Abstract
The community of microorganisms within the human gut (or microbiota) is critical to health and functions with a level of complexity comparable to an organ system. Alterations of this ecology (or dysbiosis) has been implicated in a number of disease states, the prototypical example being Clostridium difficile infection (CDI). Fecal microbiota transplantation (FMT) has been demonstrated to durably alter the gut microbiota of the recipient and has shown efficacy in the treatment of recurrent CDI. There is hope that FMT may eventually prove beneficial for treatment of other disease associated with alterations in gut microbiota, such as inflammatory bowel disease, irritable bowel syndrome and the metabolic syndrome, to name a few. Although the basic principles that underlie the mechanisms by which FMT demonstrates therapeutic efficacy in CDI are becoming apparent, further research is needed to understand the possible role of FMT in these other conditions. Though relatively simple to perform, questions regarding both short- and long-term safety, as well as the complex and rapidly evolving regulatory landscape has limited widespread utilization. Future work will focus on establishing best practices and more robust safety data than exist currently, as well as refining FMT beyond current “whole stool” transplants to increase safety and tolerability. Encapsulated formulations, full spectrum stool-based products and defined microbial consortia are all in the immediate future.
Keywords: FMT, fecal transplant, gut microbial therapeutics
Introduction
It is now understood that the gut microbiota coevolved with the human host over thousands of years and are integral to overall human physiology, playing a pivotal role in metabolism, immune system function and maintenance of gut homeostasis.1, 2 Fecal microbiota transplantation (FMT) involves administration of fecal material containing distal gut microbiota from a healthy individual (donor) to a patient with a disease or condition related to dysbiosis, or an alteration in their “normal” gut microbiota. The goal of FMT is to treat disease by restoring phylogenetic diversity and microbiota more typical of a “healthy” individual.
Though previously considered fringe-therapy and the “last resort” for patients with C. difficile infection (CDI), FMT has become more widely practiced, and interest around FMT among patients, researchers and industry has surged over the past 2–3 years. Numerous case reports, retrospective case series and a single randomized controlled trial have demonstrated benefit of FMT in patients with severe or recurrent CDI with cure rates as high as 100% and a mean cure rate of 87–90% for the over 500 cases reported in the world literature to date.3–5 Moreover, the restoration of more typical microbial communities, which come to resemble those of the donor post-transplant, persists in a durable fashion after FMT.6, 7 This has resulted in speculation that FMT may eventually prove beneficial in other conditions associated with dysbiosis, such as inflammatory bowel disease (IBD), the metabolic syndrome, and many others. This overview will describe the history of FMT, basic methodologies, and the potential mechanisms of effect in CDI and other diseases. We will present efficacy data, including a review of the real and theoretical risks of the procedure. In addition, this overview provides a discussion of the future of microbial-based therapeutics and the complex regulatory issues around this rapidly evolving field.
History of FMT
Fecal transplantation dates back to 4th century China where human fecal suspension by mouth was used to cure food poisoning and severe diarrhea8 and has been widely used in veterinary medicine to treat ruminal disorders since the 17th century.9 Anecdotal reports of the use of parental feces to treat antibiotic-associated diarrhea in children had existed, but these cases were largely unknown until the publication of the first case series in 1958 of four patients with pseudomembranous enterocolitis by the American surgeon, Ben Eiseman.10 For years, FMT remained a rarely used, if not forgotten, therapy. The first documented case of confirmed CDI treated with FMT was reported in 1983.11 Since that time, a growing number of case series and a single randomized controlled trial4 have described the administration of donor stool using various modalities to successfully treat patients, mostly with recurrent or refractory CDI.
Driven by an epidemic of increasingly virulent and severe C. difficile infections12, 13, our greater understanding of the human gut microbiome, and favorable headlines in the media, the practice of FMT has recently shown a significant increase in utilization. Some of this interest may be because the perceived “natural” properties of FMT make it appealing to both physicians and the lay public. We have also come to realize that FMT enables use of a logical, low tech, and relatively inexpensive approach to effectively treat a difficult clinical problem. Despite overwhelmingly positive anecdotal experience from the growing number of physicians who have performed this procedure, and evidence from hundreds of published cases, FMT is not yet universally available, though its acceptance is growing. The apparently high efficacy of FMT in treating CDI, compelling animal data on the impact of fecal microbiota in metabolism,14 and case reports describing successful FMT for treatment of other intestinal disorders, has led to growing interest in the potential of FMT to treat other conditions associated with dysbiosis such as the metabolic syndrome, obesity, food allergies, IBD and irritable bowel syndrome (IBS). A number of clinical trials studying FMT for these conditions and others are ongoing15.
Current Treatment Guidelines and Methods
Indications
In 2010, members of various specialty societies with an interest in FMT formed a working group for the purpose of creating a consensus on FMT for practitioners. As outlined by the workgroup,16 the primary indications for FMT are:
- Recurrent or relapsing CDI:
- Three or more episodes of mild-to-moderate CDI and failure of a 6–8 week taper with vancomycin with or without an alternative antibiotic (e.g., rifaximin, nitazoxanide or fidaxomicin).
- At least two episodes of CDI resulting in hospitalization and associated with significant morbidity.
Moderate CDI not responding to standard therapy (vancomycin or fidaxomicin) for at least a week.
Severe (even fulminant CDI) with no response to standard therapy after 48 hours.
The 2013 American College of Gastroenterology Clostridium difficile treatment guidelines also recommend FMT as a therapeutic alternative for recurrent cases of CDI which have failed to respond to a pulse/tapered regimen of vancomycin.17 The evidence supporting FMT for treatment of severe, complicated disease (e.g. toxic megacolon) is less robust, with fewer published cases, however a few case reports suggest that it may be safe and effective even in critically ill patients18–21. Certainly patients with severe CDI are at greater risk for poor outcomes and decisions to proceed with FMT, versus surgery or other standard of care modalities, should be made with caution. In all cases, primary consideration must be given to the severity and progression of the patient’s CDI when deciding whether early use of FMT is appropriate to prevent further clinical deterioration.
Donor selection
The donor may be an intimate, long-time partner, friend, or unrelated volunteer. For the purposes of informed consent, donors should be over the age of 18. However, children could also potentially serve as donors as long as both parental consent and child assent (i.e. agreement to serve as a donor) are obtained. At this time, limited data are available to suggest that any factors other than specific exclusion criteria based on medical history and laboratory testing would endorse a particular donor as optimal.
A number of advantages and disadvantages may be considered during donor selection. Intimate contacts (e.g., spouse) have the advantage of shared environmental risk factors, which may minimize the risk of transmitting an infectious agent. Maternal-line first-degree relatives may have a theoretical advantage of sharing the greatest number of microbial species in their intestinal microbiota with the recipient. Therefore, it is conceivable that adaptive immune elements in the mucosal immune system (e.g., antigen-specific antibodies) may be more tolerant of microbiota derived from such donors. Similarly, it is possible to speculate that men might be preferred donors over women as women may, theoretically, harbor microbiota that are more likely associated with autoimmune disease and IBS. Others have hypothesized that age- and gender-matched donors may be advantageous, although currently there is no data to support this hypothesis. Finally, there may be advantages in using unrelated, healthy, but rigorously screened donors, particularly when FMT is being used to treat diseases where genetics play a contributing role, such as IBD. Availability of a healthy, pre-screened donor pool may facilitate broader ability to perform FMT. These unrelated volunteer donors may even be preferable, as family members may feel coerced to donate and deny relevant infectious risk factors. Indeed, directed blood donors have higher viral marker rates than volunteer donors.22 Furthermore, as intestinal microbiota have recently been theorized to potentially be involved in the pathogenesis of a number of systemic diseases, rigorously screened, healthy volunteer donors may have advantages, especially for young patients, who may acquire additional risk factors for disease over their lifetime.
Donor Screening
Potential donors should be screened for behaviors which may confer increased risk for transmitting infection (e.g. injection drug use). Current guidelines16 recommend using a donor questionnaire that is similar to current protocols for screening blood donors, (see AABB Donor History Questionnaire Documents available at http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/ucm164185.htm Furthermore, donors should be free of diseases or conditions which may, even theoretically, be transmissible by stool. Those who meet eligibility criteria should have serologic and stool testing to screen for infectious agents, preferably within 4 weeks of donation. The donor testing described in the first two columns of Table 1 should be considered minimal and testing for additional pathogens, as also detailed, may be considered in certain clinical situations, such as when the recipient is immunocompromised or in cases of potential donor exposures.
- Donor exclusion criteria:
- A history of antibiotic treatment during the 3 months preceding donation
- A history of intrinsic gastrointestinal (GI) illnesses, including IBD, IBS, chronic constipation, Gl malignancies or major GI surgical procedures
- A history of autoimmune or atopic illnesses or ongoing immune modulating therapy
- A history of chronic pain syndromes (fibromyalgia, chronic fatigue) or of neurologic or neurodevelopmental disorders
- Metabolic syndrome, obesity (body mass index >30), or moderate-to-severe malnutrition
- A history of malignant illnesses or ongoing oncologic therapy
Table 1.
Suggested Donor Testing
| Serologic | Stool | Consider | Possibly |
|---|---|---|---|
| HAV-IgM | C. difficile toxin B (preferably by PCR) | Giardia | CMV |
| HBsAg | Culture for enteric pathogens | Cryptosporidium | Human T cell lymphoma virus |
| anti-HCV-Ab | O+P, if travel history suggests | Isospora and Cyclospora | EBV |
| HIV 1 & 2-EIA | E. coli O157 | Dientamoeba fragilis | |
| RPR | Rotavirus | Blastocystis hominus | |
| Listeria | Strongyloides stercoralis | ||
| Vibrio | Entamoeba histolytica | ||
| Norovirus | Helicobacter pylori | ||
| Schistosoma | |||
| JC virus | |||
| VRE | |||
| MRSA |
Stool Preparation and Methods of Administration
The material must be diluted and homogenized to a form that can be administered. Published data do not demonstrate a significant difference in success with FMT whether the donor stool is mixed in tap water, milk, or normal saline (sterile, nonbacteriostatic), although the latter is presumed to be less likely to affect the microbiota of the donor specimen. In general, the donor specimen is homogenized (using blender, manual effort, or other method), and filtered if necessary (e.g., gauze, coffee filter, strainer). This processed specimen is then either directly infused into the GI tract or further centrifuged, placed into gelatin capsules, and swallowed. Several series have described freezing the fecal microbiota, which can then be thawed for later use.7, 23
As with preparation of the stool, there is no clear consensus on the best method of instillation. Routes of administration have included the upper GI tract (via endoscopy, nasogastric/nasointestinal tubes, or ingestion of pills,4, 23–29 the proximal colon by colonoscopy,6, 7, 30–35 or the distal colon by enema, rectal tube, or sigmoidoscopy36–40 or a combined approach41. Nasogastric or nasointestinal routes may be uncomfortable and less appealing to the patient, require radiology assistance to confirm tube placement and carries some risk of vomiting and aspiration. Retention enema is inexpensive, with little procedural risk, but it may be difficult for some patients to retain the donor material and it may require multiple treatments. Endoscopic routes of administration are well tolerated and have the advantage of allowing examination of the colonic mucosa, and exclusion of pathology, such as IBD, which may be contributing to the patient’s symptoms and recurrent CDI. Endoscopic delivery carries some procedural risk and increases health care utilization and costs, though a cost-effectiveness study showed FMT dominated (i.e. was less costly and more effective) than vancomycin for initial CDI42. FMT is reported to be effective by all of these routes, and the preferred method may vary with the clinical situation. Less invasive methods, such as retention enema or nasointestinal infusion may be safer in patients who are frail or severely ill at the time of FMT. Ileus precludes the upper GI route of administration.
Efficacy data in CDI
FMT has been examined in both young and old, patients with limited comorbidities as well as those considered to be immunocompromised,43 and the procedure has been shown to be safe, well-tolerated, and effective. The role of FMT for the treatment of CDI in specific situations (e.g., toxic megacolon) is as yet unclear; however, of the over 500 cases reported to date3, FMT has demonstrated rapid response and a cure rate of nearly 90%, with a negligible significant adverse event rate, regardless of route. Cumulative experience is largely based on data from case reports and series. There has, to date, been only a single randomized controlled trial4. This study, conducted in the Netherlands, showed duodenal infusion of donor feces to effectively resolve recurrent CDI in 81% of patients treated, compared to only 31% efficacy of a standard course of oral vancomycin. Furthermore, FMT appeared safe, with no serious adverse events in these subjects. This study was terminated early by the safety monitoring board because it was deemed unethical to continue subjecting patients to the inferior treatment. In a systematic review, Cammarota et al. observed that lower gastrointestinal route (colonoscopy, enema) led to the achievement of higher eradication rates than upper delivery (gastroscopy, nasogastric or nasointestinal tube) (81–86% vs 84–93%, respectively)3. Conversely, a small, open label pilot study comparing upper and lower GI routes of administration did not show any differences44 A recent open-label, single group study23 at Massachusetts General Hospital assessed the efficacy of fecal material from healthy donors administered as 15 frozen pills on two consecutive days in patients with relapsing CDI. They reported an overall 90% response rate making oral administration a viable alternative to the current practice of administering fecal material via an endoscopic procedure thus decreasing both cost and potential procedural complications.
Mechanisms
C. difficile is an opportunistic organism, which generally causes disease in persons with decreased diversity of the gastrointestinal microflora (typically as a result of antibiotic use). Indeed, studies have demonstrated that patients suffering from recurrent CDI are deficient in phyla of bacteria normally dominant in the colon6,45 (Figure 1). FMT represents one of the most efficacious treatments available for recurrent CDI, though the exact mechanism of effect still remains to be elucidated.
Figure 1.
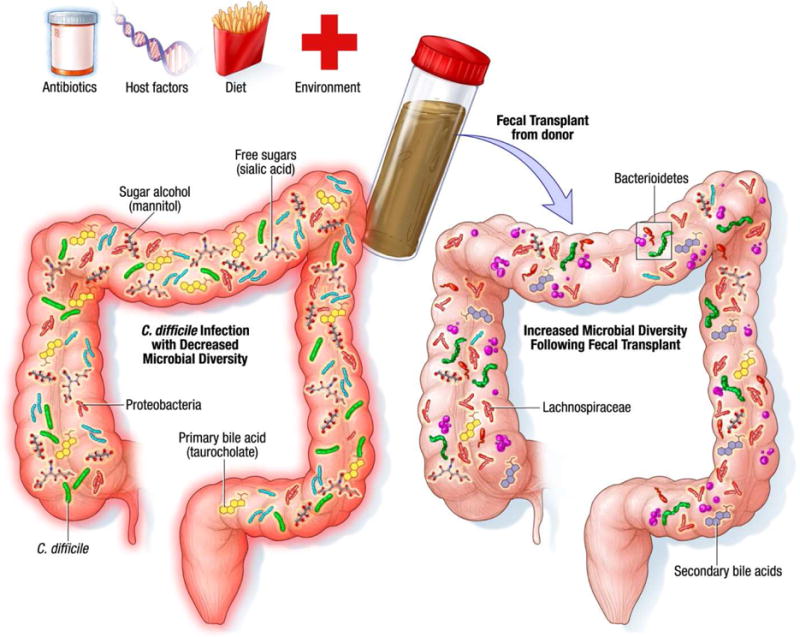
Mechanisms underlying successful treatment of recurrent CDI with FMT Improvement in symptoms following fecal microbiota transplant has been associated with change in microbial community structure such as a decrease in Proteobacteria as well as restoration of microbial diversity, increase in secondary bile acid production and niche exclusion by other bacteria.
The greatest risk factor for CDI is the use of antibiotics,46 though only a small proportion of patients on antibiotics develop the infection. Antibiotic use has been associated with alterations in both gut microbial community structure and function. An early study investigating the effect of antibiotics on gut microbiota function described decreased urobilinogen and fecal tryptic activity as well as decreased conversion of cholesterol to coprostanol following antibiotic treatment.25 Interestingly these changes were modified following fecal transplant from healthy individuals with increased appearance of coprostanol and urobilinogen.25 Several studies have since identified the effect of antibiotics on gut microbiome using sophisticated methodology and next-generation sequencing. Dethlefsen et al. investigated the effect of ciprofloxacin in three individuals and found reduced microbial taxonomic diversity, richness and evenness, although the magnitude of effect differed among individuals.47 A more recent study in mice treated with the antibiotic cefoperazone suggests that antibiotic treatment alters the fecal metabolome related to microbial function.48 The mice exhibited an increase in primary bile acids such as taurocholate, an increase in sugar alcohols mannitol and sorbitol, a decrease in free short-medium- and long-chain fatty acids, an increase in amino acids such as glycine, proline, cysteine, isoleucine, and a decrease in branched chain fatty acids, all conditions which favor C. difficile growth.48 Differences in susceptibility to infections such as CDI following antibiotic treatment may relate to differential effects of antibiotics across different individuals microbiota, to host factors, and to the type of antibiotic. While antibiotics are a major risk factor for CDI, community acquired CDI is becoming increasingly common in the absence of antibiotic use.49 There is little information on the gut microbiome in this population, though one may speculate that, in addition to host features, other factors such as diet, environment, and altered GI function may decrease microbial diversity and alter microbial function to favor growth of C. difficile.
With the advent of next-generation sequencing, we can now identify specific taxonomic changes that occur after FMT to better understand how the transplant influences gut microbial ecology. FMT appears to result in durable engraftment of new species from donor as well as augmentation of species present at only very low levels prior to FMT6. The mechanisms underlying decreased fitness of C. difficile following FMT still remain elusive but likely include niche exclusion, competition for nutrients and creation of a nutrient milieu unfavorable for growth, ability of members of healthy gut microbiota to produce antimicrobials that inhibit growth of C. difficile and an increase in secondary bile acid production. An important factor determining success of fecal transplant is restoration of microbial diversity following treatment50. Additionally, changes in microbial community structure such as restoration of key Firmicutes and Bacteroidetes species with a decrease in Proteobacteria appear to be required in order to outcompete C. difficile.50 Decrease in Lachnospiraceae has been associated with severe CDI suggesting a protective role for members of this family51 and administration of a cocktail containing a member of Lachnospiraceae was reported to cure CDI in mice52. Furthermore successful FMT restores members of Lachnospiraceae and other butyrate producing organisms supporting their potential role in outcompeting C. difficile.50 Bacillus thuringensis secretes a bacteriocin, thuricin CD, with narrow spectrum activity against gram positive bacteria including C. difficile; it is likely other members of the gut microbiota secrete similar antimicrobials.53 Bile acid composition can significantly impact C. difficile growth with primary bile acids such as taurocholate favoring germination of C. difficile spores. Weingarden and colleagues reported an increase in secondary bile acids following FMT compared to pre-transplant stool samples.19 A more recent study by Butte et al. showed treatment with either a consortium of bacteria or C. scindens both of which harbor the gene encoding 7-hydroxysteroid dehydrogenase required for secondary bile acid synthesis can ameliorate CDI in mice.54
FMT in Diseases Other than Clostridium difficile
FMT for Inflammatory Bowel Disease
Although the microbial basis of IBD is far more complex and variable than that of recurrent/refractory CDI, microbiome-based therapies are an important area of investigation for these chronic and debilitating diseases. As early as the 1900s, physicians recognized that bacteria may be playing a pivotal role in colitis.55 With over a century of research and progress, we are just beginning to understand the microbiological basis for IBD and how FMT and other microbial therapeutics may be useful for IBD.
Advanced molecular techniques have demonstrated fundamental differences in both microbial composition and function in patients with IBD.56–58 The dysbiosis is characterized by a decreased diversity at the species level, with notable decreases in the Bacteroidetes phylum and the Lachnospiraceae group within the Firmicutes phylum and increases in Proteobacteria and Actinobacteria.59 Studies have also shown lower levels of the protective Faecalibacterium prausnitzii, which has been associated with anti-inflammatory properties in patients with Crohn’s diseases.60
Conventional whole-stool FMT offers an untargeted approach to modify the underlying dybiosis in IBD. Preliminary case reports of FMT enemas in patients with ulcerative colitis (UC) and Crohn’s disease (CD) were promising, reporting that patients achieved clinical remission, and maintained remission over long-term follow-up in many of the cases61, 62 A small number of these case reports also reported endoscopic and histologic remission.63 These reports were followed by a number of small studies of FMT in children and adults with UC, CD, and pouchitis with mixed results.64–72 Unfortunately, it is difficult to infer robust conclusions about the safety and efficacy of FMT for IBD from these studies because they were under-powered, open-labeled, lacked uniformity in treatment protocols and delivery approaches, and did not include control groups. In addition, the patient populations for each study varied in disease type, phenotype, severity, and concomitant medications. Also important is the fact that although the donors were screened, they were not otherwise standardized or well-characterized.
A recent systematic review and meta-analysis of 18 studies that included 122 patients with IBD who received FMT found a 45% clinical remission rate.73 However, the remission rate fell to 36.2% when the case series were excluded in order to minimize publication bias. The pooled estimates were highest in younger patients (7–20 years old) and in patients with Crohn’s disease, with 64.1% and 60.5% achieving clinical remission, respectively. These positive result were largely driven by results at two centers with short follow up after FMT64, 74. UC patients were much less likely to achieve clinical remission (22%). This review also found that although FMT was not associated with any serious adverse events during short-term follow up, a number of patients experienced fever, chills, and gastrointestinal symptoms including bloating, flatulence, vomiting, diarrhea, and abdominal tenderness.73 Some UC patients worsened in one study of FMT71. Furthermore, flares of FMT in patients with both UC and CD have been described,43, 75, 76 raising important questions around safety and the potential to worsen disease in some patients.
Two randomized placebo-controlled trials of FMT in IBD were recently published.77, 78 In the study by Moayyedi et. al, seventy-five patients with active UC were randomized to a weekly FMT or water enema for 6 weeks, and there was a statistically significant difference in remission, the primary outcome, between the two groups. Remission (full Mayo score <3 and complete mucosal healing) was achieved in 24% of patients after FMT and 5% with placebo; stool from patients receiving FMT had greater microbial diversity than that of patients given placebo. The second study, conducted in Amsterdam78, enrolled 50 patients with mild to moderately active UC and randomized them to donor feces or autologous fecal transplant via naso-duodenal tube. FMT was administered at the start of the study and again 3 weeks later. Only 37 completed assessment for the primary endpoint, clinical remission combined with ≥1 point decrease in the Mayo endoscopic score at week 12). There was no statistically significant difference in clinical and endoscopic remission between the two groups. It is likely that this study was underpowered to detect these differences.
These initial mixed results do not rule out a possible role of FMT or other gut-microbiota products in the management of IBD. A variety of factors may influence outcome, including patients’ IBD characteristics, donor variability, dosage or frequency of FMT, and concomitant medical therapy. Future trial designs may position FMT after induction of remission instead of in the presence of clinically active inflammation. In addition, it is likely that in the future FMT will be replaced by specific manipulation and/or selective transplantation of defined microbial communities that can help restore a healthy enteric commensal community and, hopefully, alter the natural history of IBD.
Obesity
Obesity is recognized as a global epidemic79. The lack of effective, non-surgical therapies has led to investigation of potential factors contributing to the development of obesity. Several lines of evidence support the role of gut microbiota in obesity. Lean and obese individuals show marked differences in the gut microbiome.80 Transfer of gut microbiota from lean and obese individuals can recapitulate the metabolic phenotype in ex-germ free mice.14 Antibiotic treatment in early life predisposes to obesity in animal models81 and finally transfer of gut microbiota following roux-en-y gastric bypass to germ free mice leads to weight loss compared to microbiota from sham surgery.82 The exact mechanisms by which gut microbiota influence obesity still remain to be elucidated, though recent data from animal models provides valuable insight. Gut microbiota can ferment dietary carbohydrates and provide additional energy to the host in the form of short chain fatty acids. However, microbiota from lean mice produce greater amounts of short chain fatty acids than those from obese mice, suggesting the increased weight gain is not simply from increased energy harvest but rather an effect on the gut neuro-hormonal axis increasing energy expenditure or pathways affecting satiety.83 A recent double blind randomized controlled study investigated the effect of transfer of gut microbiota from lean individuals to obese individuals and found improved insulin sensitivity and increased gut microbial diversity with increased butyrate-producers following transplant.84 This pilot study is proof of principle for future randomized controlled trials using fecal transplant or defined microbial consortia for treatment of obesity, metabolic syndrome and diabetes.
Irritable bowel syndrome
IBS is a widely prevalent chronic GI disorder affecting nearly 20% of the North American population85 and is associated with significant morbidity and health care expenditure. The underlying pathophysiology of symptoms in IBS is not well defined though both central and perpipheral mechanisms have been implicated. Several studies have found alterations in the gut microbiome in patients with IBS.86–89 However, changes in microbiota composition are not consistent across studies, possibly due to heterogeneous study populations and differing methodologies for sample preparation and analysis. A consistent finding has been decreased microbial diversity, a finding which is reminiscent of alterations in patients with CDI, IBD and obesity. Additionally, recent studies suggest that changes in the gut microbiota may be responsible for underlying mechanisms associated with IBS such as visceral hypersensitivity, altered barrier function, gastrointestinal motility and the gut-brain axis90–93. Thus, gut microbiota is an important target for study in the treatment of functional disorders such as IBS, and, in fact, several currently used therapies, such as pre and probiotics, dietary restrictions and antibiotics, modulate the gut microbiota.89 A few early studies94 reported improvement in patients with IBS following FMT. For example, in a study reported in abstract form,95 nearly 90% of patients who were treated with FMT had an improvement in defecation and decreased abdominal bloating after FMT with 60% showing long term benefit from 9–19 months. While these early reports are encouraging, it is important to remember that they are from small, uncontrolled series with high risk of bias. Well-designed, large randomized controlled studies are needed to determine the role of FMT and other bacteriotherapy in the management of IBS.
Other Indications
In addition to specific indications mentioned above, FMT is being actively investigated as a therapeutic strategy for many other diseases including the metabolic syndrome, Type 2 diabetes mellitus, fatty liver disease, multidrug resistant organism eradication, hepatic encephalopathy and pediatric allergy disorders. For example, an investigator at the University of California, San Francisco is currently recruiting patients with human immunodeficiency virus (HIV) on anti-retroviral therapy to determine whether the transfer of bacterial communities from healthy donors will effect immune activation and inflammatory biomarkers in these patients96.
Safety of FMT
Evidence regarding the safety of FMT is relatively limited because the very rapid adoption of FMT as a therapeutic modality for CDI has occurred prior to the performance of large, long prospective trials that are typically done to assess the safety of new interventions. Potential adverse events can be categorized as short-term and long-term, and short-term events can further be divided into those related to the method of FMT delivery (e.g., colonoscopy, sedation) and those related to the FMT itself. Due to the recent emergence FMT, little data exists regarding long-term events and many are speculative.
FMT appears to be relatively safe in the short-term, especially when compared to its efficacy in treating recurrent CDI. However, any conclusion regarding short-term safety must be viewed with caution since the data come primarily from retrospective case series with variable assessment and follow-up for adverse events. Furthermore, determining if adverse events are related to FMT is often difficult because patients who receive FMT for recurrent CDI commonly are ill with other comorbidities. Very little information is available regarding the long-term safety of FMT. Table 2 includes reported adverse events associated with or potentially related to FMT in published studies with more than 5 patients identified in a systematic review of FMT for CDI4,27, 35, 43, 44, 97–107.
Table 2.
Adverse events in published series of more than 5 patients receiving FMT for C. difficile infection
| 1st Author | No. | Method of delivery | Follow-up | Adverse events |
|---|---|---|---|---|
| Van Nood4 | 16 | Duodenal infusion | 70 days | Diarrhea—5, abdominal cramps—5, belching—3, nausea—1; symptoms resolved in all within 3 hours |
| Youngster44 | 20 | Nasogastric tube or colonoscopy | 6 mos | Mild abdominal discomfort/bloating—4; transient fever (day 2)—1 |
| Rubin97 | 75 | Nasogastric tube | 60 days | No adverse events or deaths |
| MacConnachie27 | 15 | Nasogastric tube | 4–24 wks | No adverse events “related to transplant”; upper gastrointestinal bleeding during 1st month post-FMT |
| Aas98 | 18 | Nasogastric tube | 90 days | Peritonitis in patient on peritoneal dialysis on day 3 (died “shortly thereafter”); pneumonia in patient with COPD (died on day 14) |
| Mattila35 | 70 | Colonoscopy | 1 yr | No complications |
| Hamilton99 | 43 | Colonoscopy | 1 yr | No serious adverse events; approximately one-third noted irregular bowel movements and excessive flatulence during 1st couple of weeks after FMT |
| Patel100 | 31 | Colonoscopy | 1 yr | 1 complication: “microperforation” from colonoscopic biopsy which resolved without surgery |
| Yoon101 | 12 | Colonoscopy | 3 wks – 8 yrs | No adverse events |
| Pathak102 | 12 | Colonoscopy—11; nasoduodenal–1 | 2–29 mos | No complications of FMT |
| Dutta103 | 27 | Enteroscopy + colonoscopy | 10–34 mos | Low grade fever—5, bloating—3; resolved within 12–24 hrs |
| Lee104 | 94 | Enema | 6–24 mos | No significant adverse events; 10% experienced transient constipation and excessive flatulence |
| Emanuelsson105 | 23 | Rectal catheter | 23 | “A few” patient experienced temporary constipation (apparently soon after FMT) |
| Silverman106 | 7 | Enema | 4–14 mos | No adverse events but reported 1 patient with “post-infectious” irritable bowel syndrome (mixed pattern) |
| Schwartz107 | 13 | Colonoscopy | Not stated | Norovirus—2 (2 days and 12 days after FMT); authors speculated person-to-person rather than FMT transmission |
| Kelly43* | 80 | Mixed | 12 wks |
Potentially related adverse events Death: aspiration during colonoscopy with respiratory failure Hospitalizations: IBD flares—4, post-colonoscopy abdominal pain—1; fever, diarrhea, encephalopathy, pancytopenia in lymphoma patient—1 Non-serious adverse events: abdominal pain/bloating immediately following FMT—3; “mucosal tear” at colonoscopy—1; self-limited diarrhea-3, fever—1, IBD flare–1 |
Immunocompromised patients (e.g., immunosuppressive therapy for IBD, organ transplant, cancer with antineoplastic therapy)
Short-term adverse events
Minor events
Minor symptoms immediately following FMT are common, and include abdominal discomfort, bloating, flatulence, diarrhea, constipation, borborygmia, vomiting, and transient fever4, 70, 71, 78. The influence of route of administration on development of symptoms after FMT is uncertain. Only one prospective randomized controlled trial is available to compare adverse events with FMT vs. a control group4. Among 16 patients receiving FMT via duodenal infusion plus bowel lavage, 15 experienced diarrhea, 5 abdominal cramps, 3 belching, and 1 nausea. These symptoms, which resolved in all patients within 3 hours, were not reported in the control group receiving only bowel lavage.
Serious events
More serious adverse events related to the procedure used to administer the FMT, although rare, may occur. These include complications of endoscopy, such as perforation and bleeding, and side effects related to sedation, such as aspiration43. Transmission of enteric pathogens via FMT is also an important concern but appears to be rare with current screening. One center reported 2 cases of documented norovirus infection 2 days and 12 days after FMT107. In both cases donors were asymptomatic, and testing for norovirus in one of the donors was negative. The authors speculated that one case was related to transmission via an endoscopy unit employee and the second case was due to community exposure given the time interval between FMT and symptoms. Another potential episode of transmission comes from a case report of fever and E. coli bacteremia 24 hours after FMT via colonoscopy in a patient with IBD108. The patient had multiple previous episodes of E. coli bacteremia, the last 9 months prior to FMT. Although other serious adverse events have been reported with FMT (e.g., peritonitis in a patient undergoing peritoneal dialysis, pneumonia, IBD flares), their relationship to FMT is uncertain. In an effort to quantify adverse events associated with FMT, Kelly, et al recently published a multicenter retrospective study of immunocompromised patients who underwent FMT to treat CDI.43 Reasons for immunocompromise varied, but included IBD patients on immunosuppressive therapy, solid organ transplant recipients and patients undergoing chemotherapy. Interestingly, there were no infectious complications related to FMT in these potentially “at risk” patients. However, there were two deaths, one an aspiration event related to the procedure used to administer FMT and 17% of IBD patients experienced disease flare post-FMT. The other died 13 days post-FMT secondary to progressive pneumonia, for which she was treated with antibiotics before and after FMT. This death was felt to be unrelated to the FMT.
Potential Long-term adverse events
The greater concern regarding FMT relates to long-term safety. Such risks include the possible transmission of infectious agents via FMT or development of diseases/conditions related to changes in the gut microbiota. The theoretical possibility of transmission of unrecognized infectious agents that causes illness years later, analogous to prior experience with Hepatitis C and human immunodeficiency virus, has been raised, although one would assume that such agents would induce disease in donors as well.
A greater theoretical risk may be the induction of chronic disease based on alterations in the gut microbiota. A partial list of conditions that have been linked to the gut microbiota includes obesity, diabetes, atherosclerosis, IBD, colon cancer, non-alcoholic fatty liver disease, irritable bowel syndrome, asthma, and autism. As mentioned above, transplantation of human fecal microbiota from obese individuals to rodents has been shown to transmit an obesity phenotype109, and FMT from lean individuals to obese subjects with metabolic syndrome showed an increase in insulin sensitivity110. With respect to atherosclerosis, production of the pro-atherogenic metabolite trimethylamine-N-oxide is dependent on the gut microbiota, and increased levels of this metabolite are associated with an increase in major cardiovascular events111. Thus, legitimate concern exists regarding the long-term risk of FMT transplantation, especially when used for indications with less well-documented benefit than recurrent CDI. Clinical follow-up of patients over many years, ideally combined with analysis of banked donor and recipient specimens, will be crucial in assessing the possibility that FMT may increase (or decrease) the risk of a number of common chronic conditions.
Patient Perceptions
Patients suffering from recurrent CDI are desperate for a cure and may seek guidance from online communities. Many are willing to travel great distances to undergo FMT. Some have even resorted to performing home-enemas of donor stool when they are unable to find a physician who is willing or able to perform FMT. In fact, a YouTube video describing do-it-yourself FMT has been viewed over 45,000 times.112 Limited studies on patient perceptions regarding FMT done to date report that patients recognize the unappealing nature of FMT, yet are still open to considering it as a treatment, especially when recommended by a physician. This willingness on the part of patients to try FMT holds true regardless of their prior experience with FMT or the nature of their disease (CDI, UC or healthy patients).113–115 For example, in a survey of healthy patients who were presented with hypothetical scenarios related to treatment options for recurrent CDI, 81% of patients chose FMT.113 Similarly, a survey of UC patients at a single tertiary care center showed that, despite reporting satisfactory to excellent disease control with their treatments, the vast majority were interested in, or willing to consider, FMT.114 Subjects who had been hospitalized were more willing to undergo FMT (55% versus 34%, p=0.035) suggesting implied risk/benefit assessment by patients. The authors believed that the profound interest in FMT reflects the perception that FMT is a “natural”’ treatment for UC, and their dissatisfaction with chronic medical therapy for a variety of reasons. Brandt et al surveyed the perceptions of 77 patients who had experienced recurrences of CDI and were treated with FMT; 97% reported that they would want FMT in the event of another recurrence, and 53% of them would have opted for FMT as primary treatment prior to a trial of antibiotics for their first recurrence if given the option.32 With the advent of more aesthetically acceptable protocols, including odorless pills, FMT is likely to appeal to a majority of patients who are deemed eligible for FMT.
While no studies about potential barriers to the use of FMT by physicians have been published in full form, a recent abstract reporting a survey of physicians showed that 40% were not willing to try FMT, pending further demonstration of its efficacy, safety and perceived patient acceptance.116 Although a ‘Current Procedural Terminology’ (CPT) code for FMT does exist (44705, “preparation of fecal microbiota for instillation, including assessment of donor specimen), lack of adequate reimbursement for the time required by physicians may be a further deterrent. Thus, while patients seem willing to try FMT, it is unknown how willing physicians in various specialties are to offer, perform, or refer patients for this treatment.
Pediatric Considerations
The data regarding safety and efficacy of FMT in pediatric patients to date is quite limited. Currently, the literature is comprised of a handful of case reports and studies which include a small number of children with CDI23, 28, 97, 117 and/or IBD 67, 118, 119,74, 120, 121 who were treated with FMT. Additional larger, controlled, and prospective studies are needed to clarify both the safety and efficacy of FMT in pediatric patients.
In 2010, Russell et al. report the first pediatric patient treated with FMT via nasogastric tube.118 The case involved a 2 year old child with recalcitrant CDI that had failed an exhaustive course, including probiotics, metronidazole, pulsed-dose vancomycin, rifaximin, and nitazoxanide, who experienced complete resolution of symptoms within 36 hours of FMT. Since this report, others have reported on the safety and efficacy of FMT for children with recurrent CDI without underlying IBD via nasogastric tube, colonoscopy, and capsules with a cure rate of 92% (24/26) and no serious adverse events reported.117, 121–124 In children with recurrent CDI and underlying IBD, the data the data is mixed, highlighting the difficulty in distinguishing a clinical response to an infection that may have the same symptoms as the underlying disease.118, 121, 124 Similarly, uncontrolled data on FMT as a primary treatment for IBD, including CD and UC, shows mixed results.74, 119, 120, 125 Suskind et al. reported a clinical improvement 7 of the 9 patients with CD who received a single treatment with nasogastric FMT,74 however, the same approach in 4 patients with active UC showed no benefit.125 Although these results are promising and suggests that some pediatric patients with Crohn’s disease may respond to FMT, it is premature to draw conclusions about the role of FMT in treatment of pediatric IBD. Though no serious short-term adverse events have been reported, some speculate that children may have increased risks as the long-term effects of microbial transplantation and manipulation in children are unknown.
There has also been significant parental interest in FMT for the treatment of other childhood diseases such as food allergies, autism, and chronic diarrhea, however, currently there is no data to support its use for these conditions and studies are ongoing. Parents should be advised not to perform home FMT. They should be counselled about the potential risks of (home) FMT and referred to centers that have ongoing trials (clinicaltrials.gov).
Regulatory issues
Worldwide, regulation of FMT varies greatly between countries. The Therapeutic Goods Administration in Australia, where FMT is not considered a drug or regulated for any indication, has provided no communications regarding FMT. Though several FMT trials are ongoing in China, there has been no indication that The Ministry of Health intends to exercise authority over the procedure. In Europe, the European Medicines Agency has not regulated FMT for CDI thus far.126 However, Health Canada considers FMT and related synthetic stool therapies as a “New Biologic Drug” and requires any clinical study go through the process of a clinical trial application (CTA) to ensure that quality and safety standards are met.127 The United States Food and Drug Administration (FDA) has determined that administered stool constitutes a biological product and a drug in that it is intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease or is intended to affect the structure or function of the body128. Though fundamentally different than existing drugs and biologics, the agency has maintained that, at this point, the process of donor eligibility determination and screening, stool processing and infusion defines the FMT “product” and falls under FDA jurisdiction. As such, without large randomized controlled studies to support safety and efficacy, it is an unapproved product and an investigational new drug application (IND) is required in order to administer FMT.
The IND process is largely tailored to the pharmaceutical industry and regulatory experts. The time and administrative burden for any clinician wishing to sponsor an IND to treat his or her individual patients with FMT can be enormous. The initial application may take many weeks to prepare, and once an IND is active, follow up documentation, such as required reporting of all serious adverse events (even if unrelated), institutional review board (IRB) oversight, safety monitoring board meetings and mandatory annual reports to the FDA are similarly time consuming and burdensome. Furthermore, it is not permissible to charge for products which are being administered under IND. Requiring physicians to hold an IND to administer FMT would prevent many from offering this therapy and ensure that only commercial entities with the means to navigate the regulatory process and fund large clinical trials will control this effective therapy. The FDA announced the IND requirement in May of 2013 at a public workshop to discuss the regulatory and scientific issues around FMT. Physicians, scientists and patient stakeholders argued that the availability of FMT would be adversely affected by this requirement. The Agency agreed and subsequently stated that it would exercise “enforcement discretion” around FMT when used to by licensed physicians to treat CDI not responding to standard therapies.129 In other words, the FDA will not enforce its own requirement that FMT be done under IND, as long as providers obtain informed consent, detail risks around the procedure, and explain that FMT is considered an investigational therapy. The enforcement discretion policy does not extend to other uses of FMT such as IBD. Furthermore, clinical trials of FMT for C. difficile do not fall under enforcement discretion and require an IND.
This policy has enabled many patients suffering from recurrent CDI to receive FMT, but is probably only temporary. The position of the FDA on this matter continues to evolve. Partially in response to stool banks, which were not operating under IND and began shipping preparations of stool to providers across state lines, FDA posted a draft guidance and solicited feedback in early 2014, proposing that the FMT product must be obtained from a donor known to either the patient or to the licensed health care provider treating the patient; and the stool donor and stool are qualified by screening and testing performed under the direction of the licensed health care provider for the purpose of providing the FMT product to treat his or her patient. Although this policy has not been finalized, if fully implemented, such a policy would greatly limit FMT under the stool bank model, which uses volunteer donors who are not known to the patient or provider. Commercialized preparations of human stool are currently in clinical trials and it is conceivable that the FDA might cease to offer enforcement discretion entirely once one or more of these products is approved and commercially available. It is clear that the regulatory landscape will continue to evolve as microbiota-based therapeutics emerge.
Future of FMT & Microbial Therapeutics
While the value of FMT in treating recurrent CDI is clear, its potential long-term detrimental consequences, both beneficial and detrimental, are unknown. The gut microbiota is a complex consortium with many components that have never been characterized. A priori knowledge is not available regarding the impact of transferring these complex communities from one individual to another, although many studies in mice indicate that the composition of the gut microbiota can affect host susceptibility to diseases. Despite these safety concerns about transferring a complex and undefined microbial living microbial community from one human to another, there is very little current information about practice of FMT in the U.S. Critical methodological data such as donor/recipient screening, fecal preparation, modality of delivery, and patient consent practices are lacking. Information about the effectiveness of FMT as well as both short- and long-term safety data are not systematically collected, with the only available information being case reports published in the literature. Based on this information void, national societies have attempted to provide guidance to practitioners through published guidelines and editorials16, 130, 131 but the degree of adherence to these recommendations is unknown.
Patients need to be informed of potential risks and consent to the procedure. Protocols need to be developed about donor sample preparation, characterization, archiving (so that follow-up analyses can be performed), and host preparation/administration/dosing. Registries need to be established for collection of data on donor and recipient characteristics, FMT protocols, and short- and long-term outcomes. Best practices for FMT need to be established, and critically evaluated, and clinically-relevant recipient responses to FMT, both beneficial and adverse, need to be defined and monitored. Recognizing the importance of systematically collecting information about the practice of FMT, there has been considerable interest in the development of a national FMT registry involving collaboration between national professional and advocacy societies. Such a registry would serve as a unique national resource of information about the clinical practice of FMT in the U.S.
In the absence of other therapies FMT remains the mainstay for management of recurrent CDI not responsive to conventional therapy. In the short-term there is a need to improve delivery methods to increase accessibility of this treatment. Frozen preparations of donor material will enable eligible patients to be treated more easily and facilitate clinical research. Openbiome is a stool bank located in Medford, MA USA. This non-profit organization has already supplied over 2000 doses of donor stool to over 200 institutions in the United States at a cost of around $250 USD per dose and has recently secured an IND for this product. However, it is very likely that whole-stool FMT, as currently practiced, is a short-term bridge for the treatment of CDI that will be replaced by commercially developed products. Although the timeline for the development of these products is currently unknown, a number are in late stage development and may be available within the next few years. These include full spectrum stool-based products as well as defined microbial consortia.
Encapsulated formulations of fecal material would help make FMT accessible to many more patients, simplify clinical trials and, if proven safe, could potentially be used earlier in the course of disease. Two recent advances in this area have resulted from efforts to refine FMT in order to isolate and deliver the bacterial species responsible for therapeutic effects. Ser-109, a microbial product comprising a mixture of bacterial spores developed as an ecobiotic by Seres Health, demonstrated excellent efficacy in management of recurrent C. difficile diarrhea in a small open-label study.132 Results from the phase I/II clinical trial showed 100% success at 8 weeks. In an alternate approach using culture techniques, investigators at Queens University and Kingston General Hospital in Ontario, Canada isolated intestinal bacteria from healthy donors using modified continuous culture chemostat system. They then developed a defined microbial community of 33 strains termed RePOOPulate based on antibiotic susceptibility profiles, culturability and community robustness and administered it to two patients with relapsing C. difficile diarrhea in a proof of principle study. Both patients improved.133 It is currently believed that the development of specific combinations of bacterial strains whose biological properties have been carefully characterized by genomic sequencing and biochemical/microbiologic analyses, together with standardized methods for human inoculation, with lead to predictable host responses and reduce the risk of pathogen transmission to enhance patient safety. In turn, the safety of such products would be monitored by post-market surveillance registries as required by the FDA for biologics and drugs.
Conclusions
The high therapeutic efficacy of FMT for recurrent CDI is an important proof of concept that substantial modification of the gut microbiota can be an effective modality of the treatment of a disease in humans. Much as the discovery of H. pylori revolutionized the treatment of peptic ulcer disease, our greater understanding of the communities of microorganisms residing within the human gut and the role of dysbiosis in various disease states will certainly result in new therapeutics in the coming decade. Rather than something to be feared as disease-causing agents, “germs” are now understood to be integral to human health, and a number of diseases both within and outside the GI tract may soon be treated with microbiota. FMT is the first and crudest way to alter the intestinal microbiome, and both patients and providers have become more aware of this highly effective option for CDI. Though FMT appears to be safe, with few short-term adverse effects or complications directly attributed to the procedure yet reported, more robust safety data is certainly needed as is better understanding of the mechanisms by which FMT is effective. There is speculation that other conditions marked by dysbiosis can be treated with FMT in some form, though these conditions are more complex and the outcomes after microbial manipulation are unlikely to be as dramatic and predictably effective as with CDI. It is certain that fecal therapy will continue to be refined beyond “whole stool” transplants. Rapid advances in DNA sequencing and metabolomic technologies, together with the development of more sophisticated biocomputational tools to analyze large and complex high dimensional datasets, will greatly facilitate the development of defined microbial consortia targeted to treat specific diseases. Though challenges exist, regulatory agencies have been willing to work with stakeholders and will continue to evolve and adapt policy as therapeutics based on human gut microbiome research emerge.
Acknowledgments
AGA Gut Microbiome Center for Research and Education
Grant Support
1NIH/NIDDK 1R21DK093839-01A1
3This work was made possible by funding from NIH K08 DK100638, as well as the Global Probiotic Council, Minnesota Partnership for Biotechnology and Genomics and Center for Individualized Medicine (CIM; Mayo Clinic).
8NIH/NIGMS R01 GM103591
Abbreviations
- FMT
Fecal Microbiota Transplantation
- CDI
Clostridium difficile infection
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- GI
gastrointestinal
- UC
ulcerative colitis
- CD
Crohn’s disease
- CPT
Current Procedural Terminology
- CTA
clinical trial application
- FDA
Food and Drug Administration
- IND
investigational new drug application
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/COI
1Colleen R. Kelly-Assembly Biosciences-Research Support; Seres Health-Consultant and Site Investigator for Clinical Trial (SER-109); Volunteer on advisory boards for OpenBiome and the Fecal Transplant Foundation.
8Gary D. Wu: Scientific Advisory Boards-Chr. Hansen, Janssen, Rebiotix; Research Support-Nestle; Co-Founder-Microbiota Therapeutics, LLC
Author Contributions
Colleen R. Kelly: Planning, outlining and coordinating the work of co-authors, drafting and editing the manuscript, critical revision of the manuscript, submission of the manuscript
Stacy Kahn: drafting and editing the manuscript
Purna Kashyap: drafting and editing the manuscript
Loren Laine: drafting the manuscript, critical revision of the manuscript
David Rubin: drafting and editing the manuscript
Ashish Atreja: drafting and editing the manuscript
Thomas Moore: drafting the manuscript
Gary Wu: Planning, drafting and editing the manuscript.
Contributor Information
Colleen R. Kelly, Lifespan Women’s Medicine Collaborative, The Miriam Hospital, Alpert Medical School of Brown University, Providence, Rhode Island.
Stacy Kahn, University of Chicago, Section of Pediatric Gastroenterology, Hepatology, & Nutrition, Inflammatory Bowel Disease Center, Chicago, Illinois.
Purna Kashyap, Department of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota.
Loren Laine, Section of Digestive Diseases, Yale School of Medicine, New Haven, Connecticut; Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut.
David Rubin, University of Chicago Inflammatory Bowel Disease Center, Chicago, Illinois.
Ashish Atreja, Sinai AppLab, Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, New York.
Thomas Moore, Infectious Disease Consultants (IDC) of Kansas, Wichita, Kansas.
Gary Wu, Division of Gastroenterology, Hepatology, and Nutrition, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693–702. doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 4.Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 5.Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 6.Khoruts A, Dicksved J, Jansson JK, et al. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–60. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton MJ, Weingarden AR, Unno T, et al. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4:125–35. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Luo W, Shi Y, et al. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 9.DePeters EJ, George LW. Rumen transfaunation. Immunol Lett. 2014;162:69–76. doi: 10.1016/j.imlet.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–9. [PubMed] [Google Scholar]
- 11.Schwan A, Sjolin S, Trottestam U, et al. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2:845. doi: 10.1016/s0140-6736(83)90753-5. [DOI] [PubMed] [Google Scholar]
- 12.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 14.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ClinicalTrials.gov. Clinical Trials for Fecal Transplant. Accessed May 1 2015: https://clinicaltrials.gov/ct2/results?term=fecal+transplant&Search=Search.
- 16.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–9. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 18.You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008;148:632–3. doi: 10.7326/0003-4819-148-8-200804150-00024. [DOI] [PubMed] [Google Scholar]
- 19.Weingarden AR, Hamilton MJ, Sadowsky MJ, et al. Resolution of severe Clostridium difficile infection following sequential fecal microbiota transplantation. Journal of clinical gastroenterology. 2013;47:735–7. doi: 10.1097/MCG.0b013e31829004ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trubiano JA, Gardiner B, Kwong JC, et al. Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol. 2013;25:255–7. doi: 10.1097/MEG.0b013e32835b2da9. [DOI] [PubMed] [Google Scholar]
- 21.Neemann K, Eichele DD, Smith PW, et al. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transpl Infect Dis. 2012;14:E161–5. doi: 10.1111/tid.12017. [DOI] [PubMed] [Google Scholar]
- 22.Dorsey KA, M E, Steele WR, Eder AF, Stramer SL. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion. 2013;53:1250–6. doi: 10.1111/j.1537-2995.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 23.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. Jama. 2014;312:1772–8. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 24.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–5. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson A, Berstad A, Lund-Tonnesen S, et al. The effect of faecal enema on five microflora-associated characteristics in patients with antibiotic-associated diarrhoea. Scandinavian journal of gastroenterology. 1999;34:580–6. doi: 10.1080/003655299750026038. [DOI] [PubMed] [Google Scholar]
- 26.Rubin TA, Gessert CE, Aas J, et al. Fecal microbiome transplantation for recurrent Clostridium difficile infection: report on a case series. Anaerobe. 2013;19:22–6. doi: 10.1016/j.anaerobe.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 27.MacConnachie AA, Fox R, Kennedy DR, et al. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. Q J Med. 2009;102:781–4. doi: 10.1093/qjmed/hcp118. [DOI] [PubMed] [Google Scholar]
- 28.Russell G, Kaplan J, Ferraro M, et al. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126:e239–42. doi: 10.1542/peds.2009-3363. [DOI] [PubMed] [Google Scholar]
- 29.Kronman MP, Nielson HJ, Adler AL, et al. Fecal Microbiota Transplantation Via Nasogastric Tube for Recurrent Clostridium difficile Infection in Pediatric Patients. J Pediatr Gastroenterol Nutr. 2015;60:23–6. doi: 10.1097/MPG.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 30.Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol. 2000;95:3283–5. doi: 10.1111/j.1572-0241.2000.03302.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol. 2010;44:562–6. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 32.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–87. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 33.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol. 2010;44:567–70. doi: 10.1097/MCG.0b013e3181dadb10. [DOI] [PubMed] [Google Scholar]
- 34.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–9. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 35.Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–6. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 36.Gustafsson A, Berstad A, Lund-Tonnesen S, et al. The effect of faecal enema on five microflora-associated characteristics in patients with antibiotic-associated diarrhoea. Scand J Gastroenterol. 1999;34:580–6. doi: 10.1080/003655299750026038. [DOI] [PubMed] [Google Scholar]
- 37.Kassam Z, Hundal R, Marshall JK, et al. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172:191–3. doi: 10.1001/archinte.172.2.191. [DOI] [PubMed] [Google Scholar]
- 38.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8:471–3. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Bowden TA, Jr, Mansberger AR, Jr, Lykins LE. Pseudomembraneous enterocolitis: mechanism for restoring floral homeostasis. Am Surg. 1981;47:178–83. [PubMed] [Google Scholar]
- 40.Lee CH, Belanger JE, Kassam Z, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014;33:1425–8. doi: 10.1007/s10096-014-2088-9. [DOI] [PubMed] [Google Scholar]
- 41.Dutta SK, Girotra M, Garg S, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2014;12:1572–6. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 42.Varier RU, Biltaji E, Smith KJ, et al. Cost-effectiveness analysis of treatment strategies for initial Clostridium difficile infection. Clin Microbiol Infect. 2014;20:1343–51. doi: 10.1111/1469-0691.12805. [DOI] [PubMed] [Google Scholar]
- 43.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–71. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficle infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515–22. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased Diversity of the Fecal Microbiome in Recurrent Clostridium difficile Associated Diarrhea. Journal of Infectious Diseases. 2008;197:435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 46.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature reviews Microbiology. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 47.Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS biology. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theriot CM, Koenigsknecht MJ, Carlson PE, Jr, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature communications. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. The American journal of gastroenterology. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahinas D, Silverman M, Sittler T, et al. Toward an understanding of changes in diversity associated with fecal microbiome transplantation based on 16S rRNA gene deep sequencing. mBio. 2012;3 doi: 10.1128/mBio.00338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reeves AE, Koenigsknecht MJ, Bergin IL, et al. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infection and immunity. 2012;80:3786–94. doi: 10.1128/IAI.00647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS pathogens. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rea MC, Sit CS, Clayton E, et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9352–7. doi: 10.1073/pnas.0913554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buffie CG, Bucci V, Stein RR, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2014 doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.FC W. The Surgery of Colitis. British Medical Journal. 1909;1:10–13. doi: 10.1136/bmj.1.2505.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagalingam NA, Lynch SV. Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:968–84. doi: 10.1002/ibd.21866. [DOI] [PubMed] [Google Scholar]
- 57.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–99. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manichanh C, Borruel N, Casellas F, et al. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 59.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 62.Borody TJ, George L, Andrews P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? The Medical Journal of Australia. 1989;150:604–604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 63.Borody TJ, Warren EF, Leis S, et al. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–7. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F-M, Wang H-G, Wang M, et al. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World journal of gastroenterology: WJG. 2013;19:7213. doi: 10.3748/wjg.v19.i41.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Damman C, Brittnacher M, Hayden H, et al. Su1403 Single Colonoscopically Administered Fecal Microbiota Transplant for Ulcerative Colitis-A Pilot Study to Determine Therapeutic Benefit and Graft Stability. Gastroenterology. 2014;146:S-460. [Google Scholar]
- 66.Vaughn BP, Gevers D, Ting A, et al. Mo1228 Fecal Microbiota Transplantation Induces Early Improvement in Symptoms in Patients With Active Crohn’s Disease. Gastroenterology. 2014;146:S-591–S-592. [Google Scholar]
- 67.Suskind DL, Singh N, Nielson H, et al. Fecal Microbial Transplant via Nasogastric Tube for Active Pediatric Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2014 doi: 10.1097/MPG.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 68.Vermeire S, Joossens M, Verbeke K, et al. Sa1922 Pilot Study on the Safety and Efficacy of Faecal Microbiota Transplantation in Refractory Crohn’s Disease. Gastroenterology. 2012;142:S-360. [Google Scholar]
- 69.Kunde S, Pham A, Bonczyk S, et al. Safety Tolerability Clinical Response after Fecal Transplantation in Children and Young Adults with Ulcerative Colitis. Journal of Pediatric Gastroenterology and Nutrition. 2013 doi: 10.1097/MPG.0b013e318292fa0d. Publish Ahead of Print:10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 70.Kump PK, Grochenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–65. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 71.Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–30. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 72.Landy J, Al-Hassi HO, Mann ER, et al. Tu1985 A Prospective Controlled Pilot Study of Fecal Microbiota Transplantation for Chronic Refractory Pouchitis. Gastroenterology. 2013;144:S-897. [Google Scholar]
- 73.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. Journal of Crohn’s and Colitis. 2014;8(12):1569–81. doi: 10.1016/j.crohns.2014.08.006. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal Microbial Transplant Effect on Clinical Outcomes and Fecal Microbiome in Active Crohn’s Disease. Inflammatory bowel diseases. 2015;21:556–563. doi: 10.1097/MIB.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1036–8. doi: 10.1016/j.cgh.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 76.Kelly CR, Z H, Kahn S. New Diagnosis of Crohn’s Colits 6 weeks after Fecal Microbiota Transplantation. Inflamm Bowel Dis. 2014;20:S21. [Google Scholar]
- 77.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized, Controlled Trial. Gastroenterology. 2015 Apr 6; doi: 10.1053/j.gastro.2015.04.001. [Epub] [DOI] [PubMed] [Google Scholar]
- 78.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology. 2015 Mar 30; doi: 10.1053/j.gastro.2015.03.045. [Epub] [DOI] [PubMed] [Google Scholar]
- 79.James PT, Leach R, Kalamara E, et al. The worldwide obesity epidemic. Obes Res. 2001;9(Suppl 4):228s–233s. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 80.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 81.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liou AP, Paziuk M, Luevano JM, Jr, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Science translational medicine. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker AW, Parkhill J. Microbiology. Fighting obesity with bacteria. Science. 2013;341:1069–70. doi: 10.1126/science.1243787. [DOI] [PubMed] [Google Scholar]
- 84.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6. e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 85.Hungin AP, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Alimentary pharmacology & therapeutics. 2005;21:1365–75. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 86.Matto J, Maunuksela L, Kajander K, et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome–a longitudinal study in IBS and control subjects. FEMS immunology and medical microbiology. 2005;43:213–22. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 87.Balsari A, Ceccarelli A, Dubini F, et al. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–94. [PubMed] [Google Scholar]
- 88.Malinen E, Rinttila T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. The American journal of gastroenterology. 2005;100:373–82. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 89.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–76. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crouzet L, Gaultier E, Del’Homme C, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25:e272–82. doi: 10.1111/nmo.12103. [DOI] [PubMed] [Google Scholar]
- 91.Kashyap PC, Marcobal A, Ursell LK, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144:967–77. doi: 10.1053/j.gastro.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–42. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 93.Grover M, Camilleri M, Smith K, et al. On the fiftieth anniversary. Postinfectious irritable bowel syndrome: mechanisms related to pathogens. Neurogastroenterol Motil. 2014;26:156–67. doi: 10.1111/nmo.12304. [DOI] [PubMed] [Google Scholar]
- 94.Borody TJ, George L, Andrews P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? The Medical journal of Australia. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 95.Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation- long term follow up. Gastroenterology. 1995;108:A563. [Google Scholar]
- 96.ClinicalTrials.gov. Fecal Microbiota Transplantation in HIV. Accessed May 1 2015: https://clinicaltrials.gov/ct2/results?term=hiv+fmt&Search=Search.
- 97.Rubin TA, Gessert C, Aas J, et al. Fecal microbiome transplantation for recurrent Clostridium difficile infection: report on a case series. Anaerobe. 2013;19:22–6. doi: 10.1016/j.anaerobe.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:80–5. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 99.Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 107:761–7. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 100.Patel NC, Griesbach Cl, DiBaise JK, et al. Fecal microbiota transplant for recurrent Clostricium difficile infection: Mayo Clinic in Arizona experience. Mayo Clin Proc. 2013;88:799–805. doi: 10.1016/j.mayocp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 101.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficle-associated disease by donated stool transplanted via colonoscopy. A case series of 12 patients. J Clin Gastroenterol. 2010;44:562–6. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 102.Pathak R, Enuh HA, Patel A, et al. Treatment of relapsing Clostridium difficile infection using fecal microbiota transplantation. Clin Exp Gastroenterol. 2014;7:1–6. doi: 10.2147/CEG.S53410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dutta SK, Girotra M, Garg S, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2014;12:1572–6. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 104.Lee CH, Belanger JE, Kassam Z, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014;33:1425–8. doi: 10.1007/s10096-014-2088-9. [DOI] [PubMed] [Google Scholar]
- 105.Emanuelsson F, Claesson BEB, Ljungström L, et al. Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scan J Infect Dis. 2014;46 doi: 10.3109/00365548.2013.858181. [DOI] [PubMed] [Google Scholar]
- 106.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8:471–3. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108:1367. doi: 10.1038/ajg.2013.164. [DOI] [PubMed] [Google Scholar]
- 108.Quera R, Espinoza R, Estay C, et al. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014;8:252–3. doi: 10.1016/j.crohns.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 109.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 111.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hurst M. DIY Fecal Transplants to cure yourself of Ulcerative Colitis. YouTube. 2013 Jun 4; Accessed May 1, 2015: https://www.youtube.com/watch?v=WEMnRC22oOs.
- 113.Zipursky JS, Sidorsky TI, Freedman CA, et al. Physician attitudes toward the use of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection. Can J Gastroenterol Hepatol. 2014;28:319–24. doi: 10.1155/2014/403828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kahn SA, Vachon A, Rodriquez D, et al. Patient perceptions of fecal microbiota transplantation for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1506–13. doi: 10.1097/MIB.0b013e318281f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brandt LJ. Editorial commentary: fecal microbiota transplantation: patient and physician attitudes. Clin Infect Dis. 2012;55:1659–60. doi: 10.1093/cid/cis812. [DOI] [PubMed] [Google Scholar]
- 116.Kelly CR, O N, deLeon L, Kertstetter D. Barriers to greater utilization of fecal bacteriotherapy for chronic Clostridium difficile infection. Am J Gastroenterol. 2010;105:S135. [Google Scholar]
- 117.Kahn SA, Young S, Rubin DT. Colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection in a child. Am J Gastroenterol. 2012;107:1930–1. doi: 10.1038/ajg.2012.351. [DOI] [PubMed] [Google Scholar]
- 118.Russell GH, Kaplan JL, Youngster I, et al. Fecal transplant for recurrent Clostridium difficile infection in children with and without inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58:588–92. doi: 10.1097/MPG.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 119.Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 120.Vandenplas Y, Veereman G, van der Werff Ten, Bosch J, et al. Fecal Microbial Transplantation in a One-Year-Old Girl with Early Onset Colitis - Caution Advised. J Pediatr Gastroenterol Nutr. 2014 doi: 10.1097/MPG.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 121.Pierog A, Mencin A, Reilly NR. Fecal microbiota transplantation in children with recurrent Clostridium difficile infection. The Pediatric infectious disease journal. 2014;33:1198–1200. doi: 10.1097/INF.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 122.Rubin TA, Gessert CE, Aas J, et al. Fecal microbiome transplantation for recurrent Clostridium difficile infection: Report on a case series. Anaerobe. 2013;19:22–26. doi: 10.1016/j.anaerobe.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 123.Youngster I, Sauk J, Pindar C, et al. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin Infect Dis. 2014;58:1515–22. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kronman MP, Nielson HJ, Adler AL, et al. Fecal Microbiota Transplantation via Nasogastric Tube for Recurrent C. difficile Infection in Pediatric Patients. J Pediatr Gastroenterol Nutr. 2014 doi: 10.1097/MPG.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 125.Suskind DL, Singh N, Nielson H, et al. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. Journal of pediatric gastroenterology and nutrition. 2015;60:27–29. doi: 10.1097/MPG.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 126.van Nood E, Speelman P, Nieuwdorp M, et al. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. 2014;30:34–9. doi: 10.1097/MOG.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 127.Allen-Vercoe E, Reid G, Viner N, et al. A Canadian Working Group report on fecal microbial therapy: microbial ecosystems therapeutics. Can J Gastroenterol. 2012;26:457–62. doi: 10.1155/2012/213828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.U.S. Food and Drug Adminstration. FDA Drug Definition. FDA Glossary of Terms. Accessed May 1 2015: http://www.fda.gov/drugs/informationondrugs/ucm079436.htm#D.
- 129.U.S. Food and Drug Adminstration. Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. 2013 Jul; Accessed May 1 2015: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM361393.pdf.
- 130.Kelly CR, Kunde SS, Khoruts A. Guidance on preparing an investigational new drug application for fecal microbiota transplantation studies. Clin Gastroenterol Hepatol. 2014;12:283–8. doi: 10.1016/j.cgh.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moore T, Rodriguez A, Bakken JS. Fecal Microbiota Transplantation: A Practical Update for the Infectious Disease Specialist. Clinical Infectious Diseases. 2014;58:541–545. doi: 10.1093/cid/cit950. [DOI] [PubMed] [Google Scholar]
- 132.Khanna S, P D, Kelly C, et al. Clinical evaluation of SER–109, a rationally designed, oral microbiome–based therapeutic for the treatment of recurrent Clostridium difficile. 2014 [Google Scholar]
- 133.Petrof EO, Gloor GB, Vanner SJ, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1:3. doi: 10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


