Abstract
Cancer-related fatigue significantly disrupts normal functioning and quality of life for a substantial portion of cancer survivors, and may persist for years following cancer treatment. While the causes of persistent fatigue among cancer survivors are not yet fully understood, accumulating evidence suggests that several pathways, including chronic inflammation, autonomic imbalance, HPA-axis dysfunction, and/or mitochondrial damage, could contribute towards the disruption of normal neuronal function and result in the symptom of cancer-related fatigue. Exercise training interventions have been shown to be some of the more successful treatment options to address cancer-related fatigue. In this review, we discuss the literature regarding the causes of persistent fatigue in cancer survivors and the mechanisms by which exercise may relieve this symptom. There is still much work to be done until the prescription of exercise becomes standard practice for cancer survivors. With improvements in the quality of studies, evidenced-based exercise interventions will allow exercise scientists and oncologists to work together to treat cancer-related fatigue.
Introduction
The National Comprehensive Cancer Network defines cancer-related fatigue as a “distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual function” [1]. Fatigue is thought to be the most widespread adverse side effect of cancer in adults and children [1], with some studies placing the percent of patients suffering from fatigue as high as 75–99% [2]. Fatigue lasts longer than other treatment side-effects [3], and is the symptom reported to interfere most substantially with activities of daily living [3–6]. While it is associated with cancer itself [1], fatigue frequently worsens during treatment and is recognized as a factor limiting patient adherence to cancer therapy [2, 7]. This may explain why patients reporting high levels of fatigue during treatment have shorter disease free intervals [8]. Although symptoms frequently improve following treatment completion, fatigue persists in a substantial number of cancer survivors. It has been estimated that 19–38% of survivors experience significant levels of fatigue following treatment [1, 7, 9], which is a much higher prevalence than in the population without a cancer history. In some instances, fatigue continues for years after the cancer treatment has ended. For example, in a longitudinal study of 763 breast cancer survivors, 35% were fatigued in the first 5 years after treatment, and 34% reported fatigue 5–10 years following treatment [10]. Similar results were found in a survey of 1294 breast, prostate, or colorectal cancer survivors, where approximately one third of survivors reported fatigue 6 years after treatment [11].
The diagnostic criteria for cancer-related fatigue are presented in Table 1. Fatigue is a complex multi-dimensional phenomenon that occurs across physical, cognitive, and emotional domains [2] and is comprised of both peripheral and central aspects [12]. Peripheral fatigue refers to events that occur in the muscles and at the neuromuscular junctions, while central fatigue refers to events that originate in the brain. Central fatigue includes physical (I don’t have the strength to do it) as well as motivational (I don’t want to engage in the effort to do it) components. In a qualitative study of cancer-related fatigue, 7 of 20 patients described motivational deficits during unstructured interviews [13]. Furthermore, in studies that directly asked patients about their level of motivation or interest, the rate of reported deficits increased to 50%–65% [14–16]. Fatigue is most commonly measured through self-reports. Standardized questionnaires ask individuals to rate fatigue on a numeric scale and frequently gather additional data, such as its temporal pattern and duration and its interference with daily function [17]. Several fatigue-specific questionnaires have been developed to assess dimensions of fatigue. In addition, fatigue is often rated by patients together with other symptoms on a single multi-symptom assessment measure, such as the M.D. Anderson Symptom Inventory [18]. Nevertheless, cancer-related fatigue is thought to be underreported and underestimated, and thus undertreated [7].
Table 1.
Diagnostic criteria for cancer-related fatigue
Symptoms present every day or nearly every day during the same 2-week period in the past month:
|
And at least five of the following symptoms:
|
| The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| There is evidence from the history, physical examination, or laboratory findings that the symptoms are a consequence of cancer or cancer therapy. |
| The symptoms are not primarily a consequence of comorbid psychiatric disorders such as major depression, somatization disorder, somatoform disorder, or delirium. |
From Bennett et al (3).
Factors leading to the development of persistent cancer-related fatigue are not well understood [1, 3]. The occurrence and severity of cancer-related fatigue is not related to the type of disease or treatment variables, making it difficult to identify populations with the greatest risk for fatigue. Specifically, no reliable associations have been found between fatigue and type of cancer, disease stage at diagnosis, tumor size, number of nodes involved, presence and site of metastases, time since diagnosis, the type, extent, and length of cancer treatment, or time since treatment [9]. Several processes have been proposed to play a role in fatigue, including anemia, inflammation [19], hypothalamic-pituitary-adrenal (HPA) axis dysfunction [20], disruption of circadian rhythms [21], disturbance of monoamine pathways that regulate neurotransmitters [12], and loss of skeletal muscle [22]. Psychological variables are also thought to play a role [7]. The contributions of these factors to cancer-related fatigue are discussed in more detail below. The lack of consensus on the underlying cause(s) of cancer-related fatigue has limited its treatment options [2]. No single pharmacological or behavioral intervention has been found to be completely effective at addressing cancer-related fatigue [19]; however exercise training interventions have been some of the most successful at alleviating this symptom [23]. As presented in the current review, the multifaceted effects of exercise training, which include improvements in inflammation, brain function, fitness, and self-efficacy, may help explain the beneficial effects of exercise on cancer-related fatigue.
Mechanisms of persistent fatigue in cancer survivors
Inflammation
Chronic inflammation has received much attention as a potential mechanism leading to persistent fatigue in cancer survivors, partially due to observed associations between inflammation and fatigue in cancer survivors. Compared to nonfatigued survivors, fatigued breast cancer survivors exhibit higher levels of neopterin [24], a biomarker of macrophage activation, and C reactive protein (CRP) [25–27], an acute phase protein that is the most commonly used biomarker of inflammation. Further signs of heightened immune activity in fatigued breast cancer survivors are elevated white blood cell counts [25, 27–29], elevated T-cell counts [30], and increased production of tumor necrosis factor (TNF)-α and interleukin (IL)-6 following lipopolysaccharide stimulation [31] in comparison to nonfatigued survivors. While most of the evidence linking inflammation and fatigue in cancer survivors has been collected in breast cancer survivors, immune activation has been implicated in fatigue among other groups as well. For example, in testicular cancer survivors measured a median of 11 years post-treatment, fatigue was associated with higher levels of CRP [28]. Additionally, ovarian cancer survivors whose symptoms of fatigue improved in the year following treatment had decreases in plasma IL-6 [32]. Genetic markers also support a link between inflammation and fatigue. Significant associations between single nucleotide polymorphisms for several cytokines and fatigue have been demonstrated in lung cancer survivors [33]. Although the sample size was small, Bower et al found that fatigued breast cancer survivors had heightened gene expression for activation of pro-inflammatory cytokines, chemokine signaling, vascular growth factor, and transcriptional activation in leukocytes [34]. The same study by Bower et al also reported increased signaling by transcription factor nuclear factor-kappa B (NF-κB), which is responsible for and responds to the upregulation of many pro-inflammatory genes, and also found decreased expression of glucocorticoid receptor transcription factor [34]. Decreased glucocorticoid signaling suggests decreased sensitivity to cortisol, the main endogenous brake on the production of pro-inflammatory cytokines [12].
Causal links between inflammation and central fatigue have been demonstrated using animal models in which the symptoms of fatigue and reduced motivation appear after induction of high levels of pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β) [12]. Further evidence has come from human studies where fatigue is induced by typhoid vaccination, activation of the immune system through administration of low dose endotoxin, or in patients receiving recombinant cytokines such as interferon (IFN)-α as treatment for hepatitis C [12]. Treatments which reduce inflammation, such as antagonists of TNF-α, have also been shown to reduce fatigue in patients with rheumatoid arthritis or psoriasis [35, 36].
Fatigue is one component of ‘sickness behavior’, the coordinated set of adaptive behavioral changes that occur in infected individuals to promote survival, and include lethargy and sleepiness. These changes are orchestrated in the brain following the release of inflammatory mediators that ultimately stimulate the brain to induce feelings of sickness. Mechanistically, elevated cytokines that mediate the inflammatory response have been proposed to cause central fatigue and other symptoms by targeting the basal ganglia and dopamine function [37]. Inflammation originating in the periphery is communicated to the central nervous system (CNS) by several pathways, including activation of sensory nerves [19]. This results in the production of prostaglandins and pro-inflammatory cytokines such as IL-1β and TNF-α by endothelial cells, macrophages, and microglia in the CNS [38]. These CNS inflammatory mediators then influence neurons directly or indirectly by modifying astrocyte, oligodendrocyte, and endothelial cell functions [12], thereby contributing to instances of fatigue.
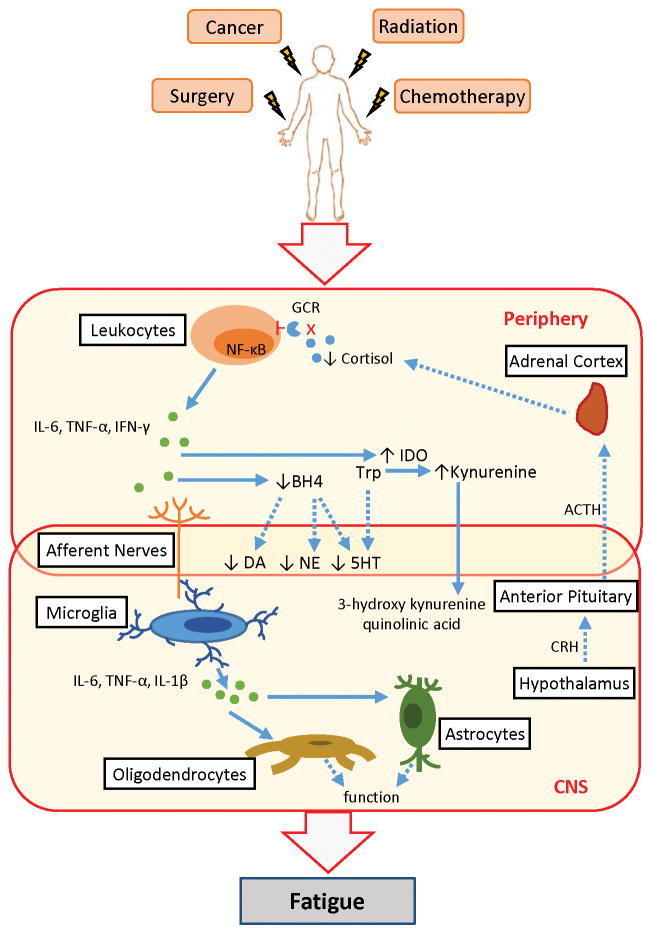
Systemic inflammation also affects the bioavailability of amino acid precursors of neurotransmitters. For example, an enzyme involved in the synthesis of neopterin by peripheral macrophages also leads to a relative deficit of an essential cofactor of aromatic amino acid hydroxylase enzymes used in the synthesis of dopamine, norepinephrine, and serotonin [39]. Serotonin neurotransmission can also be impaired during inflammation by cytokine-induced activation of indoleamine 2,3-dioxygenase, which metabolizes the serotonin precursor tryptophan into kynurenine [40–43]. Kynurenine is further metabolized into neurotoxic kynurenine metabolites that are thought to play a role in depression [44] and are associated with fatigue in lung cancer patients [45]. Thus, inflammation in the periphery impacts neural pathways that play a role in behavior, motivation, and central fatigue. The links between immune activation and changes in the central nervous system thought to contribute to fatigue are illustrated in Figure 1.
Figure 1.
Systemic inflammation contributes to cancer-related fatigue. Cancer and its treatments activate leukocytes, resulting in increased expression of the transcription factor NF-κB and production of pro-inflammatory mediators, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ). Dysregulation of the hypothalamic-pituitary-adrenal (HPA)-axis leads to altered release of cortisol; glucocorticoid receptor (GCR) function on leukocytes is also decreased. Inflammation in the periphery leads to inflammation in the central nervous system (CNS) through several immune-to-brain communication pathways. Signals from sensory nerves activate CNS cells such as microglia to produce pro-inflammatory cytokines, including IL-6, TNF-α, and interlukin-1 beta (IL-1β). These inflammatory mediators then impact neurons directly or indirectly through altered oligodendrocyte, astrocyte, and endothelial cell functions. Inflammation in the periphery also decreases the availability of precursors, such as tetrahydrobiopterin (BH4) required for the synthesis of the neurotransmitters dopamine (DA), norepinephrine (NE), and serotonin (5HT). Deficits in serotonin production also occur as tryptophan (Trp) is converted into kynurenine from an inflammation-mediated increase in indoleamine 2,3-dioxygenase (IDO). Kynurenine is further metabolized in the brain into neurotoxic kynurenine metabolites such as 3-hydroxy kynurenine and quinolinic acid. Dashed lines indicate a disrupted pathway. CRH: corticotropin release hormone; ACTH: adrenocorticotropic hormone
Autonomic nervous system and hypothalamic-pituitary-adrenal axis dysregulation
An imbalance in the autonomic nervous system may also play a role in cancer-related fatigue. Heightened sympathetic activity increases energy demands, whereas higher parasympathetic activity facilitates energy conservation [46]. Parasympathetic activity is also referred to as vagal tone, and can be assessed by measuring the fluctuation in time between consecutive heart beats, or heart rate variability (HRV). Low HRV indicates low vagal tone and thus parasympathetic underactivity [47]. In non-cancer populations, sympathetic overactivity and parasympathetic underactivity are linked to fatigue [48]. For example, healthy adults with lower HRV report more fatigue when performing cognitively demanding tasks than those with higher HRV [49], and lower HRV is associated with driver-related fatigue [50] and greater fatigue after exercise [51]. Autonomic dysfunction has been observed in breast cancer patients during and after treatment [52]. In breast cancer survivors, fatigue is associated with low HRV at rest [20, 53, 54] and in response to social stress [20]. Low vagal tone is also linked to an exaggerated pro-inflammatory profile due to a corresponding deficiency in the cholinergic anti-inflammatory pathway of the parasympathetic nervous system [55]. In a study of breast cancer survivors, low HRV was associated with greater plasma levels of IL-6 and CRP; these markers of inflammation did not however mediate the relationship between fatigue and HRV [54]. Fagundes et al also found elevated levels of norepinephrine in fatigued survivors indicating heightened sympathetic activity; this occurred independently of parasympathetic underactivity, suggesting that both aspects of the autonomic nervous system may be altered in cancer-related fatigue [20].
The HPA-axis is central to regulating inflammatory responses through glucocorticoids such as cortisol [56]. In healthy people, cortisol levels display a diurnal pattern corresponding to the rest-activity cycle. Altered diurnal cortisol secretion and disrupted circadian rhythms are indicative of HPA-axis dysfunction and are linked to increased cytokine signaling and fatigue in a variety of conditions. For example, a flattened circadian cortisol cycle is observed in chronic fatigue syndrome [57]. Fatigued breast cancer survivors have been found to have lower morning serum cortisol levels [24], flattened diurnal cortisol cycles [58], and decreased cortisol responses to acute psychological stress [59]. Ovarian cancer survivors are also reported to have altered cortisol cycles, and a return to normal cortisol cycles are associated with improvements in fatigue [32]. HPA-axis dysregulation has been reported in other cancer survivor groups, including nasopharyngeal [60], prostate [61], and leukemia [62], although fatigue was not measured in these studies. Nonetheless, there is evidence suggesting that HPA-axis dysfunction is a common occurrence following cancer treatment and that it is associated with fatigue.
Alternative mechanisms
Additional explanations for fatigue among cancer survivors include loss of fitness. Sarcopenia is a common side effect of cancer and its treatments, where alterations in skeletal muscle metabolism lead to the loss of skeletal muscle contractile strength and mass [63]. The resulting loss of muscle strength likely contributes to the physical experience of fatigue [22]. Cancer patients also exhibit lower oxygen consumption, which combined with decreased muscle strength makes the relative intensity of daily living activities closer to the anaerobic threshold [64, 65]. Certain psychological traits are also risk factors for developing fatigue. Specifically, breast cancer survivors suffering from fatigue have poor self-efficacy [66], that is, the belief in their ability to execute behaviors necessary to produce a specific performance. Engaging in catastrophizing or negative thoughts is also strongly predictive of fatigue in breast cancer survivors [7].
Despite the fact that the majority of the literature to date supports a connection between inflammation and persistent fatigue in cancer survivors, no definite proof has yet been obtained for a causal role of inflammation in this population. In addition, a few studies report negative results [67, 68], or concomitant increases in anti-inflammatory mediators among fatigued cancer survivors [24, 28]. A closer analysis of the literature on toxicities of cancer therapy and inflammation shows that there are alternative to the inflammation hypothesis. In addition to their direct cytotoxic effects on highly proliferative cells, chemotherapeutic agents generate free radicals and superoxides that contribute to cellular DNA damage and cell death. These events take place not only at the level of the tumor but also in distant organs. There, radical oxygen species induce tissue injury that triggers inflammatory pathway cascades. This mechanism is responsible for the toxic effect of chemotherapeutic agents on the gastrointestinal tract, kidney, and heart [69, 70]. However, there is no evidence for propagation of the very localized inflammatory response affecting peripheral organs.
The situation is similar with radiation. Radiotherapy can also cause inflammation in target organs, such as the lung in patients with lung cancer. However, even in this case there is no correlation between the severity of radiation-induced pneumonitis and circulating biomarkers of inflammation, other than IL-6 and this in a non-consistent manner [71]. Most chemotherapeutic agents have a limited penetrability into the nervous system, and even when they can cross the blood-nerve or the blood-brain barrier they do not cause neuronal death since neurons are terminally differentiated cells. What is observed instead is evidence of neuronal mitochondrial damage that usually manifests in the form of swelling mitochondria [72]. In summary, there is no conclusive evidence for the occurrence of systemic inflammation in response to cancer therapy and subsequent activation of immune-to-brain communication pathways. A causal role for mitochondrial dysfunction has been demonstrated for chemotherapy-induced peripheral neuropathy [73–75]. This could also be involved in the pathophysiology of other cancer-related symptoms, including fatigue, by impairing neuronal energetic metabolism and function.
Exercise training interventions to reduce cancer-related fatigue
Several treatments have been proposed to address fatigue in cancer survivors, although so far no single treatment has been shown to be fully effective. A meta-analysis of randomized controlled trials of pharmaceutical therapies for cancer-related fatigue reveal a small effect size for all drug classes [76]. Psychosocial interventions, including educational, supportive, and behavioral interventions, have been more successful, with meta-analyses showing a small to moderate effect on cancer-related fatigue [77–79]. In particular, exercise interventions are significantly associated with improvements in fatigue, both during and after treatment, for a variety of cancers, including breast, colorectal, prostate, head and neck, gynecological, and hematological cancers [65, 80–86].
A significant reduction in cancer-related fatigue was reported in a systematic review of randomized controlled trials and controlled clinical trials that compared exercise interventions with non-exercise or standard-of-care controls on quality of life in adult cancer patients [83]. This analysis included 40 trials with 3694 cancer patients; 30 of the trials were conducted post-treatment. Despite the fact that a wide range of exercise interventions were employed (including resistance training, walking, cycling, yoga, Qigong, and Tai Chi), an overall significant reduction in fatigue was found after 12 weeks (standardized mean difference: −0.82, 95% confidence interval (95% CI): −1.50 to −0.14) and at a 6 month follow-up (standardized mean difference: −0.42; 95% CI −0.02 to −0.83) [83]. A meta-analysis of randomized controlled trials of exercise interventions among cancer survivors also reported an overall significant reduction in fatigue after the interventions [82]. Data from 44 studies enrolling a total of 3254 participants of different cancer types were included and demonstrated that the interventions reduced cancer-related fatigue to a greater extent than standard-of-care controls (weighted mean effect size: 0.31, 95% CI: 0.22 to 0.4). Characteristics of the exercise interventions from individual trials were also analyzed for moderating effects on change in fatigue. Greater reductions in cancer-related fatigue were noted with interventions that used moderate-to-high intensity resistance exercise (3–6 METs, 60–80% 1-Repetition Maximum). The length and number of each exercise session included in an intervention did not significantly impact results [82].
The importance of exercise intensity has also been demonstrated by a randomized controlled trial of exercise training in breast cancer survivors [84]. In this study, 25 postmenopausal breast cancer survivors trained 3 times a week for 15 weeks on a cycle ergometer; changes in peak oxygen consumption, peak power, and fatigue were compared to untrained controls. Statistically and clinically significant improvements in fatigue were observed in the trained group; these improvements were significantly correlated with increases in peak oxygen consumption and peak power output [84]. These results suggest that improvements in cardiopulmonary fitness mediate improvements in fatigue, although a causal relationship has not been established. A more recent meta-analysis of controlled studies involving cancer patients and survivors also found a statistically significant reduction in fatigue resulting from physical exercise interventions (weighted mean effect size: −0.54, 95% CI: −0.90 to −0.19) [86]. Of the 14 studies included in the analysis that used post treatment interventions, 13 report positive results and 7 were statistically significant. The authors of the meta-analysis caution that the overall moderate effect size should be interpreted bearing in mind that the effect sizes of the individual studies were heterogeneous [86].
Thus far, variability in the study populations, exercise prescription, outcome measures, and overall study quality make results hard to translate clinically. High quality, randomized controlled trials that prescribe and monitor exercise using the best available techniques and include multiple measurements of fatigue would add to the literature. It is also important for future studies to include various modes of exercise as it is unlikely that a one-size-fits-all approach in exercise training will be successful. Rather, taking into account factors such as age, cancer site, medical comorbidities, previous exercise training, and personal preference would help target exercise interventions to individuals. One note of caution in the interpretation of these studies discussed above is the existence of publication bias [78], meaning studies with minor or negative results could be missed. Another limitation is that most exercise interventions have not included fatigue as a criterion for study entry. Individuals not experiencing fatigue are unlikely to show change in this symptom, and so the effect of exercise on fatigue may actually be larger than has been reported. Alternatively, fatigue could be a barrier for entry into exercise trials. The randomized controlled trial by Courneya et al had a 14% recruitment rate which could limit the generalizability of the findings [84]. However, once enrolled, the exercise group completed 98.4% of the prescribed exercise sessions; similar adherence has been reported by others [86].
Exercise appears to be safe for cancer survivors, as similar numbers of adverse events have been reported in interventions and control groups [86]. Overall, exercise is well-tolerated by cancer survivors, and the American College of Sports Medicine guidelines recommend most cancer survivors accrue 150 minutes/week of moderate or 75 minutes/week of vigorous intensity aerobic activity, and engage in strength training twice a week, similar to recommendations for healthy populations [87]. Together, the literature support the feasibility of exercise training in cancer survivors, and the possibility that training yields improvements in both fitness and fatigue.
Mechanisms by which exercise may reduce fatigue
Psychological well-being and physical fitness
Just as the benefits of exercise training are multifaceted in healthy populations, exercise likely acts through a variety of mechanisms to improve the quality of life and reduce fatigue in cancer survivors. Even without addressing the underlying cause(s) of cancer-related fatigue, exercise training yields psychological benefits that can reduce the symptoms of fatigue. Attaining new skills and meeting physical activity goals can improve confidence, decrease catastrophizing behavior, and increase self-efficacy [88], thus reducing the contribution of these factors towards fatigue. McAuley et al have shown that self-efficacy mediates the relationship between increased physical activity levels and reduced fatigue in breast cancer survivors [66, 89]. Exercise training can also improve quality of sleep and decrease pain and mood disturbances [90].
Training-induced gains in physical fitness may alleviate fatigue by countering physical deconditioning through increased lean muscle mass and aerobic capacity. Cancer survivors participating in exercise interventions have by and large shown improvements in cardiopulmonary fitness and muscle strength [65, 84, 91], which could decrease the effort required to complete daily living activities and thus reduce fatigue. Of note, exercise interventions with pediatric patients have shown particular benefit for improving cardiopulmonary fitness and muscle strength [81]. Exercise training can also decrease fat mass, an important consideration as high body mass index (BMI) has been shown to be predictive of persistent fatigue in breast cancer survivors [29]. Reduction in BMI may also address chronic inflammation through decreased release of pro-inflammatory adipokines from visceral fat mass [92]. However, many studies have controlled for BMI when examining the effect of exercise interventions, and have found that exercise improves fatigue independently of changes in body composition [7, 84].
Anti-inflammatory effects
The anti-inflammatory effects of exercise training may also reduce cancer-related fatigue. In healthy populations, exercise training has been shown to increase the level of anti-inflammatory cytokines such as IL-10, reduce overall TNF-α expression, decrease CRP, decrease pro-inflammatory adipokines, and reduce expression of Toll-like receptors on monocytes and macrophages (reviewed in: [92–94]). Given the role that inflammation is proposed to play in the promotion of cancer-related fatigue, it is surprising that only a few studies have examined changes in inflammatory mediators following exercise interventions with cancer survivors. One study in leukemia patients undergoing chemotherapy reported that participants in an in-hospital aerobic and resistance training program had reductions in fatigue and a trend towards reduced IL-6 and increased IL-10 circulating levels [95]. A small 3-month randomized controlled trial in breast cancer survivors found that supervised aerobic and resistance exercise training led to decreases in TNF-α and the ratio of IL-6 to IL-10, although these did not reach significance [96]. Other studies in breast cancer survivors have reported a reduction in CRP after 15 weeks of aerobic training [97], and IL-6 after 6 months of aerobic training [98]. Unfortunately, these studies did not measure fatigue, and so it is not known if the reduction in inflammation would translate to improvements in fatigue. Yoga interventions in fatigued breast cancer survivors have demonstrated decreased symptoms of fatigue and reduced markers of inflammation, such as decreased IL-6, TNF-α, IL-1β [99], as well as reduced NF-κB activity, and increased activity of glucocorticoid receptors [100]. However the effects of the physical exercise and mindfulness components of yoga are difficult to separate [100].
Conversely, a few studies have reported an increase in immune system activation in cancer survivors following an exercise intervention. A two-week moderate aerobic exercise intervention in colorectal cancer survivors found a more pro-inflammatory state in response to lipopolysaccharide, although this study lacked a control group [101]. Similarly, increased lymphocyte activation to ex vivo stimulation was observed in exercise-trained breast cancer survivors [102].
Autonomic nervous system balance
Cardiopulmonary fitness is associated with greater HRV in healthy adults, and exercise training has been shown to increase HRV [103–105]. Among cancer patients, a 16 week moderate exercise intervention during and after treatment improved HRV [106]. This suggests that exercise can restore a balance between sympathetic and parasympathetic activity. As low HRV has been linked with fatigue in cancer survivors, exercise-mediated increases in parasympathetic activity could be an additional mechanism by which exercise training addresses fatigue. However, a relationship between exercise-induced increases in HRV and fatigue has not been established in cancer survivors.
It has been proposed that cancer and its treatments accelerate aging processes, and that fatigued patients and survivors might be biologically older than their chronological age may suggest [7]. In support of this, the decreases in HRV observed in fatigued cancer survivors mimic the lower HRV found in older adults, and have been calculated to be equivalent of a 20 year difference compared to age-matched non-fatigued cancer survivors [20]. Another characteristic shared between cancer survivors and older adults is the accumulation of senescent cells. In healthy aging, this is largely driven by repeated exposure to infectious agents across the lifetime [107]. Cancer and its treatments cause DNA damage which can also induce cellular senescence, and large numbers of senescent cells have been observed in childhood cancer survivors [108]. One consequence of the accretion of senescent cells, particularly senescent immune cells, is chronic inflammation [109]. Increased numbers of senescent immune cells have been observed in other patient groups suffering from fatigue [110], but a relationship between senescent immune cells and fatigue in cancer-survivors has not yet been established. There is evidence that regular physical exercise can help prevent or possibly reverse aspects of immunosenescence including senescent immune cells [111, 112]. Reductions in pro-inflammatory senescent cells could therefore be an additional means by which fatigue is reduced through exercise training, although at this time this is speculative and an area that warrants further research.
Neurotrophic factors
Exercise also has protective effects on brain function. For example, exercise training reduces microglial activation [113, 114] and increases expression of neurotrophic factors such as brain derived neurotrophic factor [115] and adiponectin [116, 117]. As discussed above, elevated levels of the tryptophan metabolite kynurenine are associated with inflammation, depression, and fatigue [12] (Figure 1), so an exercise-induced reduction in kynurenine levels could benefit these conditions. While one study reported no exercise-induced changes in the concentration of IL-6, neopterin, tryptophan, and kynurenine with moderate-intensity exercise in depressed patients, the exercise intervention was unsupervised and lasted just one week [118].
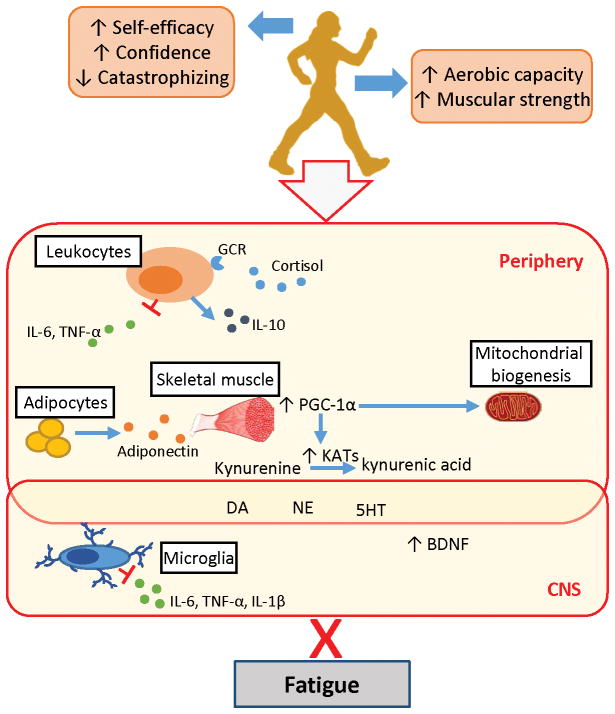
A more recent publication has provided evidence for an exercise-induced protection of brain function via modification of kynurenine levels [119]. Agudelo et al demonstrate that peroxisome proliferator-activated receptor-gamma co-activator (PGC)-1α1 overexpression in muscle promotes the expression of kynurenine aminotransferase (KAT), which prevents kynurenine from crossing the blood brain barrier [119]. Mice overexpressing PGC-1α1 also avoided the increase in neuro-inflammatory markers (such as macrophage inflammatory protein 1α (MIP1α)) following chronic stress exposure observed in wild type mice [119]. The authors further show that exercise training increased expression of both PGC-1α and KAT in mice as well as human skeletal muscle, corroborating several reports of increased PGC-1α expression in endurance-trained humans [120, 121]. Exercise-induced increases in adiponectin levels have been proposed to provide a stimulus for increased PGC-1α levels via AMPK/SIRT1-dependent pathways [122]. Thus, exercise-induced increases in adiponectin and PGC-1α1 may reduce neuro-inflammation and help maintain normal levels of tryptophan and serotonin. An alternative that still needs to be examined in the case of exercise and cancer-related symptoms is that PGC-1α activates mitochondrial biogenesis, therefore opposing the mitochondrial dysfunction induced by cancer therapy [123]. Pathways by which exercise training may reduce cancer related fatigue are summarized in Figure 2.
Figure 2.
Pathways by which exercise may ameliorate cancer-related fatigue. Exercise training confers psychological benefits, such as increased self-efficacy, and leads to improved fitness which eases the effort required for daily life activities. Exercise training decreases chronic inflammation through reductions in inflammatory cytokines, increased production of interleukin-10 (IL-10), and increased glucocorticoid receptor (GCR) function. Reduced inflammation in the periphery prevents disruption of neurotransmitter production (including norepinephrine (NE) and dopamine (DA)) and inflammation in the central nervous system (CNS). Exercise is further associated with increased neurotrophic factors such as brain derived neurotrophic factor (BDNF) and adiponectin. Exercise-induced increases in skeletal muscle PGC-1α expression, perhaps mediated by adiponectin, helps maintain levels of tryptophan (Trp) and serotonin (5HT) through activation of kynurenine aminotransferases (KATs), which favor the formation of neuroprotective kynurenine metabolites (kynurenic acid). PGC-1α may also promote mitochondrial biogenesis. Dashed lines indicate a disrupted pathway.
Areas for future research
Unfortunately, the translation of exercise interventions from the research lab to the clinic has been slow. Many fatigued cancer survivors are advised to conserve their energy expenditure and limit physical activity, which could actually worsen symptoms of fatigue through the synergistic effects of deconditioning and cancer cachexia [82, 124]. Further, some clinicians and patients fear that exercise could cause adverse events, such as lymphedema in breast cancer survivors [125]. While a meta-analysis has found no difference in adverse events including lymphedema between exercise and control groups [86], many studies have done a poor job describing adverse events [23]. Future investigations should carefully monitor and report these events so that conclusions can be accurately made about the safety of exercise. The recruitment rate and overall adherence should also be carefully described. Another difficulty in the implementation of exercise as standard practice is that although the American College of Sports Medicine recommends aerobic and resistance exercise for cancer survivors [87], there is no specific prescription for survivors suffering from fatigue [82]. This may be due in part to difficulties in comparing results across studies, as exercise interventions have differed in mode, intensity, frequency, the timing of the intervention relative to treatment, duration of each session and overall length of the intervention, and how and whether the exercise is monitored. Because associations have been found between improvements in cardiopulmonary fitness and fatigue [84], future exercise interventions should be designed using the best-available evidence from the exercise science literature to yield large fitness gains. Alternatively, the success that yoga interventions have had in reducing both inflammation and fatigue in breast cancer survivors [99, 100] suggests that yoga may provide additional options for patients who might otherwise decline to exercise.
Comparisons across different studies are also hindered by variability in outcome domains arising from the different definitions and measurements for cancer-related fatigue currently in use [2]. A key obstacle in mechanistic studies of cancer-related fatigue is the reliance on subjective feelings to assess fatigue instead of objective measurements of performance and fatigability. Besides the use of activity monitors and all their ambiguities this is an area of research that has been clearly left aside. Researchers should take note of the emergence of computerized tasks for assessing several aspects of motivated behavior in human subjects [126] and the increasing use of neuroimaging approaches in neurology, psychiatry, and cognitive psychology [127].
Future exercise intervention studies should also include outcome measures that aim to elucidate the mechanisms by which exercise yields its effect, such as longitudinal observations of changes in immune system activation and function. These measurements should go beyond measuring circulating cytokines, as serum or plasma levels of cytokines do not necessarily reflect levels in the brain where the symptoms of cancer-related fatigue likely originate. Inflammatory cytokines act as autocrine, juxtacrine, and paracrine communication signals in the microenvironment in which they are produced, and it is not clear if circulating cytokines reflect “spillover” of molecules released locally from the site of inflammation or if they arise from an inflammatory environment that influences tissue-specific activity. An alternative is to measure activation of intracellular cytokine signaling pathways in circulating immune cells or to assess the ability of these cells to produce cytokines in response to stimulation. Future exercise intervention studies could also consider measuring glucocorticoid receptor function as well as cortisol levels. In addition, it will be interesting for future studies to monitor training-induced changes in HRV in cancer survivors, and to determine if increases in HRV are linked to reductions in fatigue. Well-designed exercise interventions that measure factors known to act on central nervous system pathways involved in fatigue behavior, such as kynurenine, will also add to literature.
Overall, there is a need for higher quality randomized controlled trials with larger sample sizes and increased duration of follow-up measures. Other methodological issues in the existing literature include lack of control groups, failure to blind outcome assessments, and not controlling for potentially confounding variables such as age and previous fitness level. Inclusion criteria also need to be carefully considered. There is large inter-individual variability in the experience of cancer-related fatigue, as some patients develop high symptom levels whereas others experience very little. Using fatigue as an inclusion criterion would improve the translational potential of intervention studies, as highly fatigued individuals are likely to show the greatest improvements in this symptom following exercise training interventions. Finally, more studies should consider fatigue in pediatric cancer survivors, as their experience of fatigue and the underlying causes may differ from adult survivors. There are now fatigue measures available for use in children as young as 7 years, with ongoing efforts to validate common items in pediatric and adult questionnaires [2].
Conclusion
Awareness of the issue of cancer-related fatigue is growing, and its etiology and treatment are areas of active research. Symptom control during cancer treatment could enhance therapeutic outcomes by increasing adherence to treatment, and thus potentially increase survival. Reducing fatigue is also important following completion of cancer treatment, as persistent fatigue poses a barrier to the resumption of pre-cancer lifestyles. Research conducted in cancer survivors demonstrates that both psychological and biological factors contribute towards the development and persistence of fatigue. Although most work has focused on inflammation, the evidence is far from conclusive and there are alternatives that clearly deserve consideration. A large number of research studies support the ability of exercise training to alleviate cancer-related fatigue, especially among supervised interventions that also yield improvements in fitness parameters. While the exact mechanisms underlying this benefit are unknown, evidence is accumulating for several models. These include increases in functional capacity, reductions in chronic inflammation which can mediate sickness behavior, increases in parasympathetic nervous system activity, and the protection of neurotransmitters through mediators such as PGC-1α, all of which have been shown to accompany exercise training programs. There is still much work to be done until the prescription of exercise becomes standard practice for cancer survivors. With improvements in the quality of studies, evidenced-based exercise interventions will allow exercise scientists and oncologists to work together to treat cancer-related fatigue.
Acknowledgments
RD is supported by grants from the National Institute of Neurological Diseases and Stroke of the National Institutes of Health (Grants R01 NS073939; R01 NS074999) and the National Cancer Institute and the National Institute of Mental Health (Grants R01CA193522; R21CA183736). All research at The University of Texas MD Anderson Cancer Center is supported in part by the institution’s Cancer Center Support Grant, NCI P30 016672.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the other sponsors.
References
- 1.Berger AM, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–39. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsevick AM, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105(19):1432–40. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett B, et al. The experience of cancer-related fatigue and chronic fatigue syndrome: a qualitative and comparative study. J Pain Symptom Manage. 2007;34(2):126–35. doi: 10.1016/j.jpainsymman.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Cleeland CS, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–25. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA, et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence DP, et al. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 7.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenvold M, et al. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105(2):209–19. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 9.Prue G, et al. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006;42(7):846–63. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–8. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 11.Jones JM, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2015 doi: 10.1007/s11764-015-0450-2. [DOI] [PubMed] [Google Scholar]
- 12.Dantzer R, et al. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37(1):39–46. doi: 10.1016/j.tins.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. European journal of cancer care. 1996;5(2 Suppl):8–23. doi: 10.1111/j.1365-2354.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 14.Curt GA, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The oncologist. 2000;5(5):353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 15.Sadler IJ, et al. Preliminary evaluation of a clinical syndrome approach to assessing cancer-related fatigue. Journal of pain and symptom management. 2002;23(5):406–16. doi: 10.1016/s0885-3924(02)00388-3. [DOI] [PubMed] [Google Scholar]
- 16.Van Belle S, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2005;13(4):246–54. doi: 10.1007/s00520-004-0734-y. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza TR, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9(7):414–26. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagundes CP, et al. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36(8):1137–47. doi: 10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger AM, et al. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18(1):105–14. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 22.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–52. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 23.McNeely ML, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bower JE, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–11. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Alfano CM, et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30(12):1280–7. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orre IJ, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71(3):136–41. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Alexander S, et al. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45(3):384–92. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landmark-Hoyvik H, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J. 2009;9(5):333–40. doi: 10.1038/tpj.2009.27. [DOI] [PubMed] [Google Scholar]
- 29.Reinertsen KV, et al. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4(4):405–14. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bower JE, et al. T-cell homeostasis in breast cancer survivors with persistent fatigue. J Natl Cancer Inst. 2003;95(15):1165–8. doi: 10.1093/jnci/djg0019. [DOI] [PubMed] [Google Scholar]
- 31.Collado-Hidalgo A, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–66. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 32.Schrepf A, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun. 2013;30(Suppl):S126–34. doi: 10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rausch SM, et al. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116(17):4103–13. doi: 10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bower JE, et al. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–50. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yount S, et al. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(6):838–46. [PubMed] [Google Scholar]
- 36.Tyring S, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 37.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33(3):315–27. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 39.Neurauter G, et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008;9(7):622–7. doi: 10.2174/138920008785821738. [DOI] [PubMed] [Google Scholar]
- 40.Dantzer R, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connor JC, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182(5):3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor JC, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14(5):511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsson J, et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121(1–3):187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 44.Walker AK, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38(9):1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurz K, et al. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS One. 2012;7(5):e36956. doi: 10.1371/journal.pone.0036956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74(2):224–42. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med. 1999;50:249–61. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- 48.Tak LM, et al. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol Psychol. 2009;82(2):101–10. doi: 10.1016/j.biopsycho.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci. 2007;18(3):275–81. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- 50.Egelund N. Spectral analysis of heart rate variability as an indicator of driver fatigue. Ergonomics. 1982;25(7):663–72. doi: 10.1080/00140138208925026. [DOI] [PubMed] [Google Scholar]
- 51.Hautala A, et al. Changes in cardiac autonomic regulation after prolonged maximal exercise. Clin Physiol. 2001;21(2):238–45. doi: 10.1046/j.1365-2281.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 52.Lakoski SG, et al. Autonomic dysfunction in early breast cancer: Incidence, clinical importance, and underlying mechanisms. Am Heart J. 2015;170(2):231–41. doi: 10.1016/j.ahj.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vigo C, et al. Evidence of altered autonomic cardiac regulation in breast cancer survivors. J Cancer Surviv. 2015 doi: 10.1007/s11764-015-0445-z. [DOI] [PubMed] [Google Scholar]
- 54.Crosswell AD, et al. Low heart rate variability and cancer-related fatigue in breast cancer survivors. Psychoneuroendocrinology. 2014;45:58–66. doi: 10.1016/j.psyneuen.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicolaides NC, et al. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci. 2014;1318:71–80. doi: 10.1111/nyas.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacHale SM, et al. Diurnal variation of adrenocortical activity in chronic fatigue syndrome. Neuropsychobiology. 1998;38(4):213–7. doi: 10.1159/000026543. [DOI] [PubMed] [Google Scholar]
- 58.Bower JE, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30(1):92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67(2):277–80. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- 60.Ratnasingam J, et al. Hypothalamic pituitary dysfunction amongst nasopharyngeal cancer survivors. Pituitary. 2015;18(4):448–55. doi: 10.1007/s11102-014-0593-6. [DOI] [PubMed] [Google Scholar]
- 61.Hoyt MA, et al. Approach and avoidance coping: diurnal cortisol rhythm in prostate cancer survivors. Psychoneuroendocrinology. 2014;49:182–6. doi: 10.1016/j.psyneuen.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Follin C, et al. Moderate dose cranial radiotherapy causes central adrenal insufficiency in long-term survivors of childhood leukaemia. Pituitary. 2014;17(1):7–12. doi: 10.1007/s11102-012-0459-8. [DOI] [PubMed] [Google Scholar]
- 63.Al-Majid S, Waters H. The biological mechanisms of cancer-related skeletal muscle wasting: the role of progressive resistance exercise. Biol Res Nurs. 2008;10(1):7–20. doi: 10.1177/1099800408317345. [DOI] [PubMed] [Google Scholar]
- 64.Davis MP, Walsh D. Mechanisms of fatigue. J Support Oncol. 2010;8(4):164–74. [PubMed] [Google Scholar]
- 65.McMillan EM, I, Newhouse J. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a meta-analysis. Appl Physiol Nutr Metab. 2011;36(6):892–903. doi: 10.1139/h11-082. [DOI] [PubMed] [Google Scholar]
- 66.McAuley E, et al. Physical activity and fatigue in breast cancer and multiple sclerosis: psychosocial mechanisms. Psychosom Med. 2010;72(1):88–96. doi: 10.1097/PSY.0b013e3181c68157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knobel H, et al. High level of fatigue in lymphoma patients treated with high dose therapy. J Pain Symptom Manage. 2000;19(6):446–56. doi: 10.1016/s0885-3924(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 68.Dimeo F, et al. Physical performance, depression, immune status and fatigue in patients with hematological malignancies after treatment. Ann Oncol. 2004;15(8):1237–42. doi: 10.1093/annonc/mdh314. [DOI] [PubMed] [Google Scholar]
- 69.Vyas D, Laput G, Vyas AK. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014;7:1015–23. doi: 10.2147/OTT.S60114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee CS, Ryan EJ, Doherty GA. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J Gastroenterol. 2014;20(14):3751–61. doi: 10.3748/wjg.v20.i14.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sprung CN, et al. Immunological markers that predict radiation toxicity. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 72.Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol. 2014;10(6):326–36. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett GJ. Pathophysiology and animal models of cancer-related painful peripheral neuropathy. Oncologist. 2010;15(Suppl 2):9–12. doi: 10.1634/theoncologist.2009-S503. [DOI] [PubMed] [Google Scholar]
- 74.Krukowski K, et al. Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrin-mu. Pain. 2015;156(11):2184–92. doi: 10.1097/j.pain.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol. 2011;232(2):154–61. doi: 10.1016/j.expneurol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minton O, et al. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100(16):1155–66. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 77.Jacobsen PB, et al. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660–7. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duijts SF, et al. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology. 2011;20(2):115–26. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 79.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134(5):700–41. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 80.Speed-Andrews AE, Courneya KS. Effects of exercise on quality of life and prognosis in cancer survivors. Curr Sports Med Rep. 2009;8(4):176–81. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]
- 81.Wolin KY, et al. Exercise in adult and pediatric hematological cancer survivors: an intervention review. Leukemia. 2010;24(6):1113–20. doi: 10.1038/leu.2010.54. [DOI] [PubMed] [Google Scholar]
- 82.Brown JC, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–33. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 83.Mishra SI, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Courneya KS, et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–8. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 85.Donnelly CM, et al. A randomised controlled trial testing the feasibility and efficacy of a physical activity behavioural change intervention in managing fatigue with gynaecological cancer survivors. Gynecol Oncol. 2011;122(3):618–24. doi: 10.1016/j.ygyno.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 86.Speck RM, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 87.Schmitz KH, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 88.McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exerc Sport Sci Rev. 2000;28(2):85–8. [PubMed] [Google Scholar]
- 89.Phillips SM, McAuley E. Physical activity and fatigue in breast cancer survivors: a panel model examining the role of self-efficacy and depression. Cancer Epidemiol Biomarkers Prev. 2013;22(5):773–81. doi: 10.1158/1055-9965.EPI-12-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartescu I, Morgan K, Stevinson CD. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: a randomized controlled trial. J Sleep Res. 2015 doi: 10.1111/jsr.12297. [DOI] [PubMed] [Google Scholar]
- 91.Al-Majid S, Gray DP. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biol Res Nurs. 2009;10(4):381–91. doi: 10.1177/1099800408324431. [DOI] [PubMed] [Google Scholar]
- 92.Gleeson M, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 93.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 94.Plaisance EP, Grandjean PW. Physical activity and high-sensitivity C-reactive protein. Sports Med. 2006;36(5):443–58. doi: 10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- 95.Battaglini CL, et al. The effects of an exercise program in leukemia patients. Integr Cancer Ther. 2009;8(2):130–8. doi: 10.1177/1534735409334266. [DOI] [PubMed] [Google Scholar]
- 96.Rogers LQ, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12(4):323–35. doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fairey AS, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005;19(5):381–8. doi: 10.1016/j.bbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Jones SB, et al. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer Prev Res (Phila) 2013;6(2):109–18. doi: 10.1158/1940-6207.CAPR-12-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kiecolt-Glaser JK, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32(10):1040–9. doi: 10.1200/JCO.2013.51.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bower JE, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–9. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detect Prev. 2004;28(3):208–13. doi: 10.1016/j.cdp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Hutnick NA, et al. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005;37(11):1827–35. doi: 10.1249/01.mss.0000175857.84936.1a. [DOI] [PubMed] [Google Scholar]
- 103.Nolan RP, et al. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: a systematic review. Eur J Cardiovasc Prev Rehabil. 2008;15(4):386–96. doi: 10.1097/HJR.0b013e3283030a97. [DOI] [PubMed] [Google Scholar]
- 104.Seals DR, Chase PB. Influence of physical training on heart rate variability and baroreflex circulatory control. J Appl Physiol (1985) 1989;66(4):1886–95. doi: 10.1152/jappl.1989.66.4.1886. [DOI] [PubMed] [Google Scholar]
- 105.Levy WC, et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol. 1998;82(10):1236–41. doi: 10.1016/s0002-9149(98)00611-0. [DOI] [PubMed] [Google Scholar]
- 106.Niederer D, et al. Exercise effects on HRV in cancer patients. Int J Sports Med. 2013;34(1):68–73. doi: 10.1055/s-0032-1314816. [DOI] [PubMed] [Google Scholar]
- 107.Pawelec G, et al. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19(1):47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- 108.Ness KK, et al. Frailty in childhood cancer survivors. Cancer. 2015;121(10):1540–7. doi: 10.1002/cncr.29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hassan IS, et al. A study of the immunology of the chronic fatigue syndrome: correlation of immunologic parameters to health dysfunction. Clin Immunol Immunopathol. 1998;87(1):60–7. doi: 10.1006/clin.1997.4512. [DOI] [PubMed] [Google Scholar]
- 111.Spielmann G, et al. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav Immun. 2011;25(8):1521–9. doi: 10.1016/j.bbi.2011.07.226. [DOI] [PubMed] [Google Scholar]
- 112.Simpson RJ. Aging, persistent viral infections, and immunosenescence: can exercise “make space”? Exerc Sport Sci Rev. 2011;39(1):23–33. doi: 10.1097/JES.0b013e318201f39d. [DOI] [PubMed] [Google Scholar]
- 113.Kohman RA, et al. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masson GS, et al. Aerobic training normalizes autonomic dysfunction, HMGB1 content, microglia activation and inflammation in hypothalamic paraventricular nucleus of SHR. Am J Physiol Heart Circ Physiol. 2015;309(7):H1115–22. doi: 10.1152/ajpheart.00349.2015. [DOI] [PubMed] [Google Scholar]
- 115.Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30(2):75–9. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 116.Yau SY, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014;111(44):15810–5. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kriketos AD, et al. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27(2):629–30. doi: 10.2337/diacare.27.2.629. [DOI] [PubMed] [Google Scholar]
- 118.Hennings A, et al. Exercise affects symptom severity but not biological measures in depression and somatization - results on IL-6, neopterin, tryptophan, kynurenine and 5-HIAA. Psychiatry Res. 2013;210(3):925–33. doi: 10.1016/j.psychres.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 119.Agudelo LZ, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 120.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mahoney DJ, Tarnopolsky MA. Understanding skeletal muscle adaptation to exercise training in humans: contributions from microarray studies. Phys Med Rehabil Clin N Am. 2005;16(4):859–73. vii. doi: 10.1016/j.pmr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 122.Sanchis-Gomar F, Quilis CP, Lucia A. Antidepressant Effects of Exercise: A Role for the Adiponectin-PGC-1alpha-kynurenine Triad? J Cell Physiol. 2015;230(10):2328–9. doi: 10.1002/jcp.24964. [DOI] [PubMed] [Google Scholar]
- 123.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282(4):647–72. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 124.Lucia A, Earnest C, Perez M. Cancer-related fatigue: can exercise physiology assist oncologists? Lancet Oncol. 2003;4(10):616–25. doi: 10.1016/s1470-2045(03)01221-x. [DOI] [PubMed] [Google Scholar]
- 125.Eickmeyer SM, et al. The role and efficacy of exercise in persons with cancer. PM R. 2012;4(11):874–81. doi: 10.1016/j.pmrj.2012.09.588. [DOI] [PubMed] [Google Scholar]
- 126.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dobryakova E, et al. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. 2015;6:52. doi: 10.3389/fneur.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]