Abstract
Although there is considerable evidence that individual differences in language development are highly heritable, there have been few genome-wide scans to locate genes associated with the trait. Previous analyses of language impairment have yielded replicable evidence for linkage to regions on chromosomes 16q, 19q, 13q (within lab) and at 13q (between labs). Here we report the first linkage study to screen the continuum of language ability, from normal to disordered, as found in the general population. 383 children from 147 sib-ships (214 sib-pairs) were genotyped on the Illumina® Linkage IVb Marker Panel using three composite language-related phenotypes and a measure of phonological memory (PM). Two regions (10q23.33; 13q33.3) yielded genome-wide significant peaks for linkage with PM. A peak suggestive of linkage was also found at 17q12 for the overall language composite. This study presents two novel genetic loci for the study of language development and disorders, but fails to replicate findings by previous groups. Possible reasons for this are discussed.
Keywords: Genetics, individual differences, language development, language impairment, linkage
The ability to navigate the world according to a seemingly arbitrary set of signs and symbols is frequently cited as that which makes humans unique among species. Remarkably, children learn the complicated rules of this system (i.e. language) rapidly and seemingly effortlessly, without the need for formal instruction or explicit feedback. By the age of three, children can consistently produce and understand novel words and sentences. By the time they reach school, most have developed the sophisticated language skills they need to navigate the world, form relationships, and access formal education and learning.
These aspects of species specificity, species universality, and the ‘poverty of the stimulus’ argument have led to speculation regarding the biological bases of language learning in humans. Empirical research began with twin and family-based studies designed to address questions of heritability. Estimates of heritability for language development vary considerably, depending on the age of participants and the particular aspect of language that is studied (i.e. phonology, vocabulary or syntax; see Stromswold 2001 for a review). However, these studies are consistent in showing that correlations in language ability are larger among MZ twins than DZ ones, and there is considerable evidence that variance in language as a complex trait is influenced by genetic factors.
This evidence has spurred molecular studies aimed at identifying genetic specific loci involved in the neurobiological pathways relevant for language. Early success came with the discovery of a translocation involving the gene FOXP2 in a child (CS) with a severe speech disorder (Lai et al. 2000, 2001). Previously, linkage analysis of an independent large multigenerational pedigree displaying an unusual autosomal dominant pattern for language impairment (Hurst et al. 1990) mapped a 5.6-cM region of 7q31 between D7S2459 and D7S643 (Lai et al. 2000). Approximately half of the KE family members (n = 15) display severe language deficits similar to CS, leading to the hypothesis that FOXP2 could be a candidate gene in the disorder. Follow-up sequencing of the family found a missense mutation – R553H – in the DNA-binding domain of exon 14 in FOXP2 that co-segregates in affected family members, but was absent in unaffected relatives (Lai et al. 2001).
Since this time, FOXP2 has been associated with multiple cases of speech and language impairment via rare chromosomal abnormalities and point mutations, although this has typically been in single case studies or isolated families (see Turner et al. 2013 for a review). To date, studies looking specifically for association between FOXP2 and variation in language abilities at the level of the general population have not found a role for the gene (e.g. Meaburn et al. 2002; Newbury et al. 2002; O'Brien et al. 2003).
FOXP2 is a transcription factor important for modulating the plasticity of neural circuits in the developing brain (Groszer et al. 2008). Among its down-stream targets is CNTNAP2, a gene distal to FOXP2 on chromosome 7q that has been associated most broadly with early language development (Whitehouse et al. 2011); with phonological memory (PM; Vernes et al. 2008) and reading abilities in SLI (Newbury et al. 2011); as well as with comorbid language-related phenotypes in the form of PM in reading disorder (Peter et al. 2011) and language delay in autism (Alarcon et al. 2008). Findings from these studies suggest that CNTNAP2 may have pleiotropic effects upon both reading- and language-related measures, or that neurodevelopmental pathways are shared between these traits.
In parallel with candidate genetic studies, several genome-wide linkage scans have been conducted aimed at discovering novel genetic loci associated with language impairment.
Genome-wide linkage studies and language impairment
The SLI Consortium (2002, 2004) used a classic sib-pair design in a combined clinical/epidemiological sample. The original sample (SLI Consortium 2002) comprised 98 families selected via a proband with past or current receptive and/or expressive language abilities >1.5 SD below the mean for age. Three quantitative language phenotypes – the receptive and expressive language scales of the Clinical Evaluation of Language Fundamentals – Revised (CELF-R; Semel, Wiig and Secord 1989), and the Children's Test of Nonword Repetition (CNRep; Gathercole and Baddely 1993) – were used in a model-free non-parametric analysis. All individuals in this study (siblings, as well as probands) had non-verbal IQ (PIQ) > 80 and normal hearing. Individuals were excluded on the basis of Autism Spectrum Disorder (ASD), known neurological difficulties, as well as for English was a second language.
Findings from the initial screen showed that two loci, 16q24 (SLI1, OMIM 606711) and 19q13 (SLI2, OMIM 606712), exceeded the threshold that Lander and Kruglyak (1995) have proposed as ‘suggestive’ of linkage (i.e. LOD score >2.2). Linkpage to SLI2 yielded a maximum LOD (MLS) of 3.55 for the expressive language phenotype, which reflects an individual's ability to formulate words and sentences. In comparison, linkage to SLI1 was for the CNRep test (MLS = 3.66). Permutation analysis indicated these findings are equivalent to an empirical P = 0.0002/0.0003 and are, therefore, unlikely to be seen by chance.
The SLI Consortium followed this study with a replication sample (SLI Consortium 2004). 86 additional families were ascertained clinically using the same language and cognitive measures as the first study (now denoted Wave 1). However, in the second study (Wave 2) markers were targeted to the SLI1 and SLI2 loci on chromosomes 16 and 19 only. Both regions showed evidence of suggestive linkage to the PM phenotype (MLS 2.84 and 2.31, respectively; empiric P-values<.02). In contrast to Wave 1 of the study, the expressive language phenotype was not linked to either chromosome in Wave 2. When data from the two waves were combined, the LOD score on chromosome 16 increased to 7.46 indicating strong evidence for linkage. In contrast, the signal to chromosome 19 decreased, resulting in non-significant LOD scores for NWR (MLS =1.40) and expressive language (MLS = 1.02). This loss in signal was attributed to the fact that, in each wave of the study, linkage to chromosome 19 came from different phenotypes, which was not additive. The replication study also found suggestive evidence of linkage (MLS = 2.67) to SLI1 for a measure of spelling ability (Rust et al. 1993).
Taking advantage of the additional power that can be derived from multiple correlated phenotypes, Monaco (2007) reanalyzed data from the SLI Consortium studies in a multivariate variance-components (VC) analysis. Such an approach is appropriate when there is covariance among, and genetic correlation across, related phenotypes. Previous research indicates that measures of language are typically well correlated (Tomblin & Zhang 2006), and also show strong genetic correlation (Colledge et al. 2002). Although an initial VC screen found seven loci on chromosomes 1, 4, 5, 7, 10, 16 and 19 yielding levels of linkage P < 0.01, only the SLI1 locus on chromosome 16 remained significant once data from Wave 2 was added to the analysis. Further investigation of the SLI1 linkage peak in this study showed new evidence for linkage to reading and spelling phenotypes, as well as to non-word repetition (NWR) as before. Loci at SLI2 on chromosome 19, and a previously unreported locus on chromosome 10, showed borderline significance.
In an attempt to replicate findings from the SLI Consortium, an independent study by Falcaro et al. (2008) ascertained an additional 93 probands with SLI. Two measures of language ability, PM and past-tense marking, were treated quantitatively and dichotomously in a family-based analysis, with markers targeted to chromosomes 16 and 19. Both loci yielded LOD scores necessary for the replication of linkage: PM was linked to SLI1 (MLS=1.8) and expressive morphology was linked to the SLI2 (MLS=2.2).
Fine-mapping of the SLI1 locus has identified two candidate genes – CMIP and ATP2C2 – that are associated with PM abilities in SLI (Newbury et al. 2009). Significantly, risk variants appear to be relevant only to individuals with SLI, and further investigation of these in a large unselected cohort (the ALSPAC sample, n=3612) was not significant (Newbury et al. 2009). Subsequently, the SLI Consortium has shown that CMIP is also associated with reading abilities in SLI, although interestingly not with dyslexia (Newbury et al. 2011). Rice et al. (2009) have also directly addressed the question of shared effects between SLI and RD by performing association analyses of genes previously associated with RD in a sample selected for SLI (Rice et al. 2009). They found marginal association (0.05 > P > 0.01 prior to correction for multiple testing) with several SNPs across KIAA0319 and measures of reading, articulation, and vocabulary, and for an omnibus test of language ability. These latter studies reinforce evidence for the existence of common susceptibility genes underlying language and language-related disorders.
Bartlett et al. (2002) performed a parametric linkage analysis on five families originally ascertained as part of a study on schizophrenia (Brzustowicz et al. 2000). Each family contained at least two individuals with spoken language abilities (SLQ) > 1 SD below the mean, as measured by the age-appropriate version of the Test of Language Development (Newcomer & Hammill 1988). In this study, SLI was defined as a categorical, as opposed to a quantitative, variable. Because the mode of transmission for SLI is unknown, analyses were conducted under both dominant and recessive models. Criteria for exclusion were PIQ < 80, hearing impairment, ASD, schizophrenia, and known neurological disorders. In addition to SLI, two language-related phenotypes were also considered in this study. RD was defined similarly to SLI, on the basis of a non-word reading/IQ-based discrepancy using the non-word (Word Attack) subtest from the Woodcock Reading Mastery Test – Revised (Woodcock 1987); and clinical impairment (CI), which used the same measures as the LI/RD diagnoses although with a less stringent set of conditions.
Under the recessive model, significant evidence for linkage was found to 13q21 (SLI3) for RD (MLS 3.92), and suggestive evidence to 2p22 for SLI (MLS = 2.86). A follow-up study of 22 nuclear and extended families, which targeted chromosomes 2, 7 and 13 only, replicated linkage of RD to 13q21 (Bartlett et al. 2004). Combining samples from studies one and two resulted in a maximum LOD score of 6.18 at the same location. However, inspection of LOD scores by family showed that most of the signal in the replication sample came from four pedigrees, suggesting this locus may not be indicative of a risk factor for SLI in the general population.
Addis et al. (2010) conducted a linkage analysis of a three-generation German family displaying an apparently simple segregation of language impairment and comorbid literacy difficulties. Psychoacoustic testing also revealed evidence for auditory processing deficits. The maximum expected LOD score given the size of the family was 1.5. In the first phase of analysis, two peaks on chromosomes 4 and 12 attained this threshold. In order to narrow these regions, three more family members were added in a second wave of analysis. This resulted in the LOD score on chromosome 12 increasing to 2.1 (maximum expected LOD score =2.2), while it decreased on chromosome 4. The critical region on chromosome 12 implicated a large 58.5 Mb region, containing 600 genes. However, the specific genetic variant contributing to this signal has yet to be identified. A joint venture between members of the SLI Consortium and researchers in Chile conducted a genome-wide linkage analysis of a small isolated population with high rates of SLI (Villanueva et al. 2010, 2011). Parametric and non-parametric linkage analyses of four pedigrees yielded strong evidence of linkage to a region on chromosome 7q (SLI4, OMIM 612514), as well as signals to chromosomes 13, 17, 6q and 12. The finding to 7q is important because candidate genes for language impairment, FOXP2 and CNTNAP2, reside in this region. Segregation analysis, however, indicated a haplotype that included NOBOX and TPK1, rather than the candidate genes. The linkage peak to chromosome 13 overlapped with the SLI3 region identified previously by Bartlett et al. (2002, 2004). Previous linkage signals to SLI1 (chromosome 16) and SLI2 (chromosome 19) found by the SLI Consortium (2002, 2004), however, were not replicated.
Late language emergence is generally considered to be a hallmark of language impairment (Paul 1996; Paul et al. 1991; Poll et al. 2010). A recent linkage study detected a heterozygous 4 kb deletion at chr2q35 (SLI5, OMIM 615432) in 15 Southeast Asian probands with language delay and white-matter abnormalities (Wiszniewski et al. 2013). The mutation, which eliminated exon 3 of the TM4SF20 (transmembrane 4 L six family member gene 20) gene, co-segregated with language delay in all 15 families. As yet, the function of the gene is unknown, although it appears to represent an ancestral haplotype in Southeast Asian populations.
Genome-wide association studies and language impairment
Recently, the search for genes associated with individual differences in language has been enhanced by the use of genome-wide association methods that have the advantage of being more sensitive to genetic effects that are smaller than what can be detected by linkage. Two studies have used the data obtained from the Avon Longitudinal Study of Parents and their Children (ALSPAC) for initial discovery of associations. Luciano et al. (2013) reported an association between a PM phenotype and a pseudogene (ABCC13) on chromosome 13. Eicher et al. (2013) tested for associations of LI either comorbid with RD or without RD and found nominal associations of two genes (ZNF385D and COL4A2) in comorbid cases and in a separate replication sample found that ZNF385D on chromosome 3 was associated with LI independent of RD. More recently, Harlaar et al. (2014) reported no significant and replicable associations in a GWAS involving language measures obtained from one member of each twinship in the Twins Early Development Study (TEDS) cohort. Gialluisi et al. (2014) constructed a composite phenotype using principal components based upon language and reading measures using participants from three cohorts where language and reading had been studied. Suggestive associations were found for two SNPs in two genes (CCDC136/FLNC and RBFOX2). The findings for CCDC136/FLNC were supported by inspection of data from the Luciano et al. (2013) GWAS, although in this case the allelic effects were reversed. St Pourcain et al. (2014) performed a GWAS involving early vocabulary development in children from the Early Genetics and Lifecourse Epidemiology consortium. One SNP adjacent to ROBO2 on chromosome 3 was found to be significantly associated with vocabulary development at 24 months of age. Another member of the ROBO family (ROBO1) is also on chromosome 3 and has been associated with dyslexia and PM: however, no evidence was found for an association of ROBO1 with early vocabulary in this study. Thus, at this time GWAS investigations have yielded a small number of additional candidate regions and genes that were not found by linkage analysis. Interestingly, none of the loci that have been suggested by linkage analysis have been replicated in the association studies.
In summary, there have been multiple independent genome-wide linkage scans for language impairment, using both quantitative and binary phenotypes in single families and isolated populations. This small number stands in stark contrast to the much larger number of studies that have been conducted in other common neurodevelopmental disorders, for example, Attention-Deficit/Hyperactivity Disorder (ADHD), dyslexia and ASD, which are believed to have possible genetic overlap with language impairment (Mueller & Tomblin 2012; Newbury et al. 2011; Vernes et al. 2008). Lander and Kruglyak (1995) proposed that for the confirmation of linkage, findings need to be replicated, and should ideally be replicated by independent research groups. While SLI1, SLI2 and SLI3 have each been replicated by within-group studies, findings to these loci not been replicated between research groups. The exception to this is SLI4, which was mapped in the Chilean study and also contains the candidate genes CNTNAP2 and FOXP2. This study also replicated previous findings to SLI3 (Bartlett et al. 2002, 2004), which is surprising since it was based an isolated population comprising a highly consanguineous sample − 75% of the affected individuals in this sample were descendants of a pair of founder brothers. We would, therefore, anticipate findings to reflect rare within-family variants, which might not be found in more genetically diverse populations.
Here, we report findings from a sib-pair linkage analysis of spoken language abilities. Unlike the previous studies, the probands in this study were not limited to children with poor language.
Materials and Methods
Participants
Participants in this study comprised 391 individuals from 147 sib-ships (see Table 1), where at least one child (designated the ‘proband’) had participated in a longitudinal study on outcomes in language development and disorders (see for instance: Tomblin et al. 2000) As shown in Table 1, 14 of these sib-ships contained two probands due to each child having been ascertained independently as part of the original longitudinal study. Language status was determined via established standards (see Language phenotypes below, as well as Tomblin et al. 1996) using data obtained when the probands were in kindergarten or second grade. This resulted in 167 probands for the present study. Of these probands, 40% were children with poor language ability where their language ability was below the 16th percentile for age. Siblings of probands (either full or half) were selected without regard to their language status, and were between four and 16 years of age. Multiple siblings were included in the study if they met the age selection criterion. This resulted in 214 non-independent sib-pairs (i.e. either proband-sib pairs or sib-sib pairs). All individuals had normal hearing and were without neurodevelopmental disorders (based on parental report). Self-reported race for the parents of the probands is reported in Table 2.
Table 1. Composition of sib-ships according to their relationship with the proband.
| Relationship | Number | Proportion male | Age at test |
|---|---|---|---|
| Proband | 167 | .55 | * |
| Full sib | 174 | .51 | 9.38 (2.81) |
| Half sib | 50 | .52 | 7.86 (3.27) |
Probands were tested on multiple occasions as shown in Table 3. Phenotypes for these children represented the average across observations.
Table 2. Self-reported race of parents of probands.
| Race | Number |
|---|---|
| White | 144 |
| Black | 20 |
| Asian | 1 |
| Hispanic | 2 |
| Total | 167 |
Language phenotypes
Probands
As stated above, probands were ascertained in kindergarten as part of a cross-sectional study on the prevalence of language impairment, and were assessed again at 6, 8, 10, 14 and 16 years of age. Where possible, the same assessments were used across the span of the longitudinal study. In cases where a test used in an earlier wave of the study was no longer age-appropriate (i.e. because of ceiling effects), another test measuring the same aspect of language was substituted as replacement. Consequently, children were assessed according to four test protocols over the course of the longitudinal study (see Table 3).
Table 3. Language and nonverbal IQ measures.
| Construct | Protocol 1 (4–7 years) | Protocol 2 (7–9 years) | Protocol 3 (9–15 years) | Protocol 4 (15–18 years) |
|---|---|---|---|---|
| Vocabulary | TOLD-2:P | PPVT-R | PPVT-R | PPVT-R |
| Picture vocabulary | ||||
| Oral vocabulary | ||||
| Sentence | TOLD-2:P | CELF-III | CELF-III | CELF-III |
| Grammatic Understanding | Sentence Structure | Concepts & Following Directions | Concepts & Following Directions | |
| Grammatic Completion | Concepts & Following Directions | Recalling Sentences | Recalling Sentences | |
| Sentence Imitation | Recalling Sentences Word Structure | Formulating Sentences | ||
| Non-verbal IQ | WPPSI: | WISC-III: | WISC-III: | WISC-III: |
| Block Design | Block Design | Block Design | Block Design | |
| Picture Completion | Picture Completion | Picture Completion | Picture Completion |
CELF-III, Clinical Evaluation of Language Fundamentals (3rd edition); PPVT-R, Peabody Picture Vocabulary Test-Revised; TOLD-2:P, Test of Language Development-Primary (2nd ed.); WISC-III, Wechsler Intelligence Scale for Children (3rd. edition); WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
From these measures, three summary (composite) language scores and a score representing (non-verbal) performance IQ were computed. All individuals in this study had PIQ >70. The summary scores comprised an overall language composite, a vocabulary composite, and a composite of sentence use, which were z-scores based on age-standardized norms. Elsewhere, research on the dimensionality of these language measures does not endorse a receptive-expressive language distinction (Tomblin & Zhang 2006), so this is not made in the present study. All children over eight years of age (n=287) also received the Nonword Repetition Task (NWR; Dollaghan & Campbell 1998). The effect of chronological age on performance was removed via regression analysis and the residual score standardized to a z-score. This measure represents PM in the current study. Depending on the extent of the proband's participation in the longitudinal study a minimum of two, and as many as four, waves of data observations were available. In this case, data were taken from the wave that most closely matched to the age at which the sibling was tested.
Siblings
The siblings of probands were tested once during the longitudinal study using the same age-appropriate protocol as the probands. Composite language scores for these individuals were created in the same way as described above.
Genotyping
DNA was collected from probands, siblings and their biological parents via buccal swabs, saliva and blood. Briefly, DNA was extracted from buccal swabs using procedures described in Richards et al. (1993), and from whole blood using a modified version of Qiagens DNA Blood Maxi kit (Qiagen, Inc., Valencia, CA, USA). Samples derived from saliva were processed using Oragene's standard 0.5 ml and 4ml protocols (DNA Genotek, Ontario, Canada). Both buccal swabs and blood have been shown to provide high quality DNA and genotyping results (Hansen et al. 2007).
All 383 individuals were genotyped on the Illumina® (Illumina, San Diego, CA, USA) Linkage IVb Marker Panel at the Center for Inherited Disease Research (CIDR; http://www.cidr.jhmi.edu/human_snp.html). This panel has 5980 single nucleotide polymorphisms (SNPs) spaced at an average of ∼121 kb intervals.
Statistical analysis
Samples and genotyping calls on the Illumina® Linkage IVb Marker Panel were examined using standard quality control procedures (Pluzhnikov et al. 2010). SNPs were filtered for 5% minor allele frequency. Samples were also checked for sex and relatedness using PLINK (Purcell et al. 2007). Any samples where the genetic sex and recorded sex differed were removed. Mendelian inconsistencies were identified using PedCheck (O'Connell & Weeks 1998) and removed prior to analysis, leaving 5585 SNPs for the analysis. Linkage analysis was performed using the software Merlin (Abecasis et al. 2002). To recap, we considered four language-related traits: an overall language composite, a sentence composite, a vocabulary composite and a PM score. Multipoint non-parametric LOD scores were computed using the NPL all scoring function and the exponential model (Kong & Cox 1997). The significance of linkage peaks was determined in Merlin with 1000 simulations, by comparing the number of simulations with LOD scores at, or exceeding, those values observed in the study.
Following this, an enrichment analysis was conducted to compare the number of statistically significant SNPs in a 1-LOD linkage region under the linkage peaks on chromosomes 10 and 13 to the number expected by chance. Data for this analysis was derived from a related genome-wide association study (GWAS) of language ability using the Affymetrix 6.0 CHIP. The final GWAS data used in this analysis contains 434 unrelated children. The SNP data were filtered for MAF greater than 5%. Europeans were identified and included using principal components (Price et al. 2006). Association test P-values were calculated using PLINK (Purcell et al. 2007). QQ plots were created (Fig. 3) and the False Discovery Rate (FDR) calculated using q-value (Storey & Tibshirani 2003) and the Benjamini–Hochberg method (Benjamini & Hochberg 1995). Each QQ plot shows the expected distribution of association P-values as well as the observed distribution. The observed P-values for the SNPs in each linkage region were sorted from least significant to most significant and plotted against the uniform null distribution. Control regions for this analysis were selected from linkage studies of diabetes (Concannon et al. 2009), and lung cancer (Fang et al. 2010), both diseases that are very unlikely to be related to language ability.
Figure 3. Enrichment of chromosome 10 for the SLI phonological memory phenotype as well as the lack of enrichment for chromosome 13 for phonological memory in SLI and chromosomes 11 and 19 for type 1 diabetes.
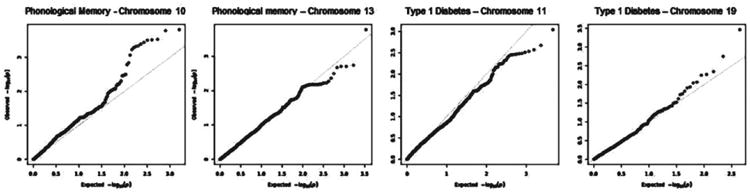
11 SNPs have an FDR < 0.10 on chromosome 10 for phonological memory.
Results
Characteristics of the phenotypes among siblings
The language abilities for each type of the sib-ship are shown in Table 4. The original longitudinal study, which forms the basis of the current one, over-sampled for individuals with poor language abilities. This is reflected in the composite language, vocabulary and sentence scores of the proband group, which were well below the expected levels for age. The NWR task was not included in the original selection protocol, and explains why proband performance was slightly better on this task. The composite language and PM scores of the full sibling group were slightly higher than the proband group, which is as expected given that siblings were not selected on the basis of language status. In contrast, composite scores for the half-sib group were lower than the full sibs which goes against the expectation that children sharing more genes (full sibs) should regress less and thus be more severe than those who share fewer genes (half sibs). However and inspection of the scores for the probands from sib-ships containing half siblings showed that they also had lower language composite scores (Mean =−0.97, SD=0.93) and PM [Mean =−0.84 (1.01)] than the probands as a group and also had lower scores than their half siblings (see Table 4). Thus, the expected regression toward the population mean in the half sibs can be seen.
Table 4. Means and standard deviations (in parentheses) of language phenotypes (in z-score units) for members of the sibships based on their relationship to the proband.
| Relationship | Composite language | Composite vocabulary | Composite sentence | Phonological memory |
|---|---|---|---|---|
| Probands | −0.79 (1.11) | −0.69 (1.0) | −0.78 (0.90) | −0.53 (1.03) |
| Full sibs | −0.48 (1.06) | −0.39 (1.12) | −0.43 (1.03) | 0.07 (0.97) |
| Half sibs | −0.79 (0.76) | −0.57 (0.99) | −0.83 (0.73) | −0.21 (0.78) |
Table 5 details correlational analyses for the phenotypic measures in this study. The high degree of correlation between the overall language composite and the vocabulary and sentence composites reflects that some language measures were common across composites. Although the vocabulary and sentence composites comprised distinct tests, the large correlation (0.75) between them is consistent with considerable evidence indicating these two skills reflect a common language and cognitive system. The correlations between PM and the language measures are lower than those among the language measures. This is likely due to two factors. First the language composite is formed from the vocabulary and sentence measures and thus the correlation is inflated due to this. Also, all of the sentence measures entail real words and thus also are sensitive to vocabulary ability, thus these are not expected to be independent traits. The PM measure used novel words and was designed to control for vocabulary ability as much as possible. Thus, these correlations appear consistent with our understanding of the constructs being measured.
Table 5. Correlations (Pearson r) among language measures within siblings.
| Relationship | Phonological memory | Language composite | Sentence composite |
|---|---|---|---|
| Language composite* | 0.53 | ||
| Sentence composite | 0.54 | 0.96 | |
| Vocabulary composite | 0.49 | 0.88 | 0.75 |
Measures in the vocabulary and sentence composites were also part of the language composite and thus the correlations are non-independent.
One assumption of sib-pair linkage analysis is that membership within familial sib-ship accounts for a substantial portion of phenotypic variance. As expected, intra-class correlation, which reflects the overall degree of familial resemblance, was strong for each of the composite phenotypes (Table 6). However, it was considerably weaker for the PM phenotype. Because this study comprised both full and half siblings, we would expect familial resemblance to be higher among sib-ships comprising full sibs compared to those comprising half. Table 6 shows that this was indeed the case, likely reflecting the heritability of the phenotypes used in this study, as well as shared environmental factors.
Table 6. Intra-class correlation coefficients (ω2) and 95% confidence intervals (italic) for composite and phonological memory phenotypes across all sib sets.
| Phenotype | All sibs | Full sibs | Half sibs |
|---|---|---|---|
| Language composite | 0.57 | 0.59 | 0.47 |
| 0.49–0.64 | 0.50–0.66 | 0.25–0.63 | |
| Vocabulary composite | 0.51 | 0.56 | 0.38 |
| 0.42–0.59 | 0.46–0.64 | 0.14–0.56 | |
| Sentence composite | 0.53 | 0.52 | 0.45 |
| 0.44–0.60 | 0.41–0.60 | 0.22–0.61 | |
| Phonological memory | 0.26 | 0.25 | 0.18 |
| 0.11–0.39 | 0.08–0.40 | 0.0–0.52 |
Linkage analysis
The non-parametric LOD scores for each phenotype are shown in Fig. 1. The largest linkage peaks were on chromosome 10 (113.13 cM, 10q23.33; MLS =4.631 for rs787652) and chromosome 13 (108.52 cM, 13q33.3; MLS =4.257 for rs1411736; Fig. 2), both to PM. Permutation analyses indicate these findings are equivalent to an empirical P-value of 0.003 and are, therefore, unlikely to be seen by chance. The largest linkage peak for the overall language composite was to chromosome 17 (62.90cM; MLS =3.102; Fig. 2). This result was nominally significant in simulations (P = 0.058).
Figure 1. Genome-wide exponential LOD scores for each of the four phenotypes examined.
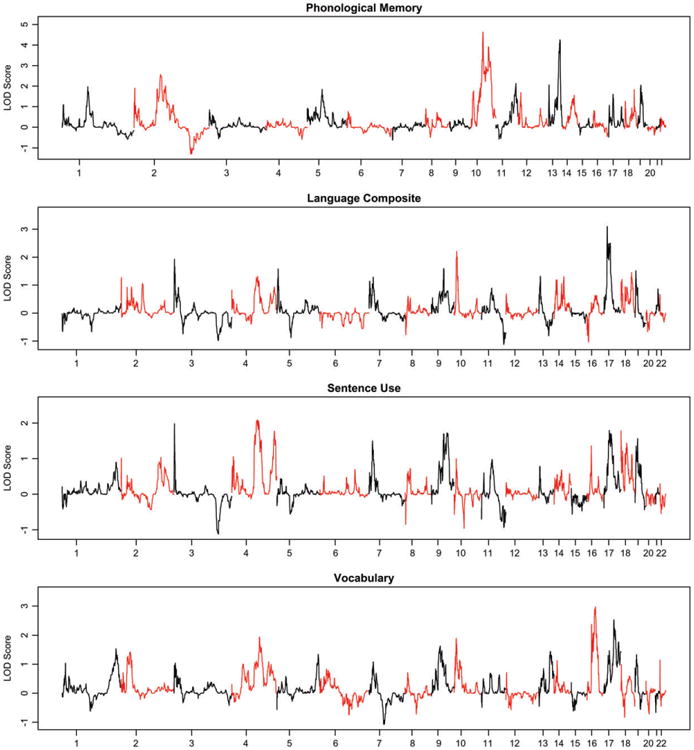
Figure 2. Linkage plots of the three regions reaching at least statistically nominal p-values for each of the phenotypes listed.
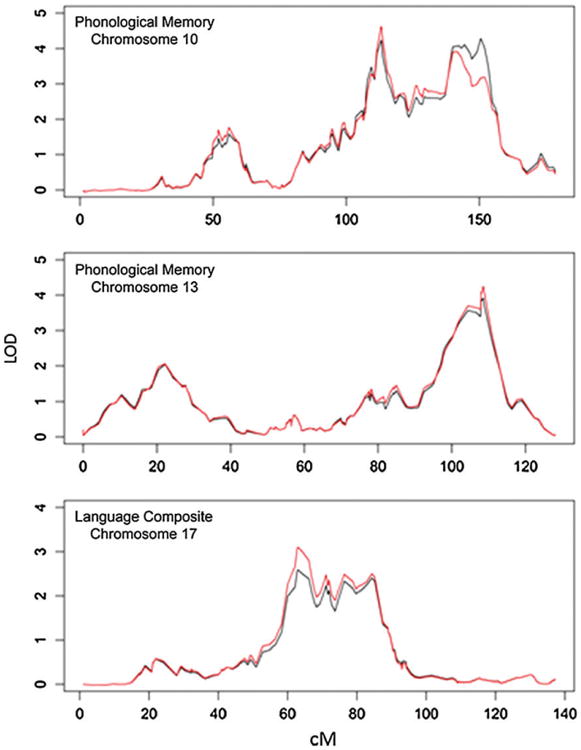
Black lines indicate a linear model, while red lines show an exponential model.
Enrichment of SNP associations at 10q23.33 and 13q33.3
The 1 LOD regions under the linkage peaks on chromosomes 10 and 13 were examined more closely for evidence of association to PM using data from a companion GWAS. About 478 individuals were genotyped using the Affymetrix 6.0 CHIP. One hundred and twenty-two of these individuals were also probands in the current study. Because both studies employed the same phenotypes, this afforded an opportunity to test whether there was an enrichment of association between statistically significant SNPs from the Affymetrix platform under the linkage peaks identified in the current study. Data for this analysis was available for 434 individuals identified as genetically European in ancestry. The P-values of SNPs from the companion GWAS within the 1-LOD intervals of four regions (the 2 significant regions from this study and 2 regions from a control study are shown) were used to generate a QQ plot. This allows the opportunity to see if the implicated regions in this study are enriched for higher P-values in the GWAS data than expected by chance, and thus provide support for the region contributing to SLI in this sample.
We found the 1-LOD region around the linkage peak on chromosome 10 contained 11 SNPs with an FDR <0.10, indicating enrichment. The deviation from the diagonal line y = x at the extreme tail suggests the presence of SNPs in this 1-LOD region that are clearly more significant than expected under the null hypothesis. However, this was not found for the chromosome 13 locus. Enrichment was not found in any of the control regions selected from other studies of complex diseases (Fig. 3; Fang et al. 2010; Concannon et al. 2009).
Discussion
Genome-wide linkage analysis was performed on 147 sets of siblings containing 214 sib-pairs. About 5927 SNPs were tested against four phenotypes: three composite measures of language, vocabulary, and sentence use and PM score. The best evidence for linkage was found on chromosomes 10 and 13 to PM (LOD scores of 4.631 and 4.257, respectively). LOD scores are equivalent to a genome-wide P-value for significance of 0.003. The chromosome 10 locus was also enriched for SNPs associated with PM, indicating it very likely contains candidate genetic loci associated with language ability.
The linkage peak on chromosome 10 contains the gene CPEB3 (cytoplasmic polyadenylation element binding protein 3), which has been previously linked to human declarative memory (Vogler et al. 2009). The declarative memory system is fundamental to language learning; in particular to word learning, a process that requires novel strings of sounds to be associated with novel semantic referents in order to form meaningful words (Gupta 2012). Individual differences in PM are also strongly related to vocabulary development (Baddeley et al. 1998). Given that both declarative memory and PM are related to the process of learning words, CPEB3 is a strong candidate for follow-up association studies. A locus for NWR (163 cM) has been previously mapped to chromosome 10 (163 cM; Monaco 2007). However, this appears to be distal to the region reported here (∼111 cM). More recently, a breakpoint on chromosome 10 has also been linked with developmental language disorder (Ercan-Sencicek et al. 2012). This breakpoint (∼97 Mb) falls between the genes ENTPD1 and CCNJ, and appears to be slightly distal to the region reported here (93–95 Mb). This finding warrants examination in the future.
The other locus significantly linked to PM in this study was on chromosome 13. Although SLI3 is also located on this chromosome, it does not overlap with the region identified here. It is, therefore, unlikely the two loci are indexing the same causal gene. One possible candidate for the linkage signal found in this study is DAOA (also known as G72). DAOA activates the DAAO enzyme that degrades D-serine and, in turn, is an activator of NMDA-type glutamate receptors. This gene has been linked to several psychiatric disorders, including schizophrenia and bipolar disorder (Chen et al. 2013; Seifuddin et al. 2012). It has also been associated with working memory, verbal fluency and the modulation of brain activity during speech (Hall et al. 2008; Jansen et al. 2009; Krug et al. 2011; Muller et al. 2011; Zuliani et al. 2009). Neither CPEB3 nor DAOA are currently candidate genes for language, so these findings now require follow-up in the form of association studies and functional analyses.
Similar to prior research, we found PM to be the most robust phenotype for genetic studies of language development and disorders (Newbury et al. 2002, 2004; Vernes et al. 2008). Replicated linkage signals for PM have been found at 6q in the DYX2 locus, for the CNTNAP2 locus on chromosome 7, and for the SLI1 and SLI2 loci on chromosomes 16 and 19. In contrast, language as a phenotype in and of itself has proven less robust. For example, Bartlett et al. (2002) found evidence suggestive of linkage to chromosome 2p22 for receptive and expressive language phenotypes, with MLS =2.86, but these findings were not replicated in their follow-up study. Falcaro et al. (2008) reported to the SLI2 region for expressive language, with a MLS =2.2, which only qualifies as suggestive of linkage according to standards set by Lander and Kruglyak (1995). In the current study, language as an overall composite was nominally significant for linkage to chromosome 17 (P =0.058). One gene in this region that is worth noting is SNIP, which is important in the regulation of dendritic spine formation and synaptic plasticity (Jaworski et al. 2009). Although this gene has not previously been associated with language, its function makes it an intriguing candidate for future study.
The present study is the fifth independent genome-wide linkage scan for language-related traits. Much of the previous research in this area has been performed with a core sample of language-impaired individuals (e.g. the SLI Consortium in the UK; Falcaro et al. 2008; Newbury et al. 2002, 2009). As shown in Table 7, the SLI Consortium has replicated linkage to chromosomes 16 and 19; however, these studies share many participants so cannot be considered fully independent. Bartlett et al. (2002) reported evidence for linkage to chromosomes 13 and 2; however, linkage was not found for either of these regions in the SLI Consortium studies. In Table 7 we have compared the phenotypes and LOD scores from the current study to those previous studies.
Table 7.
A comparison of genome-wide linkage scans for language-related phenotypes in previous and current studies. genomic locations use build hg18
| Region | Linkage results from previous studies | Replication results in current study | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Phenotype | Markers | Location | Phenotype | Marker | MaxLOD | |
| 16q (SLI1) SLI Consortium (2002, 2004) |
Phonological memory | D16S516-D16S3061 | ∼16:77681517-83492305 | Phonological memory | rs1079635 | 0.19 |
| 19q (SLI2) SLI Consortium (2002, 2004) |
Phonological memory | D19S420-D19S904 | ∼19:48500600-55468843 | Phonological memory | rs1236093 | 0.67 |
| 13q21 (SLI3) Bartlett et al. (2002) |
Reading impairment with SLI | D13S788-D13S317 | ∼13:50790624-81620315 | Language Composite | rs2274202 | 0.355 |
| 7q (SLI4) Vernes et al. (2008) |
Language impairment, phonological memory | rs851715-rs2710117 | ∼7:147829814-147904680 | Phonological memory | rs4431523 | 0.19 |
| 2q36 (SLI5) Wiszniewski et al. (2013) |
Language delay, white matter abnormalities | TM4SF20 | ∼2:227935118-227952266 | Language Composite | rs1403970 | −0.283 |
| 10q Monaco (2007) |
Phonological memory, expressive and receptive language | 163cM | ∼10:130007627-130634338 | Phonological memory | rs1255135 | 0.964 |
| 12p13.31 Addis et al. (2010) |
Language impairment, comorbid literacy difficulties, auditory processing deficit | D12S99-D12S326 | ∼12:5434815-5435185 | Language Composite | rs1558776 | 0.557 |
Given the complexity of language as a complex trait, it is perhaps not surprising to find there is little overlap between linkage studies. One obvious reason for the low level of replication may be because the sample sizes in these studies have been fairly small. It is also the case that linkage is best suited to detect genetic loci of large effect, which are typically single genes. In contrast, genetic contributions to individual differences in language development and disorders are likely small, for example comprising multiple, complex interactions among genetic, epigenetic, stochastic and environmental factors. Significantly, only two of the candidate genes currently associated with SLI have been found via linkage analysis (Newbury et al. 2009). In light of this, the novel regions identified in this study provide promising candidates for future studies of genetic loci involved in language development and disorders. Furthermore, we can expect more candidates to come from ongoing GWAS studies and now whole genome sequencing that may yield novel rare variants.
Acknowledgments
The authors of this article have no conflict of interests to report. This research was supported by grants DC00496 and DC02746 from the National Institute on Deafness and Other Communication Disorders.
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Addis L, Friederici AD, Kotz SA, Sabisch B, Barry J, Richter N, Ludwig AA, Rübsamen R, Albert FW, Pääbo S, Newbury DF, Monaco AP. A locus for an auditory processing deficit and language impairment in an extended pedigree maps to 12p13.31-q14.3. Genes Brain Behav. 2010;9:545–561. doi: 10.1111/j.1601-183X.2010.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychol Rev. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Bartlett CW, Flax JF, Logue MW, Vieland VJ, Bassett AS, Tallal P, Brzustowicz LM. A major susceptibility locus for specific language impairment is located on 13q21. Am J Hum Genet. 2002;71:45–55. doi: 10.1086/341095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett CW, Flax JF, Logue MW, Smith BJ, Vieland VJ, Tallal P, Brzustowicz LM. Examination of potential overlap in autism and language loci on chromosomes 2, 7, and 13 in two independent samples ascertained for specific language impairment. Hum Hered. 2004;57:10–20. doi: 10.1159/000077385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- Brzustowicz LM, Hodgkinson KA, Chow EWC, Honer WG, Bassett A. Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-1q22. Science. 2000;288:678–682. doi: 10.1126/science.288.5466.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xu Y, Zhang J, Liu Z, Xu C, Zhang K, Shen Y, Xu Q. A combined study of genetic association and brain imaging on the DAOA gene in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162:191–200. doi: 10.1002/ajmg.b.32131. [DOI] [PubMed] [Google Scholar]
- Colledge E, Bishop DVM, Koeppen-Schomerus G, Price TS, Happe FG, Eley TC, Dale PS, Plomin R. The structure of language abilities at 4 years: a twin study. Dev Psychol. 2002;38:749–757. doi: 10.1037//0012-1649.38.5.749. [DOI] [PubMed] [Google Scholar]
- Concannon P, Chen WM, Julier C, Morahan G, Akolkar B, Erlich HA, Hilner JE, Nerup J, Nierras C, Pociot F. Genome-wide scan for linkage to type 1 diabetes in 2,496 multiplex families from the Type 1 Diabetes Genetics Consortium. Diabetes. 2009;58:1018–1022. doi: 10.2337/db08-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollaghan C, Campbell T. Nonword repetition and child language impairment. J Speech Lang Hear Res. 1998;41:1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Eicher JD, Powers N, Miller L, Akshoomoff N, Amaral D, Bloss C, et al. Genome-wide association study of shared components of reading disability and language impairment. Genes Brain Behav. 2013;12:792–801. doi: 10.1111/gbb.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan-Sencicek AG, Davis Wright NR, Sanders SJ, Oakman N, Valdes L, Bakkaloglu B, Doyle N, Yrigollen CM, Morgan TM, Grigorenko EL. A balanced t (10; 15) translocation in a male patient with developmental language disorder. Eur J Med Genet. 2012;55:128–131. doi: 10.1016/j.ejmg.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcaro M, Pickles A, Newbury DF, Addis L, Banfield E, Fisher SE, Monaco AP, Simkin Z, Conti-Ramsden G. Genetic and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes Brain Behav. 2008;7:393–402. doi: 10.1111/j.1601-183X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Fang S, Pinney SM, Bailey-Wilson JE, de Andrade MA, Li Y, Kupert E, You M, Schwartz AG, Yang P, Anderson MW. Ordered subset analysis identifies loci influencing lung cancer risk on chromosomes 6q and 12q. Cancer Epidemiol Biomarkers Prev. 2010;19:3157–3166. doi: 10.1158/1055-9965.EPI-10-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological working-memory – a critical building-block for reading development and vocabulary acquisition. Eur J Psychol Educ. 1993;8:259–272. [Google Scholar]
- Gialluisi A, Newbury DF, Wilcutt EG, Olson RK, DeFries JC, Brandler WM, et al. Genome-wide screening for DNA variants associated with reading and language traits. Genes Brain Behav. 2014;13:686–701. doi: 10.1111/gbb.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Keays DA, Deacon RM, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA. Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008;18:354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. Word learning as the confluence of memory mechanisms: Computational and neural evidence. In: Faust M, editor. The Handbook of the Neuropsychology of Language. Wiley-Blackwell; Chichester, UK: 2012. pp. 146–163. [Google Scholar]
- Hall J, Whalley HC, Moorhead TW, Baig BJ, McIntosh AM, Job DE, Owens DG, Lawrie SM, Johnstone EC. Genetic variation in the DAOA (G72) gene modulates hippocampal function in subjects at high risk of schizophrenia. Biol Psychiatry. 2008;64:428–433. doi: 10.1016/j.biopsych.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Hansen TV, Simonsen MK, Nielsen FC, Hundrup YA. Collection of blood, saliva, and buccal cell samples in a pilot study on the Danish Nurse Cohort: comparison of the response Ratte and quality of genomic DNA. Cancer Epidemiol Biomarkers Prev. 2007;16:2072–2076. doi: 10.1158/1055-9965.EPI-07-0611. [DOI] [PubMed] [Google Scholar]
- Harlaar N, Meaburn EL, Hayiou-Thomas ME, Davis OS, Docherty S, Hanscombe KB, et al. Genome-wide association study of receptive language ability of 12-year-olds. J Speech Lang Hear Res. 2014;57:96–105. doi: 10.1044/1092-4388(2013/12-0303). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JA, Baraitser M, Auger E, Graham F, Norell S. An extended family with a dominantly inherited speech disorder. Dev Med Child Neurol. 1990;32:352–355. doi: 10.1111/j.1469-8749.1990.tb16948.x. [DOI] [PubMed] [Google Scholar]
- Jansen A, Krach S, Krug A, Markov V, Eggermann T, Zerres K, Stocker T, Shah NJ, Nothen MM, Treutlein J, Rietschel M, Kircher T. A putative high risk diplotype of the G72 gene is in healthy individuals associated with better performance in working memory functions and altered brain activity in the medial temporal lobe. Neuroimage. 2009;45:1002–1008. doi: 10.1016/j.neuroimage.2008.12.054. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Kong A, Cox NJ. Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet. 1997;61:1179–1188. doi: 10.1086/301592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Nothen MM, Georgi A, Strohmaier J, Rietschel M, Kircher T. Genetic variation in G72 correlates with brain activation in the right middle temporal gyrus in a verbal fluency task in healthy individuals. Hum Brain Mapp. 2011;32:118–126. doi: 10.1002/hbm.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Levy ER, Hodgson S, Fox M, Jeremiah S, Povey S, Jamison DC, Green ED, Vargha-Khadem F, Monaco AP. The SPCH1 region on human 7q31: genomic characterization of the critical interval and localization of translocations associated with speech and language disorder. Am J Hum Genet. 2000;67:357–368. doi: 10.1086/303011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Luciano M, Evans D, Hansell N, Medland S, Montgomery G, Martin N, et al. A genome-wide association study for reading and language abilities in two population cohorts. Genes Brain Behav. 2013;12:645–652. doi: 10.1111/gbb.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaburn E, Dale PS, Craig IW, Plomin R. Language-impaired children: No sign of the FOXP2 mutation. NeuroReport. 2002;13:1075–1077. doi: 10.1097/00001756-200206120-00020. [DOI] [PubMed] [Google Scholar]
- Monaco AP. Multivariate linkage analysis of specific language impairment (SLI) Ann Hum Genet. 2007;71:660–673. doi: 10.1111/j.1469-1809.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Tomblin JB. Examining the comorbidity of language impairment and attention-deficit/hyperactivity disorder. Top Lang Disord. 2012;32:228–246. doi: 10.1097/TLD.0b013e318262010d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DJ, Zai CC, Shinkai T, Strauss J, Kennedy JL. Association between the DAOA/G72 gene and bipolar disorder and meta-analyses in bipolar disorder and schizophrenia. Bipolar Disord. 2011;13:198–207. doi: 10.1111/j.1399-5618.2011.00905.x. [DOI] [PubMed] [Google Scholar]
- Newbury DF, Bonora E, Lamb JA, Fisher SE, Lai CS, Baird G, Jannoun L, Slonims V, Stott CM, Merricks MJ, Bolton PF, Bailey AJ, Monaco AP. FOXP2 is not a major susceptibility gene for autism or specific language impairment. Am J Hum Genet. 2002;70:1318–1327. doi: 10.1086/339931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Cleak JD, Banfield E, et al. Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am J Hum Genet. 2004;74:1225–1238. doi: 10.1086/421529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Winchester L, Addis L, et al. CMIP and ATP2C2 modulate phonological short-term memory in language impairment. Am J Hum Genet. 2009;85:264–272. doi: 10.1016/j.ajhg.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Paracchini S, Scerri TS, Winchester L, Addis L, Richardson AJ, Walter J, Stein JF, Talcott JB, Monaco AP. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behav Genet. 2011;41:90–104. doi: 10.1007/s10519-010-9424-3. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer P, Hammill D. Test of Language Development-2: Primary. Pro-Ed; Austin, TX: 1988. [Google Scholar]
- O'Brien EK, Zhang X, Nishimura C, Tomblin JB, Murray JC. Association of specific language impairment (SLI) to the region of 7q31. Am J Hum Genet. 2003;72:1536–1543. doi: 10.1086/375403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. Clinical implications of the natural history of slow expressive language development. Am J Speech-Lang Pathol. 1996;5:5–21. [Google Scholar]
- Paul R, Looney SS, Dahm PS. Communication and socialization skills at ages 2 and 3 in late-talking young children. J Speech Lang Hear Res. 1991;34:858–865. doi: 10.1044/jshr.3404.858. [DOI] [PubMed] [Google Scholar]
- Peter B, Raskind WH, Matsushita M, Lisowski M, Vu T, Berninger VW, Wijsman EM, Brkanac Z. Replication of CNTNAP2 association with nonword repetition and support for FOXP2 association with timed reading and motor activities in a dyslexia family sample. J Neurodev Disord. 2011;3:39–49. doi: 10.1007/s11689-010-9065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluzhnikov A, Below JE, Konkashbaev A, Tikhomirov A, Kistner-Griffin E, Roe CA, Nicolae DL, Cox NJ. Spoiling the whole bunch: quality control aimed at preserving the integrity of high-throughput genotyping. Am J Hum Genet. 2010;87:123–128. doi: 10.1016/j.ajhg.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll GH, Betz SK, Miller CA. Identification of clinical markers of specific language impairment in adults. J Speech Lang Hear Res. 2010;53:414–429. doi: 10.1044/1092-4388(2009/08-0016). [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Smith SD, Gayan J. Convergent genetic linkage and associations to language, speech and reading measures in families of probands with Specific Language Impairment. J Neurodev Disord. 2009;1:264–282. doi: 10.1007/s11689-009-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Parad RB, Witt D, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- Rust J, Golombok S, Trickey G. Weschler Objective Reading Dimensions. Pyschological Corporation; Sidcup: 1993. [Google Scholar]
- Seifuddin F, Mahon PB, Judy J, Pirooznia M, Jancic D, Taylor J, Goes FS, Potash JB, Zandi PP. Meta-analysis of genetic association studies on bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2012;159:508–518. doi: 10.1002/ajmg.b.32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLI Consortium. A genomewide scan identifies two novel loci involved in specific language impairment. Am J Hum Genet. 2002;70:384–398. doi: 10.1086/338649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLI Consortium. Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am J Hum Genet. 2004;74:1225–1238. doi: 10.1086/421529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pourcain B, Skuse DH, Mandy WP, Wang K, Hakonarson H, Timpson NJ, Evans DM, Kemp JP, Ring SM, McArdle WL. Variability in the common genetic architecture of social-communication spectrum phenotypes during childhood and adolescence. Mol Autism. 2014;5 doi: 10.1186/2040-2392-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromswold K. The heritability of language: a review and metaanalysis of twin and adoption studies. Language. 2001;77:647–723. [Google Scholar]
- Tomblin JB, Zhang X. The dimensionality of language ability in school-age children. J Speech Lang Hear Res. 2006;49:1193–1208. doi: 10.1044/1092-4388(2006/086). [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Zhang X. A system for the diagnosis of specific language impairment in kindergarten children. J Speech Hear Res. 1996;39:1284–1294. doi: 10.1044/jshr.3906.1284. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X, Buckwalter P. The association of reading disability, behavioral disorders, and language impairment among second-grade children. J Child Psychol Psychiatry Allied Disciplines. 2000;41:473–482. [PubMed] [Google Scholar]
- Turner SJ, Hildebrand MS, Block S, Damiano J, Fahey M, Reilly S, Bahlo M, Scheffer IE, Morgan AT. Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. Am J Genet A. 2013;161:2321–2326. doi: 10.1002/ajmg.a.36055. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH, Monaco AP, Fisher SE. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva P, Jara L, Palomino H. Association of D16S515 microsatellite with specific language impairment on Robinson Crusoe Island, an isolated Chilean population: a possible key to understanding language development. Hum Biol. 2010;82:395–408. doi: 10.3378/027.082.0404. [DOI] [PubMed] [Google Scholar]
- Villanueva P, Newbury DF, Jara L, De Barbieri Z, Mirza G, Palomino H, Fernández M, Cazier J, Monaco A, Palomino H. Genome-wide analysis of genetic susceptibility to language impairment in an isolated Chilean population. Eur J Hum Genet. 2011;19:687–695. doi: 10.1038/ejhg.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler C, Spalek K, Aerni A, Demougin P, Müller A, Huynh KD, Papassotiropoulos A, De Quervain DJF. CPEB3 is associated with human episodic memory. Front Behav Neurosci. 2009;3:1–5. doi: 10.3389/neuro.08.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV, Ang Q, Pennell CE, Fisher SE. CNTNAP2 variants affect early language development in the general population. Genes Brain Behav. 2011;10:451–456. doi: 10.1111/j.1601-183X.2011.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski W, Hunter JV, Hanchard NA, Willer JR, Shaw C, Tian Q, et al. TM4SF20 ancestral deletion and susceptibility to a pediatric disorder of early language delay and cerebral white matter hyperintensities. Am J Hum Genet. 2013;93:197–210. doi: 10.1016/j.ajhg.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Test – Revised. American Guidance Service; Circle Pines, MN: 1987. [Google Scholar]
- Zuliani R, Moorhead TW, Job D, McKirdy J, Sussmann JE, Johnstone EC, Lawrie SM, Brambilla P, Hall J, Mcintosh AM. Genetic variation in the G72 (DAOA) gene affects temporal lobe and amygdala structure in subjects affected by bipolar disorder. Bipolar Disord. 2009;11:621–627. doi: 10.1111/j.1399-5618.2009.00731.x. [DOI] [PubMed] [Google Scholar]


