and in regulating the rhythm of the heart2–4. Importantly, the expression of HCN channels in the brain is tightly regulated by an auxiliary subunit, tetratricopeptide repeat-containing Rab8b interacting protein (TRIP8b)5–9. Recent studies using knockout mice lacking HCN channel subunits and shRNA knockdown of HCN1 have linked reduced HCN channel function to antidepressant-like behavior9,10. Although these data suggest inhibiting HCN channels holds promise as a novel treatment for MDD11, HCN channels are also expressed at high levels in the heart and are critical for cardiac function12. As such, the challenge for anti-depressant therapies based on inhibition of HCN channel function is to identify a pharmacological approach that selectively targets HCN channels expressed in the brain while sparing HCN channels expressed in the heart.
TRIP8b is a brain-specific HCN channel auxiliary subunit that controls channel function and subcellular trafficking9. Genetic deletion of TRIP8b disrupts normal HCN channel trafficking within neurons and markedly reduces HCN channel function9. Consistent with an important role for HCN channel function in MDD, our lab previously noted that genetic deletion of TRIP8b also leads to antidepressant-like behavior9. As such, we reasoned that pharmacologically blocking the interaction between TRIP8b and HCN could disrupt HCN channel trafficking and function in the brain without affecting the function of HCN channels in the heart. Important from a drug-development standpoint, the brain specific expression of TRIP8b7 would preclude off target effects of this pharmacological approach on HCN channels expressed in the heart. Consistent with this, we have observed that TRIP8b knockout mice have normal cardiac function (unpublished data) indicating that cardiac HCN channels remain unaffected.
TRIP8b is expressed as several distinct splice isoforms which differ only at the N-terminus8,9. All TRIP8b splice isoforms share a conserved C-terminus that interacts with the intracellular C-terminus of pore-forming HCN channel subunits (HCN1-4) at two distinct sites (Figure 1)13,14. The first site is an interaction between the cyclic nucleotide binding domain (CNBD) of HCN and an acidic amino acid sequence located N-terminal to the tetratricopeptide (TPR) domains of TRIP8b7,13. The second interaction occurs between the C-terminal tripeptide of HCN (-SNL in HCN1, 2 and 4; -ANM in HCN3) and the TPR domains of TRIP8b15.
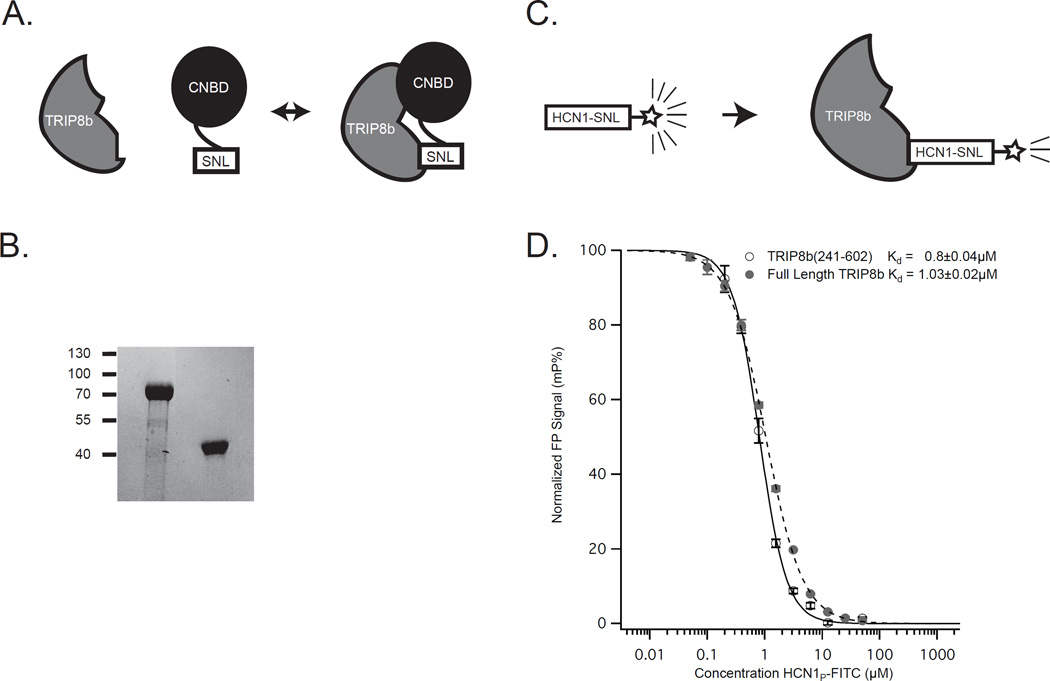
Figure 1. The interaction of TRIP8b and HCN.
A. Schematic showing the interaction between TRIP8b and HCN. Although two interaction sites exist, the downstream interaction between the TPR domains of TRIP8b and the SNL tripeptide of HCN channels is crucial for surface trafficking and function of the channel. B. Coomassie stain showing the full length TRIP8b at left and the shorter TRIP8b(241-602) on the right. Molecular weight markers are indicated on the left. C. Schematic showing the fluorescence polarization assay displayed in (D). D. The fluorescence polarization signal for each concentration of HCN1 peptide is shown. By titrating in increasing quantities of HCN1 peptide labeled with a FITC tag, we observed similar affinities of the two TRIP8b peptides for HCN1.
Our lab has previously shown that disrupting either interaction site impairs the ability of TRIP8b to interact with HCN channels and limits HCN channel function13. Furthermore, others recently demonstrated that disruption of just the interaction between the TRIP8b TPR domains and the HCN tripeptide is sufficient to impair HCN trafficking in vivo5. Given that blocking the TPR-tripeptide interaction is sufficient to inhibit HCN channel function and that the CNBD of HCN has significant homology with many other mammalian proteins16, we reasoned that targeting the TPR-tripeptide interaction would be optimal for inhibiting HCN channel function while minimizing the risk of developing molecules with off-target activity against other proteins containing CNBDs.
In this study, we examined the terminal tripeptide interaction of TRIP8b with each HCN isoform using fluorescence polarization (FP). We also developed an FP-based high throughput screening assay to identify small molecules capable of disrupting the TRIP8b-HCN1 interaction, and we employed the assay to screen a library of 20,000 small molecules. The screen identified one compound that was validated using an orthogonal AlphaScreen assay. To our knowledge, this compound represents the first described inhibitor of the TRIP8b-HCN1 interaction.
Materials and Methods
TRIP8b constructs
All restriction enzymes were purchased from New England Biolabs. All oligonucleotides for use in PCR amplification were synthesized by Integrated DNA Technologies (Coralville, IA).
A TRIP8b protein with a C terminal His tag was generated by PCR using forward primer 5’-ata gcg cca tgg aat tcg aaa ggg caa aag cgg cag, and reverse primer 5’-ctt tca att tgg atc ctt gac ccg ggc tcg agg cgg cg followed by subcloning into a modified pGS21-a vector (Genscript, Piscataway, NJ) with the deletion of GST tag at NcoI/XhoI sites. A protein expression vector containing His tagged full length TRIP8b (1b-2) isoform was generated by PCR using forward primer 5’-ata gcg cca tgg ctg aca gtg aaa tgg atg gaa g, and reverse primer 5’-ctt tca att tgg atc ctt gac ccg ggc tcg agg cgg cg followed by subcloning into the modified pGS21-a vector (Genscript, Piscataway, NJ). GST-HCN1C40 containing the last 40 C terminal amino acids was generated by PCR using forward primer 5’-gtg cga att cat ccc ccc caa ccg agg and reverse primer 5’-gtg cct cga gtc ata aat tcg aag caa aac gg followed by subcloing into pGEX-4T-1 (GE healthcare) using EcoRI/XhoI sites.
Purification of His-TRIP8b and His-HCN protein
To produce recombinant proteins, BL21 bacterial cultures (Agilent technologies, Santa Clara, CA) transformed with appropriate vectors were grown in LB broth (Invitrogen, Carlsbad, CA, USA) containing 50 mg/ml kanamycin with vigorous agitation at 37 °C. Isopropyl beta-D-1-thiogalactopyranoside (IPTG) was added to the medium at a final concentration of 1 mM after the OD 600 reached 1–1.2 followed by overnight induction at 18 °C. The bacterial cells were harvested by centrifugation at 6000 g for 15 min at 4 °C and then washed with cold phosphate buffered saline (PBS, 140mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3). Bacteria were re-suspended in buffer A (50mM NaPhosphate, 300mM NaCl, 10% Glycerol, pH 8.0). The suspended cells were disrupted by sonication on ice and centrifuged at 12,000 g for 20 min. The supernatant was applied to the affinity matrix Ni-NTA agarose column with a bed volume of 2ml, which had previously been washed and equilibrated with buffer A. The column was washed with 500 ml buffer A and 300 ml buffer B (50mM NaPhosphate, 300mM NaCl, 10% Glycerol, pH 6.0) as well as 150 ml buffer A containing 5 mM imidazole. Protein was eluted with buffer A containing 200 mM imidazole and dialyzed overnight at 4 °C with PBS.
Purification of GST-HCN1C40 protein
To produce recombinant GST–HCN1C40 protein, BL21 bacterial cultures transformed with pGEX–HCN1C40 were grown in LB broth (Invitrogen, Carlsbad, CA, USA) containing 50 mg/ml ampicillin with vigorous agitation at 37 °C. IPTG was added to the medium at a final concentration of 1 mM after the OD 600 reached 0.8 followed by an additional 1h induction. The bacterial cells were harvested by centrifugation at 6000 g for 15 min at 4 1C and then washed with cold 1X PBS (140mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) and re-suspended in lysis buffer (PBS containing 1% Triton X-100). The suspended cells were disrupted by sonication on ice and the cell solution was centrifuged at 12, 000 g for 20 min. The supernatant was applied to the affinity matrix glutathione sepharose 4B column (GE healthcare) with a bed volume of 2ml, which had previously been washed and equilibrated with PBS. The column was washed three times with 20 ml 1X PBS containing 500 mM NaCl and then the fusion protein was eluted with elution buffer (50 mM Tris-HCl, pH 8.0, containing 2 mM reduced glutathione). The eluted protein was then dialyzed overnight at 4 °C with PBS.
Fluorescence polarization
Fluorescein isothiocyanate (FITC) labeled HCN peptides (HCN1: DAEKPRFASNL; HCN2: DSARSRLSSNL; HCN3: TPRGPQISANM; HCN4: EPVRSKLPSNL) were purchased from GenScript.
For the Kd measurements, a series of two-fold dilutions of TRIP8b protein and 100 nM of final concentration of FITC labeled peptide were mixed in protein dilution buffer (50mM NaH2PO4, 50mM KCl, 1mM DTT, pH 6.5). Before reading, the plate was allowed to incubate for 15 min at room temperature in the dark. All experiments were performed in triplicate in black 384-well microtiter plates (Corning). The polarization measurements were obtained using Tecan microplate reader (Tecan) at the Structural Biology facility at Northwestern University, which is generously supported by NCI CCSG P30 CA060553 of the Robert H. Lurie Comprehensive Cancer Center. All curve fitting and data analysis were performed using Igor Pro (WaveMetrics). As described below, curves were fit by the Hill equation17 based on a previously described protocol18, with Y = (Xn) / (Kd + Xn) where Y is the fraction of binding sites occupied, X is the concentration of ligand, Kd is the dissociation constant, and n is the Hill coefficient.
To calculate the affinity of full length TRIP8b and TRIP8b(241-602) for FITC-HCN1, a serial dilution of FITC-HCN1 was added to a fixed concentration of 2 µM TRIP8b protein.
For the IC50 measurement, a serial dilution of unlabeled peptide was titrated into protein dilution buffer containing the fixed concentration of 100 nM FITC-HCN peptide and 2 µM of TRIP8b(241-602).
Z factor19 was calculated as Z = 1 − 3×( σpos + σneg) / (μneg − μpos).
High throughput screening
The mixture of purified TRIP8b(241-602) protein (2 µM) and FITC-HCN1 (50 nM) were dispensed into 384 well plates using Viafill microplate dispenser (Integra Biosciences). Two compounds were pooled into each well at 40 µM per compound using a Labcyte Echo550 acoustic liquid handler. Each plate contained 16 negative controls with DMSO only (no test compound), and 16 positive controls with FITC-HCN1 probe alone (no TRIP8b). Fluorescence polarization (mP) was measured using an AnalystGT (Molecular Devices) after a 2 hour incubation at room temperature. Values were normalized to percent inhibition using the average positive and negative controls from each plate and plotted using TIBCO Spotfire. For dose response testing, compounds were serially diluted in DMSO and dispensed using the Labcyte Echo550 into a mixture of TRIP8b (2 µM) and carboxytetramethylrhodamine (TAMRA)-conjugated HCN1 probe (50 nM) at a final DMSO concentration of 0.4%. Samples were incubated for 3 hours at room temperature before reading on the AnalystGT. IC50 values were calculated in Graphpad Prism using a four-parameter nonlinear regression model.
AlphaScreen
A mixture of GST-tagged HCN1c40 protein (20 nM) and His-tagged TRIP8b (residues 241-602, as in the FP assay, 200 nM) was incubated for 3 hours in the presence of different concentrations of a test compound (0.2–40 µM) at a final DMSO concentration of 0.25%. Anti-GST AlphaScreen Acceptor beads were then added for 1.5 hours, followed by addition of AlphaScreen Nickel chelate Donor beads for 1.5 hours. All incubations were at room temperature. A PerkinElmer EnSpire multimode plate reader was used to detect the AlphaScreen signal.
Results
Interaction of TRIP8b and HCN by fluorescence polarization
Fluorescence polarization (FP) is a sensitive, robust, and quantitative method for the study of protein interactions and for high throughput screening in drug discovery20. The technique involves exciting a ligand-tagged fluorophore with polarized light and measuring the polarity of emitted fluorescence. In the presence of a binding partner, the rotational motion of the ligand is decreased and the emitted light is polarized to a greater degree than in the absence of the binding partner.
Because we are interested in identifying inhibitors of the interaction between the TPR domains of TRIP8b and the C terminal tripeptide of HCN, we first sought to generate stable and soluble TRIP8b protein capable of mediating this interaction. We cloned and purified both full length TRIP8b and TRIP8b(241-602) (see Methods above), a fragment lacking the structurally disorganized N-terminus of the molecule that is not involved in binding HCN1-413. Additionally, we synthesized a peptide consisting of the C-terminal 11 amino acids of HCN1 conjugated to FITC. We then titrated increasing concentrations of the FITC labeled HCN1 peptide into a fixed concentration of each of the two TRIP8b proteins. As can be seen in Figure 1, the Kd was similar for the two proteins (Full length TRIP8b Kd=1.03±0.02µM, TRIP8b(241-602) Kd=0.8±0.04µM) suggesting that the TRIP8b C-terminal fragment is capable of reproducing the interaction between the TPR domain and the tripeptide tail of HCN, which is consistent with recent findings21. Because the smaller TRIP8b(241-602) peptide was more easily purified and was more stable under the conditions of our assays, we proceeded using this version.
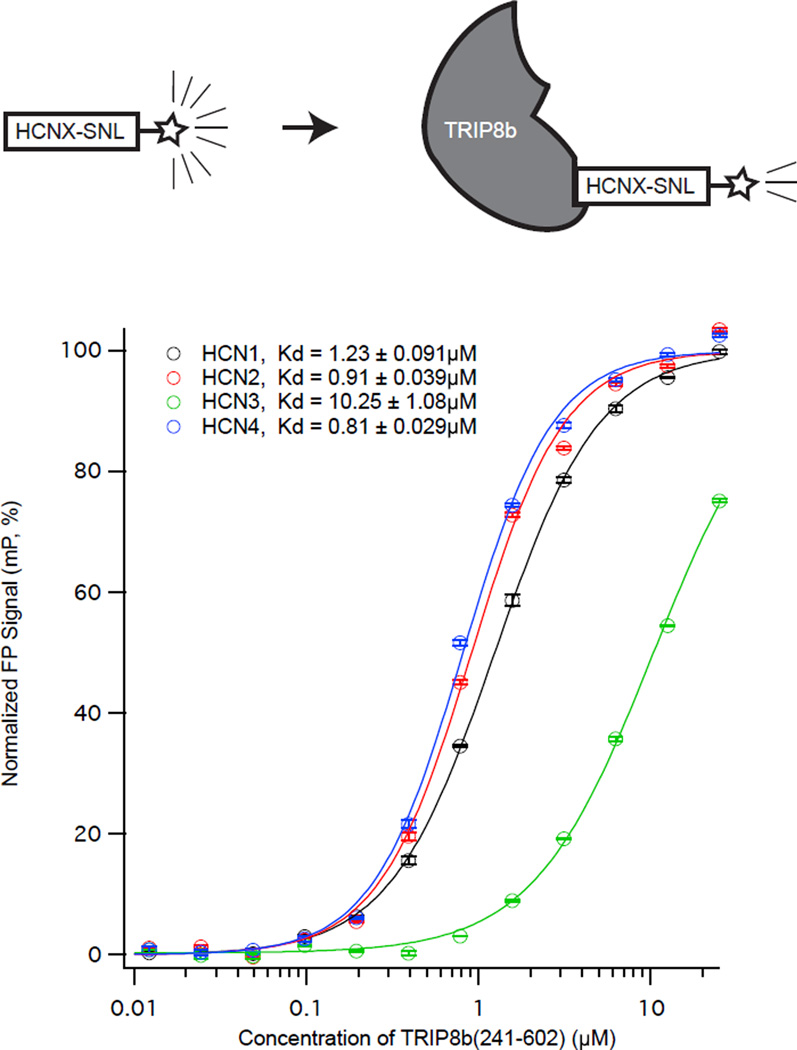
We next sought to examine the differences in interactions between the TRIP8b TPR domain and the C-termini of the distinct HCN subunits. Towards this end, we synthesized peptides consisting of the 11 C-terminal amino acids from each HCN subunit conjugated to fluorescein (see Methods, referred to with a subscripted ‘P’ for ‘Peptide’ as in HCN1P). In Figure 2, we titrated increasing concentrations of TRIP8b(241-602) in the presence of a fixed quantity of the labeled peptides. We noted that HCN1P, HCN2P, and HCN4P all had similar affinities near 1µM (HCN1P: Kd=1.23±0.09µM, HCN2P: Kd=0.91±0.03µM, HCN4P: Kd=0.81±0.02µM). In contrast, the affinity of TRIP8b(241-602) for HCN3P was lower (HCN3P: Kd=10.25±1.08µM). Given that HCN3 is expressed at low levels throughout the brain22 and has a lower affinity for TRIP8b6, we focused our subsequent efforts on HCN1, HCN2, and HCN4.
Figure 2. The interaction of TRIP8b and HCN 1, 2, 3, and 4 by FP assay.
TRIP8b(241-602) binds to HCN3 with less affinity than HCN1,2, and 4. We titrated increasing concentrations of TRIP8b(241-602) into a fixed concentration of the indicated FITC labeled HCN isoform (see the schematic above).
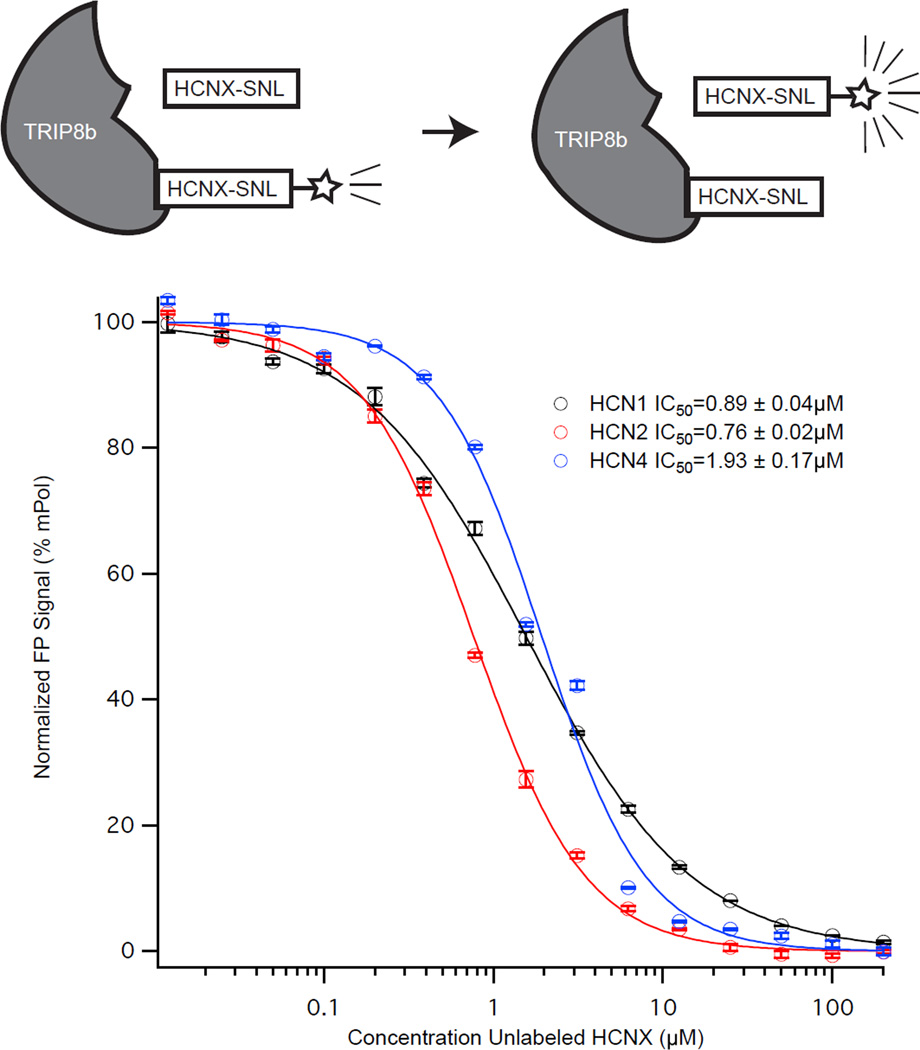
We next investigated if the interaction between the labeled HCN tripeptide and TRIP8b(241-602) could be disrupted. In addition to our fluorescein labeled peptides, we synthesized unlabeled peptides of HCN1, HCN2, and HCN4 (referred to as HCN1U for unlabeled). In Figure 3, we titrated increasing concentrations of unlabeled peptide into a fixed concentration of TRIP8b(241-602) and the corresponding labeled peptide (i.e. HCN1U against HCN1P). For all three peptides, we found roughly similar IC50 values for disrupting the interaction of TRIP8b and the corresponding HCNp (HCN1P: IC50=0.89±0.04µM, HCN2P: IC50=0.76±0.02µM, HCN4P: IC50=1.93±0.17µM). These results indicate that the interaction between the SNL tripeptide and TRIP8b can be disrupted for HCN1, 2, and 4.
Figure 3. Unlabeled HCN peptides displace labeled HCN peptides from TRIP8b(241-602).
In order to examine differences in the ability of the distinct HCN peptides to be displaced from TRIP8b(241-601), we titrated increasing concentrations of each unlabeled HCN peptide into a fixed concentration of labeled HCN peptide and TRIP8b(241-602). For example, unlabeled HCN2 peptide was titrated against FITC labeled HCN2 peptide, as in the schematic above.
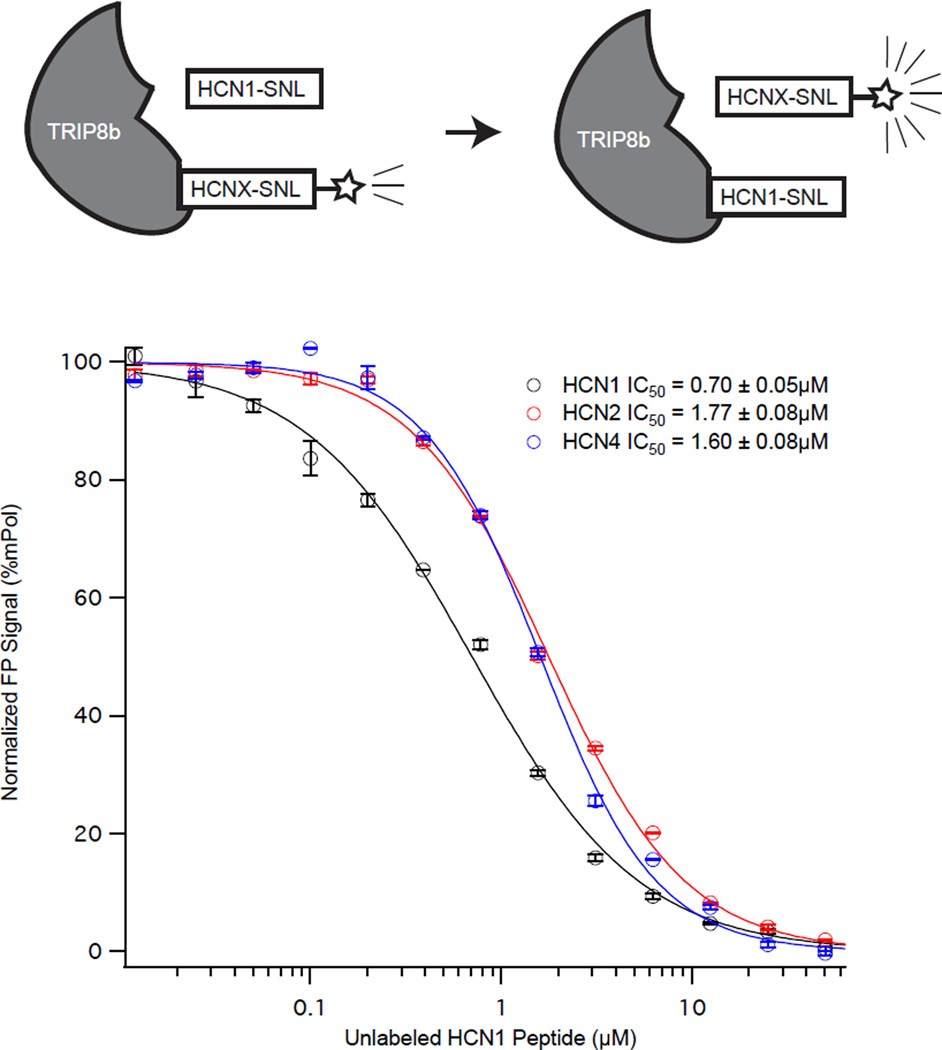
In order to identify small molecules capable of disrupting the interaction between TRIP8b and HCN, we developed an assay that could be used for high throughput compound screening. Our strategy was to use the fluorescence polarization assay with labeled HCN1 peptide and TRIP8b(241-602). To examine the utility of this approach, we first examined HCN1U as a positive control to displace HCN1P, HCN2P, and HCN4P. In Figure 4, we titrated increasing concentrations of HCN1U into fixed concentrations of TRIP8b(241-602) with the labeled HCN peptide indicated. As can be seen in Figure 4, HCN1U displaced each labeled HCN peptide with similar affinities (HCN1P: IC50=0.70±0.05µM, HCN2P: IC50=1.77±0.02µM, HCN4P: IC50=1.60±0.08µM).
Figure 4. Unlabeled HCN1 peptide displaces labeled HCN peptides from TRIP8b(241-602).
HCN1 binds TRIP8b(241-601) with less affinity than either HCN2 or HCN4. To examine differences in the binding affinity of the different HCN peptides to TRIP8b(241-602) we titrated increasing concentrations of unlabeled HCN1 peptide into a fixed concentration of FITC labeled HCN peptide and TRIP8b(241-602) (as in the schematic).
We subsequently tested the assay in 384-well format either in the presence or absence of HCN1U (to serve as a positive control for inhibition), and calculated a Z factor of 0.896 (mean ± standard deviation: no competitor 224.25 ±3.07, with competitor 44.61 ± 3.15 mP, n=384). Together, these data indicate that the assay is quantitative, robust, and well suited for primary high throughput screening (Figure 5).
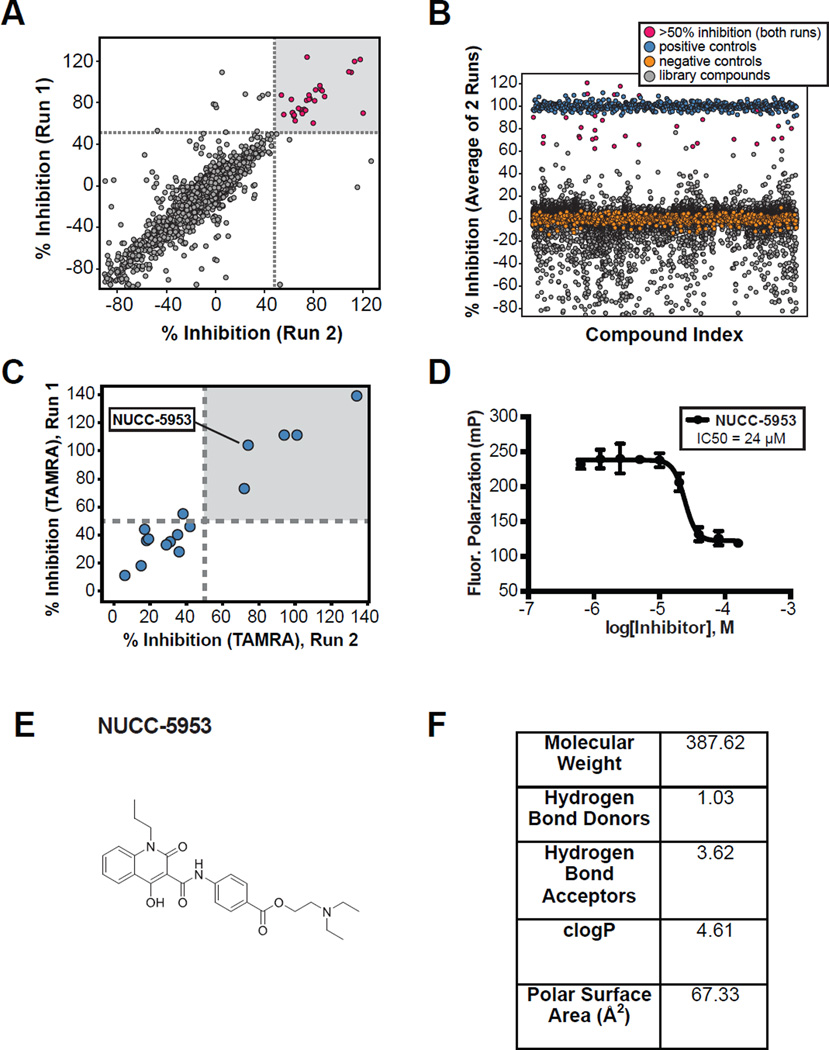
Figure 5. Summary of high throughput screening assay performance.
A. Comparison of the percent inhibition for compounds in two distinct runs of the assay. Note that linearity indicates a highly consistent assay. B. Graph showing the percent inhibition (as an average of the two runs) for the compounds screened. C. Hits identified on the FITC screen were subsequently assayed using a TAMRA labeled HCN peptide to rule out compounds that interact with FITC. D. NUCC-5953 was identified as an inhibitor in both the FITC screen and follow up TAMRA screen. Subsequent dose response testing using the TAMRA labeled HCN probe is shown. E. Structure for compound NUCC-5953 is shown. F. Table with properties of the screening library.
FP-based High throughput screen
To evaluate the FP assay in a high throughput screening (HTS) setting, we conducted a screen of a 20,000-compound diverse drug-like library (see Figure 5F for details) at the Northwestern University High Throughput Analysis (HTA) Lab. The high throughput screen was run in duplicate using pools of two compounds in each well, each at 40 µM. Compounds were incubated with a mixture of TRIP8b(241-602) protein (2 µM) and FITC-HCN1P (50 nM). The addition of DMSO (no compound) served as a negative control, and HCN1u peptide served as a positive control. Our HTS identified 32 active pools that showed >50% inhibition of the interaction in both HTS runs (Figure 5). Figure 5B is an alternate visualization of the HTS data plotting the average percent inhibition of the TRIP8b-HCN interaction from both HTS runs against all compound pools from the screen. Positive and negative controls are plotted to illustrate data consistency. Active compounds (pink) were selected from the plot in Figure 5A, and therefore not every compound with average percent inhibition >50% was considered a hit. To determine which individual compound had activity in a given active pool, the compounds were re-tested in duplicate at a single concentration using a TAMRA-conjugated HCN1 probe. The TAMRA probe is an alternate readout that emits in a distinct spectral region compared to the FITC probe, and was used to eliminate artifact hit compounds that interact with fluorescein or emitted in an overlapping spectral region. Using the TAMRA HCN1 probe, we found that five of our original hits exhibited >50% inhibition of binding in both replicates (Figure 5A). Thus, we observed an overall validated hit rate of 0.16% (in the pooled format) and a validated hit rate (compounds showing >50% inhibition at 40 µM with both FITC and TAMRA conjugated probes) of 0.025%. From this set of hits, we identified one compound with a favorable structure for downstream medicinal chemistry optimization, NUCC-5953. We therefore characterized the potency of NUCC-5953 by performing a dose response titration of this compound using the TAMRA-conjugated HCN1 probe and obtained an IC50 value of 24 µM (Figure 5D).
AlphaScreen assay
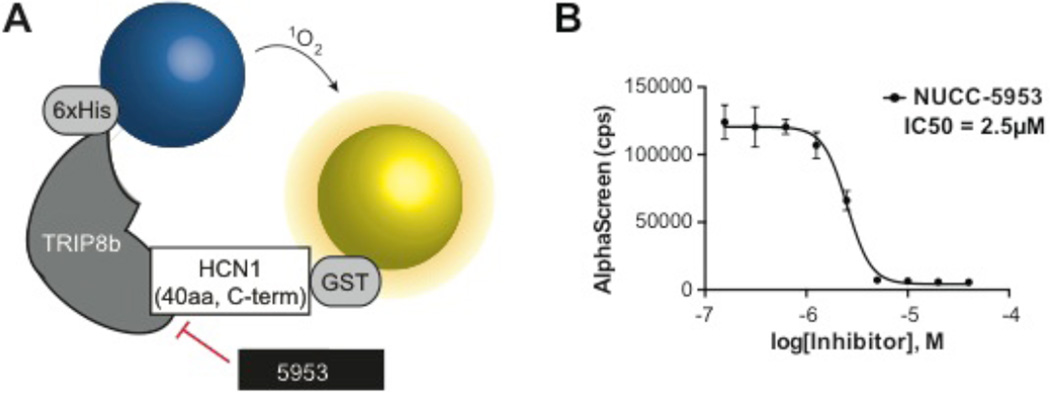
To confirm that our active compound, NUCC-5953, disrupts the TRIP8b-HCN1 interaction (i.e., its activity in the FP assay is not due to fluorescence interference), we developed an orthogonal assay utilizing AlphaScreen technology. In an AlphaScreen assay, signal is generated when Donor and Acceptor beads are brought within proximity of each other (~200 nm); red laser excitation causes singlet oxygen to “tunnel” from the Donor to the Acceptor, generating a luminescent signal that is proportional to the number of proximal bead pairs in solution23. Since AlphaScreen enables monitoring of larger protein complexes than FP, we employed a larger more physiologically relevant HCN1-based peptide in this assay that contained 40 residues from the C-terminal end (including the –SNL), called HCN1C40 (Figure 6A). For detection, a mixture of GST-tagged HCN1C40 protein and TRIP8b(241-602) was incubated at room temperature for 3 hours in the presence of a range of test compound concentrations (0.2–40 µM). In this assay, NUCC-5953 yielded an IC50 value of 2.5 µM (Figure 6B), thus confirming it as an inhibitor of the TRIP8b-HCN interaction.
Figure 6. AlphaScreen testing.
A. Schematic showing the mechanism underlying the AlphaScreen assay sees text for details. B. Dose response curve generated from compound NUCC-5953 using the AlphaScreen assay with IC50 value of 2 µM.
Discussion
In this study, we developed an FP assay for identifying molecules that inhibit the interaction between TRIP8b and HCN and developed a secondary AlphaScreen assay to confirm the validity of FP-identified hits..
In the course of developing the FP screening assay, we made several observations regarding the structure of the interaction between HCN and TRIP8b. When examining the differences in binding the C-terminal peptides of distinct HCN subunits to TRIP8b, we found that HCN1, 2, and 4 C-termini all had nearly equal affinities for TRIP8b while HCN3 had a significantly lower affinity. This is consistent with predictions from the crystal structure for the interaction between the terminal tripeptide of HCN2 and TRIP8b15. In particular, HCN3 has an ‘ANM’ tripeptide instead of the ‘SNL’ found in HCN1, 2, and 4. The serine to alanine change is predicted to cause a loss of a stabilizing interaction with Tyr-472 of TRIP8b. The terminal leucine residue of HCN2 ordinarily rests in a hydrophobic pocket formed by Val-343, Thr-346, Phe-348, and Thr-458 of TRIP8b. The presence of a hydrophobic methionine at this position in HCN3 likely preserves this interaction albeit with lower affinity given the change from a branched residue (leucine) to a linear one (methionine). Consistent with these results, we did not notice any substantial differences when using an unlabeled HCN1 peptide to displace the labeled HCN peptides from TRIP8b (Figure 4).
We subsequently developed a high throughput assay based on the FP screen to identify compounds that are capable of disrupting the tripeptide interaction between TRIP8b and HCN1. Our assay has significant utility for screening given its high Z factor value (0.896). After screening 20,000 compounds, we identified 1 hit (0.005%) with favorable chemical properties that we validated using our novel AlphaScreen binding assay. Although our hit molecule has limited potency (2.5µM, in our AlphaScreen assay), it provides proof of concept that the TRIP8b-HCN interaction can be pharmacologically modulated, and it will serve as a starting point for subsequent hit-to-lead optimization to generate more potent probe molecules.
The utility of identifying an inhibitor of the TRIP8b-HCN interaction is as a novel anti-depressant. We previously demonstrated that that genetic deletion of TRIP8b, HCN1 or HCN2 (the predominant channel subunits in the brain22) leads to an increase in antidepressant-like behavior in mice9. Consistent with our results, others found that shRNA knock down of HCN1 in the dorsal hippocampus produced a similar antidepressant-like phenotype in rats10. These results strongly suggest that limiting HCN channel function in the brain produces antidepressant behavior11.
With respect to treating depression, the benefit of developing an inhibitor of the TRIP8b-HCN interaction as opposed to targeting the HCN channel pore is in limiting potential cardiac effects. Given that HCN channels are expressed in the heart without TRIP8b, inhibitors of this interaction should not produce cardiac effects. In contrast, subcellular trafficking of HCN by TRIP8b is critical for channel function in the brain, and the C-terminal tripeptide interaction with TRIP8b is required for normal channel trafficking5. In the CA1 region of the hippocampus, where inhibition of HCN channels leads to antidepressant-like effects10, TRIP8b creates a gradient of HCN1 in the pyramidal neuron dendrites that requires the terminal tripeptide interaction with TRIP8b6. We thus reasoned that inhibitors of the interaction between the terminal tripeptide of HCN and the TPR domains of TRIP8b will block brain HCN channel function without affecting cardiac HCN channels that lack TRIP8b.
Our work has identified the first small molecule inhibitor of the interaction between HCN and TRIP8b, an interaction that has been shown to be a potential drug target for MDD. In addition to structure optimization of our identified hit, future work will focus on screening additional compound libraries using the workflow described here to identify more potent inhibitors with novel chemotypes. These efforts will aid in the development of new research tools as well as possible therapies for the treatment of MDD.
Acknowledgments
This work was supported by National Institutes of Health grant R21MH104471 (DMC), Brain Research Foundation SG 2012-01 (DMC), Northwestern University Clinical and Translational Sciences Institute 8UL1TR000150 (YH), Chicago Biomedical Consortium HTS-004 (YH and DMC), and National Institutes of Health grants 2T32MH067564 (KL). A part of this work was performed by the Northwestern University Medicinal and Synthetic Chemistry Core (ChemCore) at the Center for Molecular Innovation and Drug Discovery (CMIDD), which is funded by the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust and Cancer Center Support Grant P30 CA060553 from the National Cancer Institute awarded to the Robert H. Lurie Comprehensive Cancer Center.
Abbreviations
- HCN
Hyperpolarization-activated cyclic nucleotide-gated
- CNBD
cyclic nucleotide binding domain
- TPR
tetratricopeptide repeat
- TRIP8b
tetratricopeptide repeat-containing Rab8b interacting protein
- FP
fluorescence polarization
- cAMP
3’,5’-cyclic adenosine monophosphate
- FITC
fluorescein isothiocyanate
- TAMRA
tetramethylrhodamine
- GST
glutathione-S-transferase
- His
histidine
- PBS
phosphate buffered saline
- IPTG
isopropyl beta-D-1-thiogalactopyranoside
References
- 1.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis AS, Chetkovich DM. HCN channels in behavior and neurological disease: too hyper or not active enough? Mol. Cell. Neurosci. 2011;46(2):357–367. doi: 10.1016/j.mcn.2010.11.007. PMCID: PMC3073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis AS, Estep CM, Chetkovich DM. The fast and slow ups and downs of HCN channel regulation. Channels (Austin) 2010;4(3):215–231. doi: 10.4161/chan.4.3.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biel M, Wahl-Schott C, Michalakis S, et al. Hyperpolarization-activated cation channels: from genes to function. Physiological Reviews. 2009;89(3):847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 5.Piskorowski R, Santoro B, Siegelbaum SA. TRIP8b splice forms act in concert to regulate the localization and expression of HCN1 channels in CA1 pyramidal neurons. Neuron. 2011;70:495–509. doi: 10.1016/j.neuron.2011.03.023. PMCID: PMC3107038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro B, Piskorowski RA, Pian P, et al. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron. 2009;62(6):802–813. doi: 10.1016/j.neuron.2009.05.009. PMCID: PMC2720631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J. Neurosci. 2004;24(47):10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis AS, Schwartz E, Chan CS, et al. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J. Neurosci. 2009;29(19):6250–6265. doi: 10.1523/JNEUROSCI.0856-09.2009. PMCID: PMC2730639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis AS, Vaidya SP, Blaiss CA, et al. Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice. J. Neurosci. 2011;31(20):7424–7440. doi: 10.1523/JNEUROSCI.0936-11.2011. PMCID: PMC3169171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CS, Chang PY, Johnston D. Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors. Neuron. 2012;75(3):503–516. doi: 10.1016/j.neuron.2012.05.027. PMCID: PMC3418514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah MM. HCN1 channels: a new therapeutic target for depressive disorders? Sci Signal. 2012;5(244):pe44. doi: 10.1126/scisignal.2003593. [DOI] [PubMed] [Google Scholar]
- 12.Postea O, Biel M. Exploring HCN channels as novel drug targets. Nat Rev Drug Discov. 2011;10(12):903–914. doi: 10.1038/nrd3576. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Noam Y, Lewis AS, et al. Trafficking and gating of hyperpolarization-activated cyclic nucleotide-gated channels are regulated by interaction with tetratricopeptide repeat-containing Rab8b-interacting protein (TRIP8b) and cyclic AMP at distinct sites. J. Biol. Chem. 2011;286(23):20823–20834. doi: 10.1074/jbc.M111.236125. PMCID: PMC3121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saponaro A, Pauleta SR, Cantini F, et al. Structural basis for the mutual antagonism of cAMP and TRIP8b in regulating HCN channel function. Proc. Natl. Acad. Sci. U.S.A. 2014;111(40):14577–14582. doi: 10.1073/pnas.1410389111. PMCID: PMC4210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bankston JR, Camp SS, DiMaio F, et al. Structure and stoichiometry of an accessory subunit TRIP8b interaction with hyperpolarization-activated cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. U.S.A. 2012;109(20):7899–7904. doi: 10.1073/pnas.1201997109. PMCID: PMC3356637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu. Rev. Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- 17.Goutelle S, Maurin M, Rougier F, et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam Clin Pharmacol. 2008;22(6):633–648. doi: 10.1111/j.1472-8206.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 18.Rossi AM, Taylor CW. Analysis of protein-ligand interactions by fluorescence polarization. Nat Protoc. 2011;6(3):365–387. doi: 10.1038/nprot.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Chung T, Oldenburg K. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 20.Roehrl MHA, Wang JY, Wagner G. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of protein-protein interactions by fluorescence polarization. Biochemistry. 2004;43(51):16056–16066. doi: 10.1021/bi048233g. [DOI] [PubMed] [Google Scholar]
- 21.Zolles G, Wenzel D, Bildl W, et al. Association with the Auxiliary Subunit PEX5R/Trip8b Controls Responsiveness of HCN Channels to cAMP and Adrenergic Stimulation. Neuron. 2009;62(6):814–825. doi: 10.1016/j.neuron.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J. Comp. Neurol. 2004;471(3):241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- 23.Eglen RM, Reisine T, Roby P, et al. The use of AlphaScreen technology in HTS: current status. Curr Chem Genomics. 2008;1(1):2–10. doi: 10.2174/1875397300801010002. PMCID: PMC2775125. [DOI] [PMC free article] [PubMed] [Google Scholar]