Abstract
Despite their controversial physiology, sigma-1 (σ1) receptors are intriguing targets for the development of therapeutic agents for central nervous system diseases. With the aim of providing versatile pharmacological tools to study σ1 receptors, we developed three σ1 fluorescent tracers by functionalizing three well characterized σ1 ligands with a fluorescent tag. A good compromise between σ1 binding affinity and fluorescent properties was reached, and the σ1 specific targeting of the novel tracers was demonstrated by confocal microscopy and flow cytometry. These novel ligands were also successfully used in competition binding studies by flow cytometry, showing their utility in nonradioactive binding assays as an alternative strategy to the more classical radioligand binding assays. To the best of our knowledge these are the first σ1 fluorescent ligands to be developed and successfully employed in living cells, representing promising tools to strengthen σ1 receptors related studies.
Keywords: Sigma receptors, Fluorescent ligand
1. Introduction
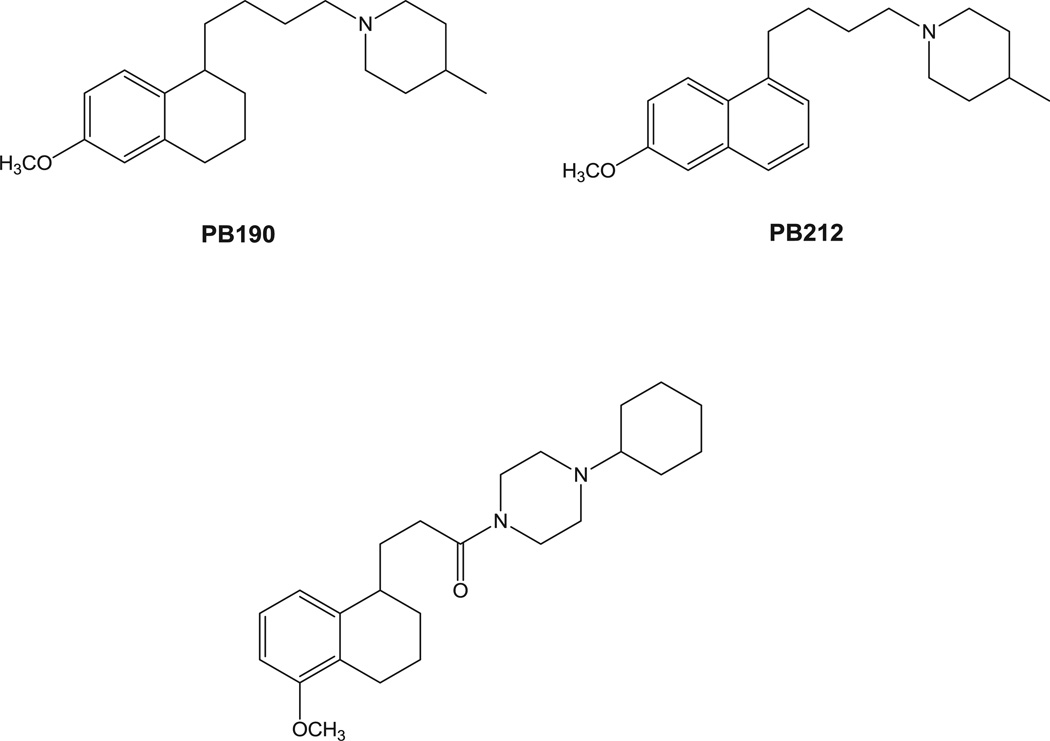
The existence of sigma (σ) receptors was first reported in 1976 when they were considered as subtypes of opiate receptors [1]. Later studies clarified that they are a unique set of proteins divided into two subtypes, namely σ1 and σ2 [2]. The σ2 subtype is the lesser known: it has not yet been cloned, and different hypotheses about its identity have been formulated [3–5]. Despite this paucity of information, interest in σ2 receptor research is on the increase because this subtype is overexpressed in several cancers and its activation through ligands leads cancer cells to death [6–9]. Worthy of note is also the efficacy of σ2 ligands in resistant tumors that shed light on novel strategies for tumor treatments [10]. On the other hand, σ1 receptor was cloned right after the two subtypes were discovered [11]. Several structures were proposed for this 223 amino-acids protein and the most accepted model represents the σ1 receptor made of three hydrophobic regions two of which are trans-membrane spanning segments. The NH2 and the COOH termini of the σ1 protein are on the same side of the cell compartment where a possible endogenous ligand, not yet identified, regulates its function [12]. Progesterone, sphingolipid-derived amines (D-erythro-sphingosine) and more recently N,N-dimethyltryptamines have been proposed as σ1 endogenous ligands [13–15]. Because of the important therapeutic potentials (e.g. treatment of anxiety, depression, schizophrenia, etc) linked to σ1 receptor targeting, interest in σ1 receptor research is on the increase [16]. Recent evidence has shown that σ1 receptor can naturally form dimers and/or oligomer and that heteromers between σ1 and D2 receptor play an important role in cocaine addiction [17,18]. These dimers/oligomers may lead to different functional forms of the σ1 receptor (such as agonist or antagonist activity). All these lines of evidence make novel pharmacological tools appealing for the study of the still controversial σ1 receptor physiology. With this aim, we produced three σ1 receptor fluorescent tracers starting from previously well characterized σ1 receptor ligands. We adopted the same strategy that was shown to be successful for σ2 fluorescent ligands: the appropriate σ1 pharmacophore was linked through a spacer to a green emitting fluorescent tag [19–21]. In particular, as σ1 pharmacophores we selected 4-methyl-1-[4-(6-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)butyl]piperidine PB190 and 4-methyl-1-[4-(6-methoxynaphthalen-1-yl)butyl]piperidine PB212 (Fig. 1) which were shown to be respectively a σ1 selective agonist and a σ1 selective antagonist by both in vitro and in vivo assays [22–26]. We also selected 4-cyclohexyl-1-[3-(5-methoxy-1,2,3,4-tetrahydronaph thalen-1-yl)propionyl]piperazine (Fig. 1) which showed excellent σ1 affinity and σ1 vs σ2 selectivity [27]. The methoxy group present in these σ1 ligands was replaced by a hexamethylenoxy chain carrying in the ω position a 4-N,N-dimethylphthalimide moiety. These choices were made on the basis of data obtained from σ2 receptor fluorescent ligands: the position on the naphthalene or tetralin ring and the nature of the linker showed to be well tolerated with 4-N,N-dimethylphthalimide conferring optimal fluorescent properties that allowed successful experiments in living cells by flow cytometry and confocal microscopy.
Fig. 1.
σ1 Receptor lead compounds exploited for the development of σ1 fluorescent ligands.
2. Results and discussion
2.1. Chemistry
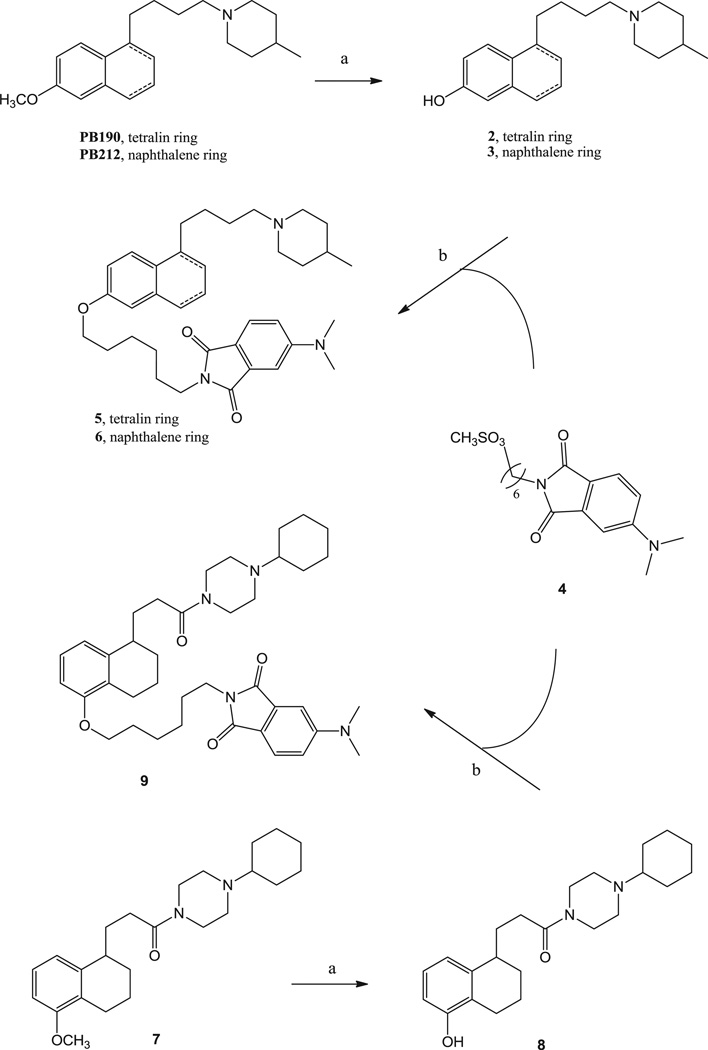
The synthetic pathway for final compounds 5, 6 and 9, is depicted in Scheme 1. The already known piperidines PB190 and PB212 [22] and the piperazine 1 [27] were demethylated by reaction with BBr3 to provide the corresponding phenolic intermediates 2, 3 and 8, although phenol 3 was previously obtained through another synthetic pathway [28]. Alkylation of the phenolic intermediates 2, 3 and 8 with the already known mesyl derivative 4 [20] provided final fluorescent compounds 5, 6 and 9. Final compounds 6 and 9 were converted into the corresponding hydrochloride salts with gaseous HCl, whereas 5 was converted into the corresponding oxalate salt.
Scheme 1.
Reagents and Conditions: (a) BBr3, CH2Cl2, from −78 °C to room temperature, overnight; (b) K2CO3, DMF, 150 °C, overnight.
2.2. σ Receptors radioligand binding
Results from binding assays are expressed as inhibition constants (Ki values) in Table 1. As in the σ2 receptors fluorescent ligands, the introduction of the fluorescent tag connected through the hexamethylenoxy linker at the 2- or 5-position of the naphthalene and tetralin ring respectively, produced a decrease in the σ1 receptor affinity with respect to the corresponding lead compounds. Only compound 5 presented affinity values at both σ receptors (Ki = 3.61 nM at the σ1, and Ki = 48.3 nM at the σ2) comparable to those of lead compound PB190 (Ki = 1.01 nM at the σ1, and Ki = 48.7 nM at the σ2). Final compounds 6 and 9 presented a 100-fold drop in the σ1 affinity (Ki = 5.20 nM and Ki = 13.1 nM, respectively) in comparison to the lead compounds PB212 and 1, respectively (Ki = 0.03 nM and Ki = 0.11 nM, respectively). By contrast, the affinity at the σ2 receptors only slightly decreased for 6 (2.5-fold) in comparison to its lead compound PB212, whereas σ2 receptor affinity of 9 was 5-fold higher than σ2 receptor affinity displayed by 1. Despite the decrease in the σ1 affinity compared to the lead compounds, the three fluorescent ligands still demonstrated strong nanomolar σ1 binding, and compounds 5 and 6 also kept a certain σ1 versus σ2 receptor selectivity (10-fold), which was lower (3-fold) in 9. Taking these data together, the strategy adopted to obtain σ2 receptor fluorescent ligands showed to be successful also for σ1 receptor fluorescent ligands.
Table 1.
σ Receptor affinities of final and lead-compounds and fluorescence properties.
| Ki nM ± SEM (nM)a | ||||||
|---|---|---|---|---|---|---|
| Compound | σ1 | σ2 | λex nmb | λem nmb | QY EtOHc |
QY CHCl3c |
| PB190d | 1.01 ± 0.41 | 48.7 ± 9.2 | ||||
| PB212d | 0.03 ± 0.013 | 17.9 ± 5.3 | ||||
| 1e | 0.11 ± 0.01 | 179 ± 56 | ||||
| 5 | 3.61 ± 0.4 | 48.3 ± 6.0 | 390 | 510 | 0.02 | 0.5 |
| 6 | 5.20 ± 0.17 | 45.6 ± 4.6 | 390 | 520 | 0.02 | 0.4 |
| 9 | 13.1 ± 5.02 | 38.4 ± 10.9 | 390 | 520 | 0.02 | 0.4 |
| DTG | 35.4 ± 5.8 | |||||
| (+)-Pentazocine | 4.52 ± 0.7 | |||||
2.3. Fluorescent ligands studies
2.3.1. Fluorescent properties
The fluorescent properties are listed in Table 1. The three compounds displayed very similar maximum excitation wavelength (λex = 390 nm) and maximum emission wavelength (λem ~ 520 nm), with an important difference between λex and λem (i.e., Stokes shift) in accordance to the spectra recorded for the σ2 ligands with the same fluorescent tag [20,21]. Measurement of quantum yields (QY) demonstrated the environment sensitive properties (low fluorescence intensity in polar solvent such as EtOH, but high fluorescence in nonpolar solvents such as CHCl3), proper for the 4-N,N-dimethylphthalimide fluorescent tag, as recorded for σ2 ligands.
2.3.2. Imaging of ARPE19 cells with compound 9 by confocal microscopy
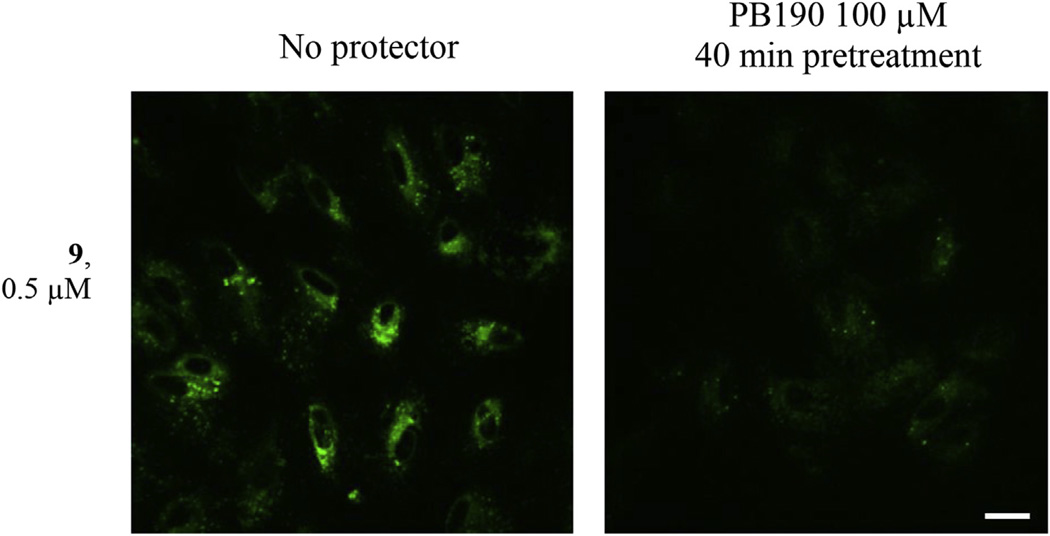
In Fig. 2 the labeling of human retinal pigment epithelia ARPE19 cells with compound 9 shows fluorescent signals in intracellular membranes. The fluorescent signals were protected by PB190, indicating the specificity of binding to σ receptors. In these same cells, the cytoprotective properties of compound 9 were assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) viability assay upon toxicity mediated by paraquat (Fig. 1, in the supporting information), making clear that compound 9 protects against such toxicity and therefore may be useful for oxidative stress treatments.
Fig. 2. Labeling of ARPE19 Cells with compound 9.
Signal is localized to intracellular membranes. Lower intensity of labeling is detected when cells were prelabeled with PB190 100 µM. Scale bar = 20 µm.
2.3.3. Detection of σ1 receptors by flow cytometry
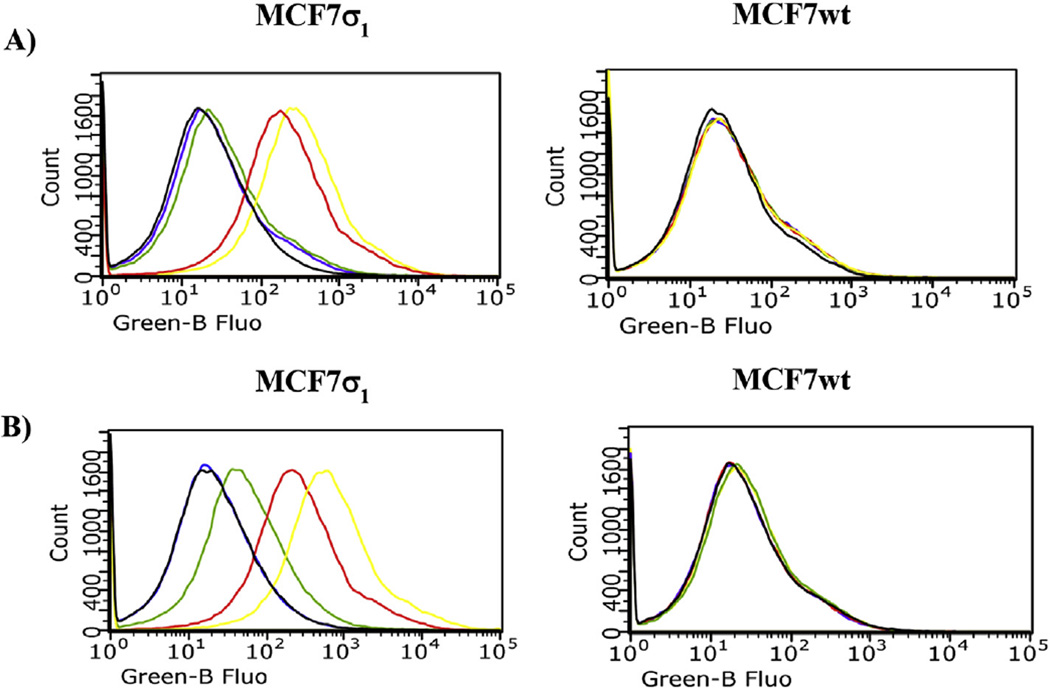
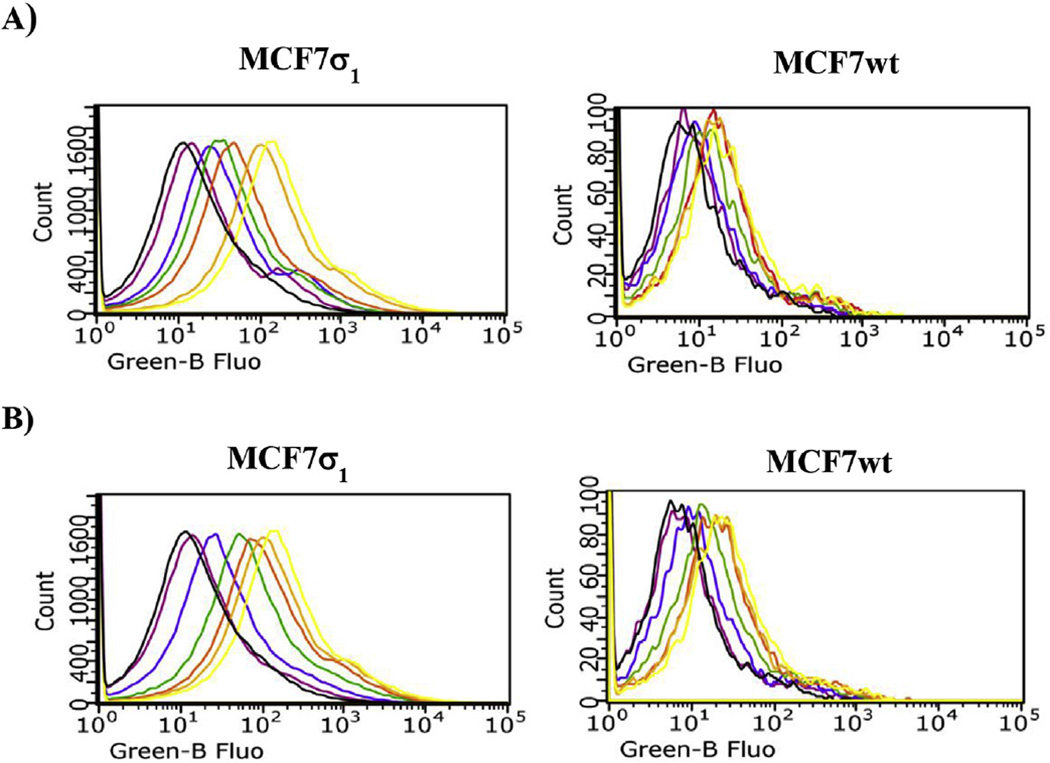
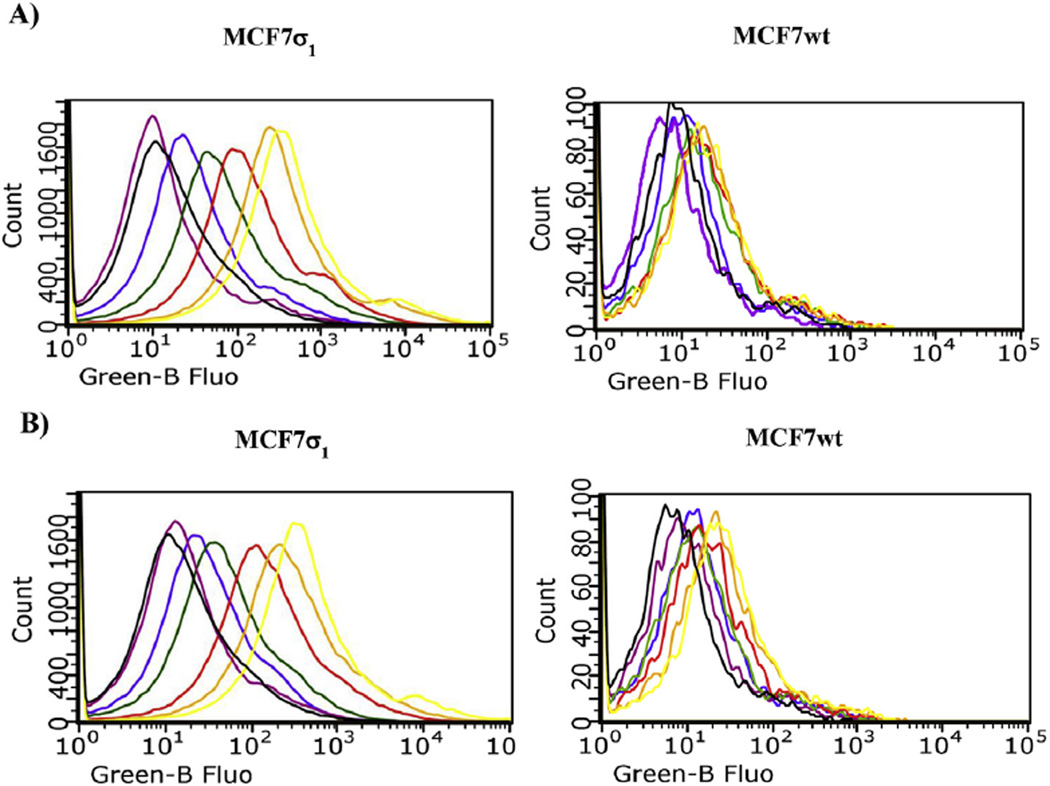
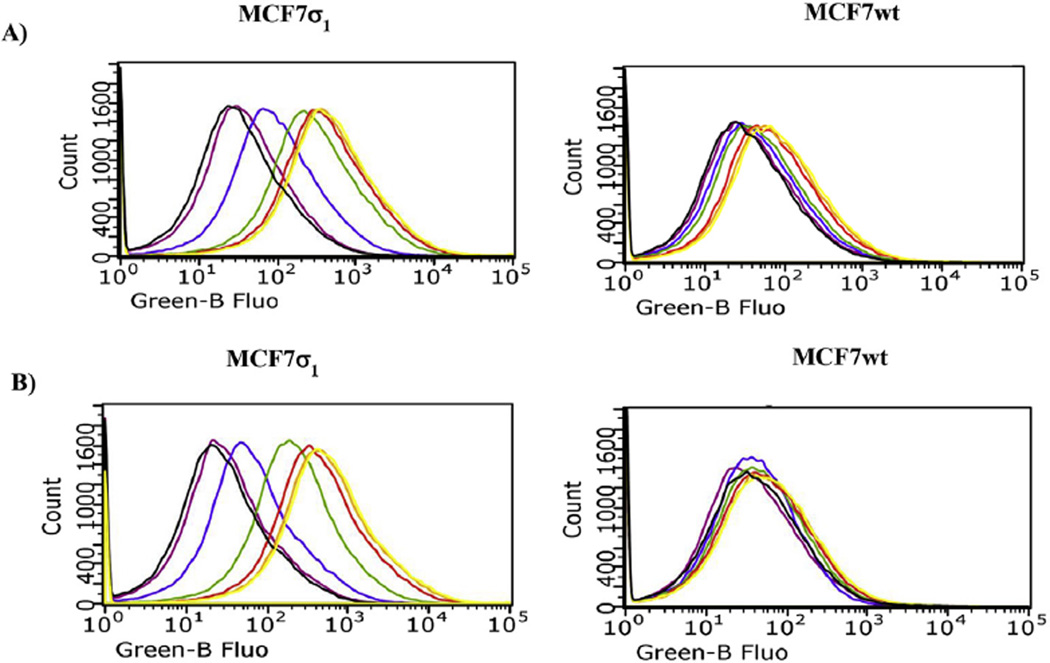
Cellular uptake of compounds 5 and 6 was studied by flow cytometry in two human breast tumor MCF7 cell lines: MCF7wt and MCF7σ1. Both these cell lines were thoroughly and previously characterized: the former over expresses σ2 receptors (Bmax = 2.02 pmol/mg protein) [5,20] whereas σ1 receptors' expression is very low (Bmax = 0.17 pmol/mg protein) [5,29]; the latter is obtained by stable transfection of MCF7 cells with σ1 c-DNA to obtain a good expression of σ1 receptors (Bmax = 3.45 pmol/mg protein) [21,29]. All of the experiments were performed by incubation of the two cell lines for 45 and 75 min with either compound 5 or 6 at 100 nM, as the fluorescence signal increased in a dose-dependent manner, reaching a plateau at 100 nM. Despite their 10-fold σ1 vs σ2 selectivity, compounds 5 and 6 still display σ2 receptor affinity (Ki = 48.3 nM for 5 and Ki = 45.6 nM for 6). Therefore, the σ2 selective ligand 2-(3-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)propyl)-5-methoxy-3,4-dihydroisoquinolin-1(2H)-one 10 [30] (Ki = 2435 nM at the σ1, and Ki = 4.24 nM at the σ2) was used at 10 µM to mask the possible fluorescent signal generated by the σ2 targeting of the fluorescent compounds. The σ1 specificity of the fluorescent tracers (100 nM) was demonstrated by comparison of the fluorescent signals by MCF7σ1 and MCF7wt, (Fig. 3) upon treatment with (+)-pentazocine and 10. In MCF7wt cells, where the fluorescence intensity was very low in each experimental condition, no change in the fluorescent signal by either 5 or 6 was detected upon administration of (+)-pentazocine or 10 (Fig. 3, right panels). In MCF7σ1 cells the administration of (+)-pentazocine (10 µM) strongly decreased the fluorescent signal by both 5 and 6 (with the signal by 5 completely abated) whereas, treatment with compound 10 (10 µM) only slightly decreased fluorescence by both the tracers. As, expected, co-administration of both (+)-pentazocine and 10 completely abated the fluorescent signals by both the tracers (Fig. 3, left panels). These results reflected the expression of σ1 receptors in the MCF7 cell lines and suggested that these tracers, administered at 100 nM, bind σ1 receptors rather specifically, with a negligible interference by σ2 receptors. In MCF7σ1, either 5 (100 nM) or 6 (100 nM) were dose dependently displaced (as demonstrated by the progressive decrease of fluorescence) by pre-incubation with increasing concentrations of either PB190 or PB212 (Figs. 4 and 5A and B; left panels; basal fluorescence of MCF7 cells in the presence of PB190 or PB212 reported in Fig. 2 in the supporting information), which were used as σ1 reference non-fluorescent compounds (from 1 nM to 10 µM). The same experiment was performed with (+)-pentazocine and a dose-dependent displacement were again recorded, with more linear results obtained by 45 min incubation time (Fig. 6). By contrast, no differences were shown between the two incubation times studied (45 min or 75 min) when 5 or 6 were displaced by PB190 and PB212. As expected from a cell line with very low amount of σ1 receptors, the displacement was far more modest in MCFwt (Figs. 4–6, A and B; right panels). The fluorescence intensity results obtained by either 5 or 6 in MCF7σ1 cells by dose-dependent displacement of the σ1 receptor ligands studied, served to generate binding curves for PB190, PB212 and (+)-pentazocine allowing the measurement of the IC50 values of these compounds for the human σ1 receptors in MCF7 cells (Table 2). Differences between radioligand binding assays performed on animal tissues and human cells homogenates have already been reported for σ1 receptors, with slightly lower binding affinities from the latter experiment [31]. The IC50 values obtained by flow cytometry on MCF7σ1 cells were in accordance with such trend, demonstrating the utility of σ1 receptors fluorescent ligand as tools to detect their presence in living cells and to conveniently performσ1 binding assays of novel ligands instead of the radioligand binding assays.
Fig. 3.
Flow Cytometry analysis (cell associated fluorescence vs cell count) of MCF7σ1 and MCF7wt, exposed to compound 5 or 6 and treated with compound 10 (10 µM) to mask σ2 receptors, or (+)-pentazocine (10 µM) to mask σ1 receptors, or both 10 (10 µM) with (+)-pentazocine (10 µM). A) Displacement of 5 (100 nM, yellow curve): black curve: control; red curve: 10 µM 10; green curve: 10 µM (+)-pentazocine; violet curve: 10 µM 10 with (+)-pentazocine 10 µM. B) Displacement of 6 (100 nM, yellow curve): black curve: control; red curve: 10 µM 10; green curve: 10 µM (+)-pentazocine; violet curve: 10 µM 10 with (+)-pentazocine 10 µM.
Fig. 4.
Flow Cytometry analysis (cell associated fluorescence vs cell count) of MCF7σ1 and MCF7wt, exposed to 5 and treated with 10 (10 µM) to mask σ2 receptors. A) Displacement of 5 (100 nM, yellow curve) with increasing concentrations of PB190: black curve: control; orange curve: 1 nM PB190; red curve: 10 nM PB190; green curve: 100 nM PB190; blue curve: 1 µM PB190; violet curve: 10 µM PB190. B) Displacement of 5 (100 nM, yellow curve) with increasing concentrations of PB212: black curve: control; orange curve: 1 nM PB212; red curve: 10 nM PB212; green curve: 100 nM PB212; blue curve: 1 µM PB212; violet curve: 10 µM PB212.
Fig. 5.
Flow cytometry analysis (cell associated fluorescence vs cell count) of MCF7σ1 and MCF7wt, exposed to 6 and treated with 10 (10 µM) to mask σ2 receptors. A) Displacement of 6 (100 nM, yellow curve) with increasing concentrations of PB190: black curve: control; orange curve: 1 nM PB190; red curve: 10 nM PB190; green curve: 100 nM PB190; blue curve: 1 µM PB190; violet curve: 10 µM PB190. B) Displacement of 6 (100 nM, yellow curve) with increasing concentrations of PB212: black curve: control; orange curve: 1 nM PB212; red curve: 10 nM PB212; green curve: 100 nM PB212; blue curve: 1 µM PB212; violet curve: 10 µM PB212.
Fig. 6.
Flow cytometry analysis (cell associated fluorescence vs cell count) of MCF7σ1 and MCF7wt, exposed to 5 or 6 and treated with 10 (10 µM) to mask σ2 receptors. A) Displacement of 5 (100 nM, yellow curve) with increasing concentrations of (+)-pentazocine: black curve: control; orange curve: 1 nM (+)-pentazocine; red curve: 10 nM (+)-pentazocine; green curve: 100 nM (+)-pentazocine; blue curve: 1 µM (+)-pentazocine; violet curve: 10 µM (+)-pentazocine. B) Displacement of 6 (100 nM, yellow curve) with increasing concentrations of (+)-pentazocine: black curve: control; orange curve: 1 nM (+)-pentazocine; red curve: 10 nM (+)-pentazocine; green curve: 100 nM (+)-pentazocine; blue curve: 1 µM (+)-pentazocine; violet curve: 10 µM (+)-pentazocine.
Table 2.
σ1 Receptor affinities of reference σ1 receptor ligands by flow-cytometry with novel σ1 fluorescent ligands.
| σ1; IC50 nM ± SEMa | ||
|---|---|---|
| compound | 5b | 6b |
| PB190c | 11.3 ± 2.4 | 12.1 ± 1.4 |
| PB212c | 15.5 ± 2.3 | 10.6 ± 1.2 |
| (+)-Pentazocined | 8.24 ± 1.2 | 7.59 ± 0.8 |
Values are the means of n ≥ 2 separate experiments, in duplicate.
IC50 values from binding curves obtained by dose dependent displacement of 5 (100 nM) or 6 (100 nM) by σ1 reference compounds (PB190, PB212 and (+)-pentazocine) in MCF7σ1 cells by flow cytometry.
Data from 75 min incubation time.
Data from 45 min incubation time. The experiments were performed in the presence of the σ2 masking agent 10 (10 µM).
3. Conclusions
Three σ1 fluorescent tracers were synthesized by functionalization of three well characterized σ1 ligands with a fluorescent tag. A good compromise between binding affinity at the σ1 subtype and fluorescent properties was obtained, with all the compounds displaying nanomolar σ1 receptor affinity. Their σ1 receptor specificities were demonstrated by protection with σ1-specific compounds in different cell lines through different techniques: confocal microscopy (compound 9) and flow cytometry (compounds 5 and 6). We also demonstrated that through the use of these fluorescent tracers in flow cytometry, σ1 binding assays of novel ligands can be conveniently performed in spite of the more classical radioligand binding assays that involves the use of radioligands such as [3H]-(+)-pentazocine. To the best of our knowledge, these compounds are the first σ1 fluorescent ligands to be produced and successfully employed, and they emerge as useful tools to empower σ1 receptors studies.
4. Experimental section
4.1. Chemistry
Both column chromatography and flash column chromatography were performed with 60 Å pore size silica gel as the stationary 300 phase (1:30 w/w, 63–200 µm particle size, from ICN and 1:15 w/w, 15–40 µm particle size, from Merck, respectively). Melting points were determined in open capillaries on a Gallenkamp electrothermal apparatus. Purity of tested compounds was established by combustion analysis, confirming a purity >95%. Elemental analyses (C, H, N) were performed on an Eurovector Euro EA 3,000 analyzer; the analytical results were within ±0.4% of the theoretical values, unless otherwise indicated.1H NMR spectra were recorded on a Mercury Varian 300 MHz using CDCl3 as solvent, unless otherwise reported. The following data were reported: chemical shift (δ) in ppm, multiplicity (s, singlet; d, doublet; t, triplet; q, quadruplet; m, multiplet), integration and coupling constants in hertz. Recording of mass spectra was done on an Agilent 6890-5973 MSD gas chromatograph/mass spectrometer and on an Agilent 1100 series LC–MSD trap system VL mass spectrometer; only significant m/z peaks, with their percentages are reported in parentheses. Chemicals were from Aldrich, TCI and Alfa Aesar and were used without any further purification.
4.2. General procedure for the synthesis of phenolic intermediates (2, 3, 8)
A solution of one among the methoxy derivatives PB190, PB212 and 1 (1.0 mmol) in anhydrous CH2Cl2 (10 mL) and under Argon was cooled to −78 °C and then added in a dropwise manner with BBr3 (3.0 mmol, 0.3 mL). The mixture was stirred overnight under argon while it slowly reached room temperature. After cooling in an ice bath, H2O was added to the mixture which was basified with Na2CO3. The resulting mixture was extracted with CH2Cl2 (3 × 10 mL) and the organic phases collected, dried (Na2SO4) and concentrated under reduced pressure to afford crude oils which provided the title compounds upon purification through column chromatography (CH2Cl2/MeOH, 95:5).
4.2.1. 5-[4-(4-Methylpiperidine-1-yl)butyl]-5,6,7,8-tetrahydronaphthalen-2-ol (2)
was obtained in 69% yield (0.204 g) as beige solid: mp = 184–186 °C; GC–MS m/z: 301 (M+, 20), 112 (100); LC-MS (ESI−) m/z: 300 [M−H]−.
4.2.2. 5-[4-(4-Methylpiperidine-1-yl)butyl]naphthalen-2-ol (3)
was obtained in 69% yield (0.202 g) as beige solid: mp = 182–184 °C (literature [28] mp = 183–185 °C); GC–MS m/z: 297 (M+, 20), 112 (100); LC-MS (ESI−) m/z: 296 [M−H]−; LC-MS-MS 296: 156.
4.2.3. 4-Cyclohexyl-1-[3-(5-hydroxy-1,2,3,4-tetrahydronaphthalen-1-yl)propionyl]piperazine (8)
was obtained in 90% yield (0.33 g) as sticky solid.1H NMR δ 1.05–1.35 [m, 6H, cyclohexyl), 1.55–2.05 (m, 10H, COCH2CH2CHCH2CH2 and cyclohexyl CH2), 2.20–2.45 (m, 3H, benzyl CH and CH2), 2.50–2.58 (m, 4H, CH2 piperazine), 2.60–2.75 (m, 2H, CH2CO), 2.80–2.85 (m, 1H, CHN), 4.38–4.70 [m, 4H, CON(CH2)2], 5.20 (broad s, 1H, OH), 6.60 (d, J = 7.15 Hz, 1H aromatic), 6.78 (d, J = 7.7 Hz, 1H, aromatic), 7.00 (t, J = 7.8 Hz, 1H, aromatic); GC–MS m/z: 370 (M+, 30), 138 (70), 125 (100), 112 (80).
4.3. General procedure for the synthesis of final fluorescent compounds (5, 6 and 9)
A solution in DMF (10 mL) of one among the phenol intermediates 2 or 3 or 8 (1.0 mmol) was added with the mesyl derivative 4 (1.0 mmol, 0.37 g) and K2CO3 (1.2 mmol, 0.16 g) to provide a mixture which was stirred at 150 °C overnight. After concentration under reduced pressure the crude residue which was taken up with water was extracted with CH2Cl2 (3 × 10 mL) and the organic phases collected were dried (Na2SO4) and evaporated under reduced pressure to provide a crude oil which was purified by column chromatography (CH2Cl2/MeOH, 95:5) to afford the title compounds.
4.3.1. 5-(Dimethylamino)-2-(6-((5-(4-(4-methylpiperidin-1-yl) butyl)-5,6,7,8-tetrahydronaphthalen-2-yl)oxy)hexyl)isoindoline-1,3-dione (5)
The title compound was obtained in a 50% yield (0.286 g) as a bright yellow oil.1H NMR δ 0.98 (d, J = 5.7 Hz, 3H, CHCH3), 1.25–2.00 [m, 19H, N(CH2)2, CH2(CH2)4, CH(CH2)3 e NH], 2.25–2.40 (m, 3H, benzyl CH and CH2), 2.60–2.70 (m, 4H, N(CHH)2 and CH2CH2CH2N), 2.85–2.95 (m, 2H, N(CHH)2), 3.10 [s, 6H, N(CH3)2], 3.62 (t, J = 7.15 Hz, 2H, (CO)2NCH2), 3.88 (t, J = 6.6 Hz, 2H, OCH2), 6.50–7.65 (m, 6H, aromatic); LC–MS (ESI+) m/z: 574 [M+H]+; LC–MS–MS 574: 203. Anal. (C36H51N3O3·C2O4H2·½H2O) C, H, N.
4.3.2. 5-(Dimethylamino)-2-{6-[(5-(4-(4-methylpiperidin-1-yl) butyl)naphthalen-2-yl)oxy]hexyl}isoindole-1,3-dione (6)
The title compound was obtained in a 50% yield (0.284 g) as a bright yellow oil.1H NMR δ 0.95–1.00 (m, 3H, NHCH3), 1.15–2.05 [m, 17H, CH(CH2)2, (CO)2NCH2(CH2)4CH2 and ArCH2(CH2)2], 2.60–2.75 [m, 2H, N(CHH)2], 2.85–2.95 [m, 2H, N(CHH)2], 2.98–3.05 [m, 4H, Ar(CH2)3CH2N], 3.10 [s, 6H, N(CH3)2], 3.65 (t, J = 7.15 Hz, 2H, (CO)2NCH2), 4.05 (t, J = 6.6 Hz, 2H, OCH2), 6.70–8.05 (m, 9H, aromatic); LC–MS (ESI+) m/z: 570 [M+H]+; LC–MS–MS 570: 203. Anal. (C36H47N3O3·HCl·1.75H2O) C, H, N.
4.3.3. 2-(6-((5-(3-(4-Cyclohexylpiperazin-1-yl)-3-oxopropyl)-5,6,7,8-tetrahydronaphthalen-1-yl)oxy)hexyl)-5-(dimethylamino) isoindole-1,3-dione (8)
The title compound was obtained in a 57% yield as a yellow oil (0.366 g).1H NMR δ 1.05–2.05 [m, 24H, piperazine CH2, OCH2(CH2)4, CH(CH2)3], 2.20–2.85 [m, 10H, CHN(CH2)2, CH2CO, benzyl CH and CH2], 3.11 [s, 6H, (NCH3)2], 3.38–3.42 [m, 2H, CON(CHH)2], 3.58-3.45 [m, 4H, (CO)2NCH2 and CON(CHH)2], 3.90 (t, 2H, J = 6.1 Hz, OCH2), 6.60–6.65 (m, 6H, aromatic); LC–MS (ESI+) m/z: 665 [M+Na]+; LC–MS–MS 665: 497, 393. Anal. (C39H54N4O4·2HCl) C, H, N.
4.4. Biology
4.4.1. Materials
(+)-Pentazocine was purchased from Sigma–Aldrich (Milan, Italy), and 1,3-di-2-tolylguanidine (DTG) from Tocris Cookson Ltd., U.K. [3H]-DTG (50 Ci/mmol), and (+)-[3H]-pentazocine (30 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Male Dunkin guinea pigs and Wistar Hannover rats (250–300 g) were from Harlan, Italy. Cell culture reagents were purchased from EuroClone (Milan, Italy).
4.4.2. Competition binding assays
All the procedures for the binding assays were previously described. σ1 And σ2 receptor binding were carried out according to Berardi et al. [27]. [3H]-DTG, 1,3-di-2-tolylguanidine (30 Ci/mmol) and (+)-[3H]-pentazocine (34 Ci/mmol) were purchased from PerkinElmer Life Sciences (Zavantem, Belgium). DTG was purchased from Tocris Cookson Ltd. (UK). (+)-Pentazocine was obtained from Sigma–Aldrich-RBI s.r.l. (Milan, Italy). Male Dunkin guinea pigs and Wistar Hannover rats (250–300 g) were from Harlan (Italy). The specific radioligands and tissue sources were, respectively: (a) σ1 receptor, (+)-[3H]-pentazocine, guinea pig brain membranes without cerebellum; (b) σ2 receptor, [3H]-DTG in the presence of 1 µm (+)-pentazocine to mask σ1 receptors, rat liver membranes. The following compounds were used to define the specific binding reported in parentheses: (a) (+)-pentazocine (73–87%), (b) DTG (85–96%). Concentrations required to inhibit 50% of radioligand specific binding (IC50) were determined using six to nine different concentrations of the drug studied in two or three experiments with samples in duplicate. Scatchard parameters (Kd and Bmax) and apparent inhibition constants (Ki values) were determined by nonlinear curve fitting, using Prism GraphPad software (version 3.0) [32].
4.4.3. Cell culture
Human breast adenocarcinoma cell line (MCF7) was purchased from ICLC (Genoa, Italy), and were stably transfected with σ1-receptor c-DNA (MCF7σ1) as already described [29]. Cells were grown in DMEM high-glucose medium supplemented with fetal bovine serum (10%), and penicillin-streptomycin (1%) in a humidified incubator at 37 °C with a CO2 atmosphere. Human retinal pigment epithelia ARPE19 cells were purchased from ATCC and grown in complete growth medium (DMEM supplemented with fetal bovine serum 10%) and penicillin-streptomycin (1%) in cell culture incubator with 5% CO2.
4.4.4. ARPE19 cell imaging
Drug treatment and imaging of ARPE cells was done in HBSS. For control, some wells were pretreated with 100 µMPB190 for 40 min. Cells were further treated with compound 9 0.5 µMfor 40 min, then rinsed twice in HBSS and imaged upon excitation wavelength of 408 nm and emission detection of 525 nm under a Nikon A1R laser confocal microscope (Nikon, Tokyo, Japan) supplied with green 488 nm Argon laser, red 561 nm DPSS laser through a Apo60X VC oil-immersion objective with NIS elements software.
4.4.5. MTT cell viability assay
ARPE19 cells were seeded to 96 well plates to a density of 30,000 cells/well and grown in complete growth medium to reach 100% confluency. Then 1 mM toxin Paraquat was applied with or without various concentrations of compound 9 and cells were incubated for 24 h in complete growth media. Then wells were quickly aspirated and MTT (Sigma–Aldrich M2128) was applied under the dim light at concentration 0.5 mg/mL in phenol red free DMEM (100 µL/well) supplemented with fetal bovine serum 0.5%. After 3 h of incubation 100 µL of 10% Sodium Dodecyl Sulfate (SDS) was added per each well. Plates were left overnight in cell culture incubator to dissolve precipitate. Absorbance at λ = 540 nm was detected in 96 well plate reader.
4.4.6. Flow cytometry studies
MCF7 and MCF7σ1 cells were incubated with increasing concentrations (0.1,1,10, and 100 nmol/L and 1 and 10 µmol/L) of either PB190 or PB212 followed by 100 nmol/L of either compounds 5 or 6 for 45 or 75 min at 37 °C. To mask σ2 receptors, compound 10 (10 µmol/L) was co-incubated. When indicated, cells were treated with increasing concentrations (1, 10, and 100 nmol/L and 1 and 10 µmol/L) of (+)-pentazocine or PB190 or PB212, followed by 100 nmol/L of compounds 5 or 6 for 45 or 75 min at 37 °C. At the end of the incubation periods, cells were washed twice with PBS, detached with 200 µL of Cell Dissociation Solution (Sigma Chemical Co.) for 10 min at 37 °C, centrifuged at 13,000 g for 5 min and resuspended in 500 µL of PBS. The fluorescence was recorded using a Bio-Guava® easyCyte™ 5 Flow Cytometry System (Millipore, Billerica, MA), with a 530 nm band pass filter. For each analysis, 50,000 events were collected and analyzed with the InCyte software (Millipore).
4.5. Fluorescence spectroscopy
Emission and excitation spectra of compounds 5, 6 and 9, were determined in CHCl3 and in PBS buffer solution as previously reported. Fluorescence quantum yields were calculated with respect to quinine sulfate as previously reported [19].
Supplementary Material
Abbreviations
- DTG
1,3-di-2-tolylguanidine
- DMEM
Dulbecco's modified Eagle's medium
- PBS
Phosphate buffered saline
- SDS
Sodium dodecyl sulfate
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2015.12.014.
References
- 1.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effect of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 2.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 3.Colabufo NA, Berardi F, Abate C, Contino M, Niso M, Perrone R. Is the σ2 receptor a histone binding protein? J. Med. Chem. 2006;49:4153–4158. doi: 10.1021/jm0600592. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, Johnston F, Spitzer D, Chang KC, Hotchkiss RS, Hawkins WG, Wheeler KT, Mach RH. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011;2:380. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abate C, Niso M, Infantino V, Menga A, Berardi F. Elements in support of the ‘non-identity’ of the PGRMC1 protein with the σ2 receptor. Eur. J. Pharmacol. 2015;758:16–23. doi: 10.1016/j.ejphar.2015.03.067. [DOI] [PubMed] [Google Scholar]
- 6.Zeng C, Rothfuss J, Zhang J, Chu W, Vangveravong S, Tu Z, Pan F, Chang KC, Hotchkiss R, Mach RH. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br. J. Cancer. 2012;106:693–701. doi: 10.1038/bjc.2011.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornick JR, Vangveravong S, Spitzer D, Abate C, Berardi F, Goedegebuure P, Mach RH, Hawkins WG. Lysosomal membrane permeabilization is an early event in sigma-2 receptor ligand mediated cell death in pancreatic cancer. J. Exp. Clin. Cancer Res. 2012;31:41. doi: 10.1186/1756-9966-31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abate C, Perrone R, Berardi F. Classes of sigma2 (σ2) receptor ligands: structure affinity relationship (SAfiR) studies and antiproliferative activity. Curr. Pharm. Des. 2012;18:938–949. doi: 10.2174/138161212799436485. [DOI] [PubMed] [Google Scholar]
- 9.Abate C, Niso M, Contino M, Colabufo NA, Ferorelli S, Perrone R, Berardi F. 1-Cyclohexyl-4-(4-arylcyclohexyl)piperazines: mixed σ and human Δ8–Δ7 sterol isomerase ligands with antiproliferative and P-glycoprotein inhibitory activity. Chem. Med. Chem. 2011;6:73–80. doi: 10.1002/cmdc.201000371. [DOI] [PubMed] [Google Scholar]
- 10.Niso M, Abate C, Contino M, Ferorelli S, Azzariti A, Perrone R, Colabufo NA, Berardi F. Sigma-2 receptor agonists as possible antitumor agents in resistant tumors: hints for collateral sensitivity. Chem. Med. Chem. 2013;8:2026–2035. doi: 10.1002/cmdc.201300291. [DOI] [PubMed] [Google Scholar]
- 11.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal A, Chu UB, Ramachandran S, Grawoig D, Guo LW, Hajipour AR, Ruoho AE. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the σ1 receptor. J. Biol. Chem. 2008;283:19646–19656. doi: 10.1074/jbc.M802192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB. Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels. Am. J. Physiol. Cell Physiol. 2011;300:328–337. doi: 10.1152/ajpcell.00383.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruoho AE, Chu UB, Ramachandran S, Fontanilla D, Mavlyutov T, Hajipour AR. The ligand binding region of the sigma-1 receptor: studies utilizing photoaffinity probes, sphingosine and N-alkylamines. Curr. Pharm. Des. 2012;18:920–929. doi: 10.2174/138161212799436584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banister SD, Kassious M. The therapeutic potential of sigma (σ) receptor for the treatment of central nervous system diseases: evaluation of the evidence. Curr. Pharm. Des. 2012;18:884–901. doi: 10.2174/138161212799436539. [DOI] [PubMed] [Google Scholar]
- 17.Chu UB, Ramachandran S, Hajipour AR, Ruoho AE. Photoaffinity labeling of the sigma-1 receptor with N-[3-(4-nitrophenyl)propyl]-N-dodecylamine: evidence of receptor dimers. Biochemistry. 2013;52:859–868. doi: 10.1021/bi301517u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro G, Moreno E, Bonaventura J, Brugarolas M, Farré D, Aguinaga D, Mallol J, Cortés A, Casado V, Lluis C, Ferre S, Franco R, Canela E, McCormick PJ. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS One. 2013;8:61245. doi: 10.1371/journal.pone.0061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abate C, Hornick JR, Spitzer D, Hawkins WJ, Niso M, Perrone R, Berardi F. Fluorescent derivatives of σ receptor ligand 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)-propyl]piperazine (PB28) as a tool for uptake and cellular localization studies in pancreatic tumor cells. J. Med. Chem. 2011;54:5858–5867. doi: 10.1021/jm200591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abate C, Niso M, Marottoli R, Riganti C, Ghigo D, Ferorelli S, Ossato G, Perrone R, Lacivita E, Lamb DC, Berardi F. Novel derivatives of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) with improved fluorescent and σ receptors binding properties. J. Med. Chem. 2014;57:3314–3323. doi: 10.1021/jm401874n. [DOI] [PubMed] [Google Scholar]
- 21.Niso M, Riganti C, Pati ML, Ghigo D, Berardi F, Abate C. Novel and selective fluorescent σ2-receptor ligand with a 3,4-dihydroisoquinolin-1-one scaffold: a tool to study σ2 receptors in living cells. Chem. Bio. Chem. 2015;16:1078–1083. doi: 10.1002/cbic.201402712. [DOI] [PubMed] [Google Scholar]
- 22.Berardi F, Ferorelli S, Abate C, Pedone MP, Colabufo NA, Contino M, Perrone R. Methyl substitution on the piperidine ring of N-[ω-(6-methoxynaphthalen-1-yl)alkyl] derivatives as a probe for selective binding and activity at the σ1 receptor. J. Med. Chem. 2005;48:8237–8244. doi: 10.1021/jm050654o. [DOI] [PubMed] [Google Scholar]
- 23.Gasparre G, Abate C, Berardi F, Cassano G. The sigma-1 receptor antagonist PB212 reduces the Ca2+-release through the inositol (1,4,5)-trisphosphate receptor in SK-N-SH cells. Eur. J. Pharmacol. 2012;684:59–63. doi: 10.1016/j.ejphar.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Skuza G, Szymanska M, Budziszewska B, Abate C, Berardi F. Effects of PB190 and PB212, new σ receptor ligands, on glucocorticoid receptor-mediated genetranscription in LMCAT cells. Pharmacol. Rep. 2011;63:1564–1568. doi: 10.1016/s1734-1140(11)70722-8. [DOI] [PubMed] [Google Scholar]
- 25.Skuza G, Sadaj W, Kabzinski M, Cassano G, Gasparre G, Abate C. The effects of new sigma (σ) receptor ligands, PB190 and PB212, in the models predictive of antidepressant activity. Pharmacol. Rep. 2014;66:320–324. doi: 10.1016/j.pharep.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Moritz C, Berardi F, Abate C, Peri F. Live imaging reveals a new role for the sigma-1 (σ1) receptor in allowing microglia to leave brain injuries. Neurosci. Lett. 2015;591:13–18. doi: 10.1016/j.neulet.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Berardi F, Abate C, Ferorelli S, Uricchio V, Colabufo NA, Niso M, Perrone R. Exploring the importance of piperazine N-atoms for σ2 receptor affinity and activity in a series of analogs of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) J. Med. Chem. 2009;52:7817–7828. doi: 10.1021/jm9007505. [DOI] [PubMed] [Google Scholar]
- 28.Gao M, Wang M, Hutchins GD, Zheng QH. Synthesis of carbon-11-labeled piperidine ring of N-[ω-(6-methoxynaphthalen-1-yl)alkyl] derivatives as new selective PET σ1 receptor probes. Appl. Radiat. Isot. 2010;68:459–465. doi: 10.1016/j.apradiso.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Abate C, Ferorelli S, Niso M, Lovicario C, Infantino V, Convertini P, Perrone R, Berardi F. 2-Aminopyridine derivatives as potential σ2 receptor antagonists. Chem. Med. Chem. 2012;7:1847–1857. doi: 10.1002/cmdc.201200246. [DOI] [PubMed] [Google Scholar]
- 30.Abate C, Selivanova SV, Müller A, Krämer SD, Schibli R, Marottoli R, Perrone R, Berardi F, Niso M, Ametamey SM. Development of 3,4-dihydroisoquinolin-1(2H)-one derivatives for the Positron Emission Tomography (PET) imaging of σ2 receptors. Eur. J. Med. Chem. 2013;69:920–930. doi: 10.1016/j.ejmech.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Berardi F, Abate C, Ferorelli S, Colabufo NA, Perrone R. 1-Cyclohexylpiperazine and 3,3-dimethylpiperidine derivatives as sigma-1 (σ1) and sigma-2 (σ2) receptor ligands: a review. Cent. Nerv. Syst. Agents Med. Chem. 2009;9:205–219. doi: 10.2174/1871524910909030205. [DOI] [PubMed] [Google Scholar]
- 32.Prism Software, Version 3.0 for Windows. San Diego, CA: GraphPad Software, Inc; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.