Abstract
Background
Patients with a low platelet count (thrombocytopenia) often require the insertion of central lines (central venous catheters (CVCs)). CVCs have a number of uses; these include: administration of chemotherapy; intensive monitoring and treatment of critically‐ill patients; administration of total parenteral nutrition; and long‐term intermittent intravenous access for patients requiring repeated treatments. Current practice in many countries is to correct thrombocytopenia with platelet transfusions prior to CVC insertion, in order to mitigate the risk of serious procedure‐related bleeding. However, the platelet count threshold recommended prior to CVC insertion varies significantly from country to country. This indicates significant uncertainty among clinicians of the correct management of these patients. The risk of bleeding after a central line insertion appears to be low if an ultrasound‐guided technique is used. Patients may therefore be exposed to the risks of a platelet transfusion without any obvious clinical benefit.
Objectives
To assess the effects of different platelet transfusion thresholds prior to the insertion of a central line in patients with thrombocytopenia (low platelet count).
Search methods
We searched for randomised controlled trials (RCTs) in CENTRAL (The Cochrane Library 2015, Issue 2), MEDLINE (from 1946), EMBASE (from 1974), the Transfusion Evidence Library (from 1950) and ongoing trial databases to 23 February 2015.
Selection criteria
We included RCTs involving transfusions of platelet concentrates, prepared either from individual units of whole blood or by apheresis, and given to prevent bleeding in patients of any age with thrombocytopenia requiring insertion of a CVC.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
One RCT was identified that compared different platelet transfusion thresholds prior to insertion of a CVC in people with chronic liver disease. This study is still recruiting participants (expected recruitment: up to 165 participants) and is due to be completed in December 2017. There were no completed studies. There were no studies that compared no platelet transfusions to a platelet transfusion threshold.
Authors' conclusions
There is no evidence from RCTs to determine whether platelet transfusions are required prior to central line insertion in patients with thrombocytopenia, and, if a platelet transfusion is required, what is the correct platelet transfusion threshold. Further randomised trials with robust methodology are required to develop the optimal transfusion strategy for such patients. The one ongoing RCT involving people with cirrhosis will not be able to answer this review's questions, because it is a small study that assesses one patient group and does not address all of the comparisons included in this review. To detect an increase in the proportion of participants who had major bleeding from 1 in 100 to 2 in 100 would require a study containing at least 4634 participants (80% power, 5% significance).
Plain language summary
Comparison of different platelet transfusion thresholds prior to insertion of central lines in people with a low platelet count
Review question
We evaluated the evidence about whether people with a low platelet count require a platelet transfusion prior to insertion of a central line (central venous catheter (CVC)), and if so what is the platelet count level at which a platelet transfusion is required.
Background
Patients with a low platelet count often require the insertion of central lines. Central lines are catheters whose tip usually lies in one of two main veins returning blood to the heart. They have a number of uses including: giving chemotherapy; intensive monitoring and treatment of critically‐ill patients; giving nutrition into a vein (when the patient cannot eat); and when patients require long‐term repeated treatments in to a vein. Current practice in many countries is to increase the platelet count above a pre‐specified level with platelet transfusions to prevent serious bleeding due to the procedure. However, the platelet count level recommended prior to central line insertion varies significantly from country to country. This means that clinicians are uncertain about which is the correct platelet count level, or if a platelet transfusion is required. The risk of bleeding after a central line insertion appears to be low if the clinician uses ultrasound to guide insertion of the line. Patients may, therefore, be exposed to the risks of a platelet transfusion without any obvious clinical benefit.
Study characteristics
The evidence is current to February 2015. In this review one randomised controlled trial was identified that compared giving platelet transfusions at a low platelet count (25 x 109/l) versus giving platelet transfusions at a higher platelet count (50 x 109/l) prior to insertion of a central line to prevent bleeding. This trial is still recruiting and is due to complete recruitment in December 2017. There were no trials that compared no platelet transfusions versus giving platelet transfusions at a prespecified platelet count.
Key results
There are no results from the one eligible study because it is still recruiting participants. This ongoing study (expected to recruit 165 participants) will be unable to provide sufficient data for this review's primary outcomes because major bleeding and mortality are uncommon. We would need to design a study with at least 4634 participants to be able to detect an increase in the number of people who had major bleeding from 1 in 100 to 2 in 100.
Quality of the evidence
There is no evidence from randomised controlled trials to answer our review questions.
Background
Description of the condition
Patients with a low platelet count (thrombocytopenia) often require the insertion of central lines (central venous catheters (CVCs)). CVCs are catheters with tips that lie within the proximal third of the superior vena cava (large vein which returns blood to the heart), the right atrium or the inferior vena cava (Bishop 2007; Smith 2013). They can be inserted through a superficial vein (e.g. the basilic or cephalic veins in the arm) or a central vein (most commonly the jugular, subclavian or femoral veins) (Bishop 2007; Smith 2013). There are four main types: 1) a non‐tunnelled line into a central vein (short‐term use); 2) a line inserted into a superficial vein (medium‐term use); 3) a tunnelled line (long‐term use); and 4) a totally implanted device (long‐term use) (Bishop 2007; Smith 2013). They have a number of uses; these include: administration of chemotherapy and other irritant drugs with fewer complications; intensive monitoring and treatment of critically‐ill patients; administration of total parenteral nutrition; and long‐term intermittent intravenous access for patients requiring repeated treatments (Smith 2013). Patients requiring CVCs can have a variety of conditions, and include: patients with haematological malignancies, patients receiving chemotherapy, patients with liver failure, and patients who are critically ill (Bishop 2007; Smith 2013).
CVCs are associated with complications, these include bleeding, thrombosis, infection, misplacement of the CVC and pneumothorax (Bishop 2007; Smith 2013).
A low platelet count is a relative contraindication to the insertion of a CVC due to the risk of bleeding (Bishop 2007; Smith 2013). Platelet transfusions are used in modern clinical practice to prevent and treat bleeding in thrombocytopenic patients. Administration of platelet transfusions to patients with haematological disorders now constitute a significant proportion (up to 67%) of all platelet components issued (Cameron 2007; Greeno 2007; Pendry 2011), and 15% of these are given to prevent bleeding prior to a procedure (Estcourt 2012).
Central line insertion is the most common intervention that requires prophylactic platelet transfusions (to prevent bleeding) in patients with haematological disorders (Estcourt 2012). Critically‐ill patients usually require central line insertion to administer treatments. A large United Kingdom (UK) study of patients admitted to the intensive care unit (ICU) reported that 9% developed thrombocytopenia (Stanworth 2013).
Description of the intervention
Current practice in many countries is to correct thrombocytopenia with platelet transfusions prior to CVC insertion, in order to mitigate the risk of serious peri‐ or post‐procedural bleeding. The platelet count threshold recommended prior to CVC insertion varies significantly from country to country. For example, in the UK the current threshold is 50 x 109/L (BCSH 2003), in Belgium the threshold is 30 x 109/L (Bosly 2007), in the United States (US) the threshold is 20 x 109/L (Kaufman 2015), and in Germany the threshold is 10 x 109/L, unless there are risk factors for bleeding (GMA 2009).
There is no standard platelet count that alternative thresholds can be compared against. Therefore we will make the following two main comparisons.
The most common thresholds recommended by guidelines from different countries (10 x 109/L, 20 x 109/L, 30 x 109/L, 50 x 109/L) versus no prophylactic platelet transfusion prior to the procedure.
The lower thresholds recommended by guidelines from different countries (10 x 109/L, 20 x 109/L, 30 x 109/L) versus the highest commonly‐used threshold (50 x 109/L).
Platelet transfusions are associated with adverse events. Mild to moderate reactions to platelet transfusions include rigors, fever, and urticaria (an allergic reaction) (Heddle 2009). These reactions are not life‐threatening but can be extremely distressing for the patient. Rarer, but more serious sequelae include: anaphylaxis (life‐threatening allergic reaction); transfusion‐transmitted infections; transfusion‐related acute lung injury; and immunomodulatory effects (Benson 2009; Blumberg 2009; Bolton‐Maggs 2012; Heddle 2009; Khan 2007; Knowles 2011; Pearce 2011; Popovsky 1985; Silliman 2003; Taylor 2010). The requirement to administer platelet transfusions to correct thrombocytopenia prior to central line insertion may additionally delay the start of treatment, which may be time‐critical in a patient in intensive care. It remains unclear whether platelet transfusions in thrombocytopenic non‐bleeding patients, despite improving the platelet count, reduce the incidence of clinically‐important bleeding or improve other meaningful patient‐oriented outcomes, such as mortality.
How the intervention might work
Platelet transfusions are administered to thrombocytopenic patients in order to increase the platelet count and therefore reduce the incidence of bleeding. However, the risk of bleeding after a central line insertion appears to be low if an ultrasound‐guided technique is used (Cavanna 2010; Hind 2003). A systematic review showed that ultrasound guidance significantly reduced the failure rate of cannulating the internal jugular vein (risk ratio (RR) 0.14, 95% confidence interval (CI) 0.06 to 0.33) compared to using an anatomical landmark method (Hind 2003). In Cavanna 2010, 1978 ultrasound‐guided CVC procedures were performed in 1660 patients who had a solid or haematological malignancy, of whom 116 had a platelet count below 50 x 109/L, and 70 had a platelet count below 20 x 109/L. None of the patients experienced major bleeding. Patients may therefore be exposed to the risks of a platelet transfusion without any obvious clinical benefit.
Why it is important to do this review
As discussed above, the platelet count threshold recommended prior to CVC insertion varies significantly from country to country (BCSH 2003; Bosly 2007; GMA 2009; Kaufman 2015). This indicates significant uncertainty among clinicians of the correct management for these patients.
Several non‐randomised studies have demonstrated the safety of performing invasive procedures without clinically‐significant bleeding in patients with thrombocytopenia who did not receive prophylactic platelet transfusions (Foster 1992; Haas 2010; Hong Pheng Loh 2007; Ray 1997). The use of a platelet count threshold above which a platelet transfusion is required prior to CVC insertion has therefore been called into question. It is uncertain whether platelet transfusions are effective at preventing bleeding in patients with thrombocytopenia undergoing an invasive procedure. If effective, the platelet count threshold above which platelet transfusions are clinically effective is also uncertain.
Objectives
To assess the effects of different platelet transfusion thresholds prior to the insertion of a central line in patients with thrombocytopenia (low platelet count).
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs), irrespective of publication status.
Types of participants
We included patients of any age with thrombocytopenia (as defined by the studies' own definitions) requiring insertion of a central venous catheter (CVC) (tunnelled or untunnelled), or portacath. We excluded patients who were experiencing clinically‐significant bleeding at the time of the catheter insertion, because such patients are routinely given platelet transfusions to treat the bleeding.
Types of interventions
We included RCTs comparing the following two types of platelet transfusion regime.
1) No platelet transfusion prior to central line insertion versus platelet transfusion prior to central line insertion when:
platelet count is less than 10 x 109/L or;
platelet count is less than 20 x 109/L or;
platelet count is less than 30 x 109/L or;
platelet count is less than 50 x 109/L.
2) Platelet transfusion prior to central line insertion when platelet count is less than 50 x 109/L versus platelet transfusion prior to central line insertion when:
platelet count is less than 10 x 109/L or;
platelet count is less than 20 x 109/L or;
platelet count is less than 30 x 109/L.
We planned to report each analysis separately, as subgroups within the main comparisons, had relevant studies been identified.
Types of outcome measures
Primary outcomes
Major procedure‐related bleeding within 24 hours of the procedure.
For example: a significant fall in haemoglobin (Hb), e.g. 20 g/L or greater in the absence of another cause; a fall in systolic blood pressure (SBP) by at least 20 mmHg or increase in heart rate by at least 20 beats per minute (BPM) or greater; haemothorax; requiring an intervention such as a transfusion to treat bleeding; or major bleeding (not further defined) as reported by individual studies.
All‐cause mortality up to 30 days after the procedure.
Secondary outcomes
Minor procedure‐related bleeding within 24 hours of the procedure (defined as prolonged bleeding at the insertion site which only requires treatment with a pressure bandage, or haematoma at the insertion site), or minor bleeding (not further defined) as reported by individual studies.
-
Serious adverse events.
Transfusion‐related complications within 24 hours of the procedure (including transfusion‐related acute lung injury (TRALI), transfusion‐transmitted infection, transfusion‐associated circulatory overload (TACO), transfusion‐associated dyspnoea (TAD), acute transfusion reactions).
Line‐related complications within seven days of the procedure (infection, thrombosis, other).
Duration of hospital stay (total number of days in hospital).
Proportion of patients receiving platelet transfusions and red cell transfusions within 24 hours of the procedure.
Quality of life, as defined by the individual studies.
Search methods for identification of studies
The Systematic Review Initiative’s Information Specialist (CD) formulated the search strategies in collaboration with the Cochrane Haematological Malignancies Group.
Electronic searches
We limited our searches to five main electronic databases and two ongoing trial databases.
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2015, issue 2) (Appendix 1).
MEDLINE (1946 to 23 February 2015) (Appendix 2).
EMBASE (1974 to 23 February 2015) (Appendix 3).
PubMed (e‐publications only on 23 February 2015) (Appendix 4).
Transfusion Evidence Library (www.transfusionevidencelibrary.com) (1950 to 23 February 2015) (Appendix 5).
WHO International Clinical Trials Registry Platform (ICTRP) (on 23 February 2015) (Appendix 6).
ClinicalTrials.gov (on 23 February 2015) (Appendix 6).
We combined searches in MEDLINE with the Cochrane RCT search filter, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We combined searches in EMBASE with the relevant Scottish Intercollegiate Guidelines Network (SIGN) RCT studies filter (www.sign.ac.uk/methodology/filters.html). We excluded studies published in languages other than English. We did not limit searches by year of publication or publication type.
Searching other resources
We handsearched reference lists of included studies in order to identify further relevant studies. We contacted the lead author of the included study to identify any unpublished information regarding the ongoing study.
Data collection and analysis
Selection of studies
We selected studies according to Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The Systematic Review Initiative’s Information Specialist (CD) initially screened all search hits for relevance against the eligibility criteria and discarded all those that were clearly irrelevant. Thereafter, two review authors (MD, LE) independently screened all of the remaining references for relevance against the full eligibility criteria using DistillerSR software. We retrieved full‐text articles for all references for which a decision on eligibility could not be made from title and abstract alone. We requested additional information from study authors as necessary to assess the eligibility for inclusion of individual studies. The two review authors discussed the results of study selection and resolved any discrepancies between themselves without needing to refer to a third review author (SS). We have reported the results of study selection using a PRISMA flow diagram (Moher 2009).
Data extraction and management
As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), two review authors (MD, LE) planned to independently extract data onto standardised forms using DistillerSR software. However, no completed study was included in this review.
We planned to extract the following information for each study.
Source: Study ID; report ID; review author ID; date of extraction; ID of author checking extracted data; citation of paper; contact authors details.
General study information: Publication type; study objectives; funding source; conflict of interest declared; other relevant study publication reviewed.
Study details and methods: Location; country; setting; number of centres; total study duration; recruitment dates; length of follow‐up; power calculation; primary analysis (and definition); stopping rules; method of sequence generation; allocation concealment; blinding (of clinicians, participants and outcome assessors); and any concerns regarding bias.
Characteristics of interventions: Number of study arms; description of experimental arm; description of control arm; type of platelet component (e.g. apheresis or pooled); dose of platelet component.
Characteristics of participants: Age; gender; primary diagnosis; type of catheter inserted; route and method of catheter insertion; platelet count.
Participant flow: Total number screened for inclusion; total number recruited; total number excluded; total number allocated to each study arm; total number analysed (for review outcomes); number of allocated patients who received planned treatment; number of drop‐outs with reasons (percentage in each arm); protocol violations; missing data.
Outcomes: Major procedure‐related bleeding within 24 hours of the procedure; minor procedure‐related bleeding within 24 hours of the procedure; transfusion‐related complications within 24 hours of the procedure; line‐related complications within 7 days of the procedure; duration of hospital stay; proportion of patients receiving platelet and red cell transfusions within 24 hours of the procedure; all‐cause mortality up to 30 days from the procedure; quality of life (as defined by the individual studies).
Assessment of risk of bias in included studies
We planned to perform an assessment of all RCTs using the Cochrane 'Risk of bias' tool according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). This tool includes the following domains:
Selection bias: random sequence generation and allocation concealment.
Performance bias: blinding of participants and personnel.
Detection bias: blinding of outcome assessment.
Attrition bias: incomplete outcome data.
Reporting bias: selective reporting.
Other bias.
However, no completed study was identified in this review and therefore no assessment of risk of bias could be performed.
Measures of treatment effect
We did not perform any of the planned analyses because no completed study was included in this review. We planned to record the following data for this review:
Continuous outcomes: mean, standard deviation and total number of participants in both the treatment and control groups.
Dicotomous outcomes: number of events and total number of participants in both the treatment and control groups.
The following analyses were planned for this review and will be performed in future updates of this review:
For continuous outcomes using the same scale: analyses using the mean difference (MD) with 95% confidence intervals (CIs).
For continuous outcomes measured with different scales: analyses using the standardised mean difference (SMD).
Extraction and reporting of hazard ratios (HRs) for mortality data or, if HRs were not available, every effort would be made to estimate the HR as accurately as possible using the available data and a purpose‐built method based on the Parmar and Tierney approach (Parmar 1998; Tierney 2007).
For dichotomous outcomes: reporting the pooled risk ratio (RR) with a 95% CI. Where the number of observed events was small (< 5% of sample per group), and where trials have balanced treatment groups, we planned to report the Peto's Odds Ratio (OR) with 95% CI (Deeks 2011).
If data allowed, we planned to undertake quantitative assessments using Review Manager 5 (RevMan 2014).
Where appropriate, we planned to report the number needed to treat to benefit (NNTB) and the number needed to treat to harm (NNTH) with CIs.
If we could not report the available data in any of the formats described above, we planned to present a narrative report, and if appropriate we planned to present the data in tables.
Unit of analysis issues
We planned to treat any unit of analysis issues in accordance with the advice given in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). However, no completed study was identified in this review and there were therefore no unit of analysis issues.
Dealing with missing data
Where data were identified to be missing or unclear in published literature, we contacted study authors directly.
Assessment of heterogeneity
We did not perform any of the planned analyses because no completed study was included in this review.
We had planned to combine the data to perform a meta‐analysis if the clinical and methodological characteristics of individual studies were sufficiently homogeneous. We planned to assess statistical heterogeneity of treatment effects between studies using a Chi2 test with a significance level at P < 0.1. We planned to use the I2 statistic to quantify the degree of potential heterogeneity, and classify it as moderate if I2 > 50%, or considerable if I2 > 80%. We perceived that we would identify at least moderate clinical and methodological heterogeneity within the studies selected for inclusion; in such cases, we planned to use the random‐effects model. If statistical heterogeneity was considerable, we planned not to report the overall summary statistic. We planned to assess potential causes of heterogeneity by sensitivity and subgroup analyses (Deeks 2011).
Assessment of reporting biases
We did not perform a formal assessment of potential publication bias (small trial bias) by generating a funnel plot and statistically test using a linear regression test (Lau 2006; Sterne 2011), because there were no completed trials within this review.
Data synthesis
We planned to perform analyses according to the recommendations of Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions, using aggregated data for analysis (Deeks 2011).
We did not perform any of the planned analyses because no completed study was included in this review.
Summary of findings
We planned to use the GRADE approach to create a 'Summary of findings' table, as suggested in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b). We planned to use the GRADE approach to rate the quality of the evidence as 'high', 'moderate', 'low', or 'very low' using the five GRADE considerations.
Risk of bias: serious or very serious
Inconsistency: serious or very serious
Indirectness: serious or very serious
Imprecision: serious or very serious
Publication bias: likely or very likely
We planned to report separate 'Summary of findings' tables for: prophylactic platelet transfusion versus no prophylactic platelet transfusion prior to the procedure; and a lower platelet count threshold versus the highest commonly used threshold (50 x 109/L). We planned to report the subgroup for each comparison that contained the largest number of studies.
The outcomes we planned to include in a 'Summary of findings' table are listed below.
Major procedure‐related bleeding within 24 hours of the procedure.
All‐cause mortality up to 30 days after the procedure.
Minor procedure‐related bleeding within 24 hours of the procedure.
Respiratory deterioration attributable to transfusion‐associated circulatory overload (TACO), transfusion‐related acute lung injury (TRALI), or transfusion‐associated dyspnoea (TAD) within 24 hours of the procedure.
Line‐related complications within 7 days of the procedure (infection, thrombosis, other).
Proportion of patients receiving platelet transfusions within 24 hours of the procedure.
Quality of life.
However, no completed study was identified in this review and therefore we could not produce a 'Summary of findings' table nor assess the quality of the evidence.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for each of the following outcomes in order to assess the effect on heterogeneity.
Type of central line inserted (venous tunnelled, venous untunnelled, portacath, whether an emergency or elective procedure).
Type of patient (intensive care, liver disease, leukaemia, other).
Age of patient (neonate, child (1 to 15 years), adult (16 years or older)).
Whether patients had associated clotting abnormalities, including disseminated intravascular coagulation (DIC).
If appropriate, we also planned to investigate heterogeneity between studies as follows.
Type of platelet component.
Dose of platelet component.
However, no completed study was identified in this review and therefore no subgroup analyses could be performed.
Sensitivity analysis
We planned to assess the robustness of our findings by performing the following sensitivity analyses where appropriate.
Including only those studies with a ‘low risk of bias’ (for example, RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation).
Including only those studies with less than a 20% drop‐out rate.
However, no completed study was identified in this review and therefore no sensitivity analyses could be performed.
Results
Description of studies
See Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
See the PRISMA Flow Diagram (Figure 1). The search (conducted on 23 February 2015) identified a total of 520 potentially‐relevant records. There were 480 records after duplicates were removed. Two review authors (LE and MD) excluded 462 records on the basis of the abstract. Eighteen full text articles were retrieved for assessment by the same two review authors. Sixteen studies reported in 17 papers were excluded.
1.
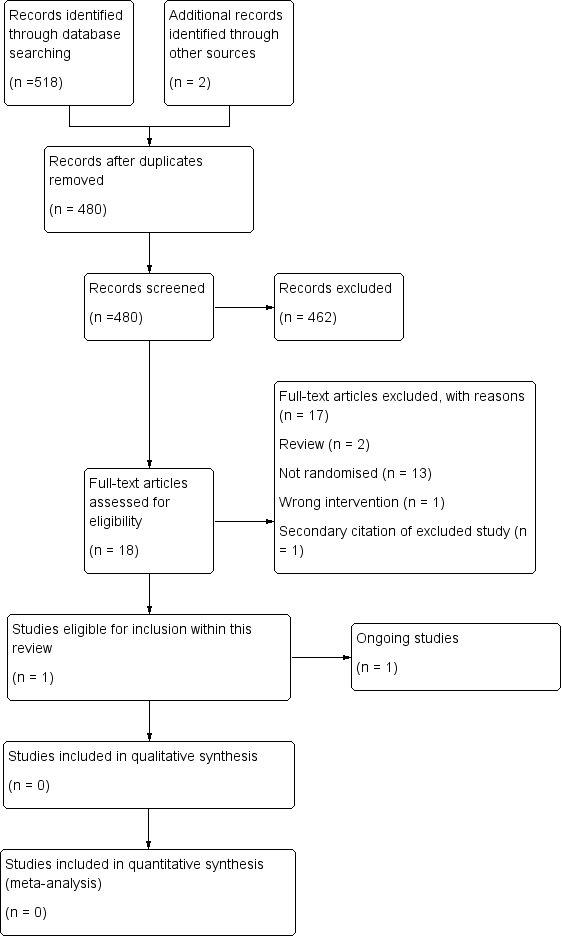
Study flow diagram.
Included studies
One study was eligible for inclusion, this study is ongoing (POCKET (NCT02311985)). There were no completed studies.
Ongoing studies
See Characteristics of ongoing studies for further details.
Design
The POCKET (NCT02311985) study is a single‐centre, blinded, parallel assignment three‐arm trial comparing different transfusion strategies prior to central line insertion.
Sample sizes
The planned sample size is 165 participants. Recruitment started in 2014 and is planned to be completed in 2017.
Setting
The study is being conducted in Brazil.
Participants
The participants will be adults with chronic liver disease who have been admitted to intensive care and require insertion of a central line.
Intervention
Arm 1: Coagulogram‐based protocol.
This arm is based on a standard coagulation tests protocol to guide blood transfusion before central venous catheterisation.
Arm 2: Thromboelastometry‐based protocol
The interventions for this protocol include transfusion of fresh frozen plasma, platelets (random or apheresis) and/or cryoprecipitate, based on rotational thromboelastometry (ROTEM(R)).
Arm 3: Restrictive strategy
The interventions for this protocol include transfusion of fresh frozen plasma and/or platelets (random or apheresis), based on international normalised ratio (INR) and platelet count, but more restrictive than Arm 1.
Outcome
This study's primary outcome is the proportion of patients receiving a blood component transfusion ‐ i.e. fresh frozen plasma, platelets and/or cryoprecipitate ‐ before central venous catheterisation.
Excluded studies
We excluded the 17 remaining papers we assessed in full text; 1 study (Weigand 2009) was reported in 2 papers. See Characteristics of excluded studies for further details.
Thirteen studies were non‐randomised studies (Adorno 1999; Amarapurkar 2014a; Amarapurkar 2014b; Barrera 1996; Barrett 2004; Blumberg 2002; Napolitano 2012; Napolitano 2013; Natalia 2012; Potet 2013; Ray 1997; Weigand 2009; Zeidler 2011)
Two studies were reviews (Schiffer 2011; Slichter 2011).
One study compared the wrong intervention (OPTIMAL).
Risk of bias in included studies
We did not perform a 'Risk of bias' assessment because there were no completed studies.
Effects of interventions
We could not assess the effects of interventions because there were no completed studies.
Discussion
Summary of main results
One randomised controlled trial (RCT) was identified that compared different platelet transfusion thresholds prior to insertion of a central venous catheter (CVC) in patients with chronic liver disease. This study is still recruiting patients and is due to be completed in December 2017. There were no completed studies. There were no studies that compared no platelet transfusions to a platelet transfusion threshold.
Overall completeness and applicability of evidence
This review did not identify any completed RCTs and therefore there is no evidence that can be assessed.
The one ongoing study (expected recruitment 165 participants) will be too small to provide sufficient data for this review’s primary outcomes. For example, if we assumed that major bleeding occurred in 1 out of 100 people who had a central line when their platelet count was raised to 50 x 109/L or above, and that the risk of major bleeding doubled to 2 out of 100 people when their platelet count was only raised to 20 x 109/L or above, we would need to design a study with at least 4634 participants to detect this difference with 80% power and 5% significance (6202 participants required to detect a difference with 90% power) (calculated using a power calculator at http://www.sealedenvelope.com/power/binary‐superiority/).
Quality of the evidence
This review did not identify any completed RCTs and therefore there is no evidence that can be assessed.
Potential biases in the review process
To our knowledge, our review process is free from bias. We conducted a comprehensive search, searching data sources (including multiple databases, and clinical trial registries) to ensure that all relevant trials would be captured. The relevance of each paper identified was carefully assessed and all screening and data extractions were performed in duplicate. We had planned to exclude any non‐English language publications but no relevant publications were identified in our search.
We prespecified all outcomes and subgroups prior to analysis.
Only one small study was included in this review and this is still recruiting patients.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other systematic reviews that report on this topic.
Authors' conclusions
Implications for practice.
This review provided no evidence to guide practice.
Implications for research.
The ongoing trial that compares two different platelet count thresholds and is due to be completed in December 2017 will be unable to answer the primary questions of this review because the study is too small. To detect a doubling in the number of participants with major bleeding from 1% to 2% would require a study with over 4600 participants; the ongoing study is only planning to recruit 165 participants. No trials have been identified that compared no platelet transfusions versus a prespecified platelet count threshold. Further randomised controlled clinical trials are now required, in order to develop the optimal transfusion strategy for patients who are thrombocytopenic and require a central line insertion.
What's new
| Date | Event | Description |
|---|---|---|
| 10 August 2020 | Amended | Following correspondence between the editorial base and the funding institution of one of the authors, the acknowledging statement was updated. |
History
Protocol first published: Issue 6, 2015 Review first published: Issue 12, 2015
Notes
This review was a rapid review (definition of a rapid review as previously agreed with the Cochrane Haematological Malignancies Group), we only included English language publications. No relevant non‐English language reports were identified during the searches.
Acknowledgements
We thank the editorial base of the Cochrane Haematological Malignancies Review Group.
We thank the National Institute of Health Research (NIHR). This review is part of a series of reviews that have been funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. This research was also supported by the NIHR Oxford Biomedical Research Centre Programme.The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
We thank Marialena Trivella who was an author of the protocol.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Blood Platelets] explode all trees #2 (platelet* or thrombocyte*):ti #3 MeSH descriptor: [Platelet Transfusion] explode all trees #4 MeSH descriptor: [Plateletpheresis] explode all trees #5 ((platelet* or thrombocyte*) near/5 (prophyla* or transfus* or infus* or administ* or requir* or need* or product or products or component* or concentrate* or apheres* or pooled or single donor* or random donor*)) #6 thrombocyt?pheres* or plateletpheres* #7 ((platelet* or thrombocyte*) near/5 (protocol* or trigger* or threshold* or schedul* or dose* or dosing or usage or utilisation or utilization)) #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Catheterization, Central Venous] this term only #10 MeSH descriptor: [Catheters, Indwelling] this term only #11 MeSH descriptor: [Central Venous Catheters] this term only #12 MeSH descriptor: [Vascular Access Devices] this term only #13 hickman* or "port catheter*" or port‐a‐cath* or "invasive line*" or portacath* or TIVAD* #14 ((central* or venous* or vascular* or intravenous* or tunnel* or indwelling or "in‐dwelling" or implant* or placement* or subclavian or femoral or jugular) near/5 (catheter* or line* or cannul* or port*)) #15 ((vascular or venous) next (access* or reservoir*)) #16 #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 #8 and #16
Appendix 2. MEDLINE (OvidSP) search strategy
1. Catheterization, Central Venous/ 2. Catheters, Indwelling/ 3. Central Venous Catheters/ 4. Vascular Access Devices/ 5. (hickman* or port‐a‐cath* or port catheter* or port‐a‐cath* or invasive line* or portacath* or TIVAD*).tw. 6. ((central* or venous* or vascular* or intravenous* or tunnel* or indwelling or "in‐dwelling" or implant* or placement* or subclavian or femoral or jugular) adj5 (catheter* or line* or cannul* or port*)).tw. 7. ((vascular or venous) adj2 (access* or reservoir*)).tw. 8. or/1‐7 9. Platelet Transfusion/ 10. Plateletpheresis/ 11. Blood Platelets/ 12. ((platelet* or thrombocyte*) adj5 (prophyla* or transfus* or infus* or administ* or requir* or need* or product* or component* or concentrate* or apheres* or pooled or single donor or random donor)).tw. 13. (thrombocytopheres* or plateletpheres*).tw. 14. ((platelet* or thrombocyte*) adj5 (protocol* or trigger* or threshold* or schedul* or dose* or dosing or usage or utili?ation)).tw. 15. (platelet* or thrombocyte*).ti. 16. or/9‐15 17. 8 and 16 18. randomized controlled trial.pt. 19. controlled clinical trial.pt. 20. randomi*.tw. 21. placebo.ab. 22. clinical trials as topic.sh. 23. randomly.ab. 24. groups.ab. 25. trial.tw. 26. or/18‐25 27. exp animals/ not humans.sh. 28. 26 not 27 29. 17 and 28
Appendix 3. EMBASE (OvidSP) search strategy
1. exp Central Venous Catheterization/ 2. exp Indwelling Catheter/ 3. exp Central Venous Catheter/ 4. Vascular Access Devices/ 5. (hickman* or port‐a‐cath* or port catheter* or port‐a‐cath* or invasive line* or portacath* or TIVAD*).tw. 6. ((central* or venous* or vascular* or intravenous* or tunnel* or indwelling or "in‐dwelling" or implant* or placement* or subclavian or femoral or jugular) adj5 (catheter* or line* or cannul* or port*)).tw. 7. ((vascular or venous) adj2 (access* or reservoir*)).tw. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. Thrombocyte Transfusion/ 10. Thrombocytopheresis/ 11. Thrombocyte/ 12. ((platelet* or thrombocyte*) adj5 (prophyla* or transfus* or infus* or administ* or requir* or need* or product* or component* or concentrate* or apheres* or pooled or single donor or random donor)).tw. 13. (thrombocyt?pheres* or plateletpheres*).tw. 14. ((platelet* or thrombocyte*) adj5 (protocol* or trigger* or threshold* or schedul* or dose* or dosing or usage or utili?ation)).tw. 15. (platelet* or thrombocyte*).ti. 16. or/9‐15 17. 8 and 16 18. Randomized Controlled Trial/ 19. Randomization/ 20. Single Blind Procedure/ 21. Double Blind Procedure/ 22. Crossover Procedure/ 23. Placebo/ 24. exp Clinical Trial/ 25. Prospective Study/ 26. (randomi* or double‐blind* or single‐blind* or RCT*).tw. 27. (random* adj2 (allocat* or assign* or divid* or receiv*)).tw. 28. (crossover* or cross over* or cross‐over* or placebo*).tw. 29. ((treble or triple) adj blind*).tw. 30. or/18‐29 31. Case Study/ 32. case report*.tw. 33. (note or editorial).pt. 34. or/31‐33 35. 30 not 34 36. (animal* or cat or cats or dog or dogs or pig or pigs or sheep or rabbit* or mouse or mice or rat or rats or feline or canine or porcine or ovine or murine or model*).ti. 37. 35 not 36 38. limit 37 to embase 39. 17 and 38
Appendix 4. PubMed search strategy (epublications only)
#1 hickman* OR port catheter* OR port‐a‐cath* OR "invasive line" OR portacath* OR TIVAD* #2 ((central* OR venous* OR vascular* OR intravenous* OR tunnel* OR indwelling OR "in‐dwelling" OR implant OR implants OR placement* OR subclavian OR femoral OR jugular) AND (catheter* OR line OR lines OR cannul* OR port OR ports)) #3 ((vascular OR venous) AND (access* OR reservoir*)) #4 #1 OR #2 OR #3 #5 ((platelet* OR thrombocyte*) AND (prophyla* OR transfus* OR infus* OR administ* OR requir* OR need* OR product* OR component* OR concentrate* OR apheres* OR pooled OR single donor* OR random donor*)) #6 (thrombocytopheres* OR plateletpheres*) #7 ((platelet* OR thrombocyte*) AND (protocol* OR trigger* OR threshold* OR schedul* OR dose* OR dosing OR usage OR utilisation OR utilization)) #8 platelet*[TI] OR thrombocyte*[TI] #9 #5 OR #6 OR #7 OR #8 #10 #4 AND #9 #11 (random* OR blind* OR control group OR placebo OR controlled trial OR controlled study OR groups OR trials OR systematic review OR meta‐analysis OR metaanalysis OR literature search OR medline OR cochrane OR embase) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]) #12 #10 AND #11
Appendix 5. Transfusion Evidence Library search strategy
Clinical Specialty: Haematology and Oncology AND Subject Area: Platelets OR (All fields: vascular OR venous OR invasive OR intravenous OR tunnel OR indwelling OR implant OR subclavian OR femoral OR jugular OR hickman OR catheter OR line OR access OR reservoir OR port‐a‐cath OR portacath OR cannula OR port OR ports) AND (Keywords: platelets OR platelet transfusion)
Appendix 6. Ongoing Trial Register search strategies
WHO ICTRP (Title: vascular OR venous OR invasive OR intravenous OR tunnel OR indwelling OR implant OR subclavian OR femoral OR jugular OR hickman OR catheter OR line OR access OR reservoir OR port‐a‐cath OR portacath OR cannula OR port OR ports) AND (Intervention: platelets)
ClinicalTrials.gov (Title: hickman OR catheter OR line OR access OR reservoir OR port‐a‐cath OR portacath OR cannula OR port OR ports) AND (Intervention: platelets OR platelet transfusion)
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adorno 1999 | Non‐randomised study |
| Amarapurkar 2014a | Non‐randomised study |
| Amarapurkar 2014b | Non‐randomised study |
| Barrera 1996 | Non‐randomised study |
| Barrett 2004 | Non‐randomised study |
| Blumberg 2002 | Non‐randomised study |
| Napolitano 2012 | Non‐randomised study |
| Napolitano 2013 | Non‐randomised study |
| Natalia 2012 | Non‐randomised study |
| OPTIMAL | Wrong intervention |
| Potet 2013 | Non‐randomised study |
| Ray 1997 | Non‐randomised study |
| Schiffer 2011 | Review |
| Slichter 2011 | Review |
| Weigand 2009 | Non‐randomised study |
| Zeidler 2011 | Non‐randomised study |
Characteristics of ongoing studies [ordered by study ID]
POCKET (NCT02311985).
| Study name | Point‐of‐care Versus Standard Coagulation Tests Versus Restrictive Strategy to Guide Transfusion in Chronic Liver Failure Patients Requiring Central Venous Line: Prospective Randomized Trial (POCKET) |
| Methods | Allocation: Randomised Endpoint Classification: Safety/Efficacy Study Intervention Model: Parallel Assignment (three arms) Masking: Double Blind (Subject, Caregiver) Primary Purpose: Treatment |
| Participants |
Inclusion criteria: Adults (aged 18 years or older) with chronic liver failure (cirrhosis and/or chronic liver graft dysfunction) admitted to the intensive care unit and requiring insertion of a central line. Exclusion criteria:
|
| Interventions |
Arm 1:Coagulogram‐based protocol Arm based on standard coagulation tests protocol to guide blood transfusion before central venous catheterisation. The possible components to be used include fresh frozen plasma, platelets (random or apheresis) and/or cryoprecipitate, based on international normalised ratio (INR), partial thromboplastin time (PTT), platelet count and/or fibrinogen.
Arm 2: Thromboelastometry‐based protocol The interventions for this protocol include transfusion of fresh frozen plasma, platelets (random or aphaeresis) and/or cryoprecipitate, based on rotational thromboelastometry (ROTEM(R)).
Arm 3: Restrictive strategy The interventions for this protocol include transfusion of fresh frozen plasma and/or platelets (random or aphaeresis), based on INR and platelet count.
|
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | September 2014 |
| Contact information | Leonardo L Rocha, MD +55‐11‐21511500 lrocha23@gmail.com Thiago D Correa, MD, PhD +55‐11‐21511500 thiagodct@gmail.com |
| Notes |
Estimated Enrollment: 165 Estimated Study Completion Date: December 2017 Estimated Primary Completion Date: September 2017 (Final data collection date for primary outcome measure) Location of trial: Hospital Israelita Albert Einstein, Sao Paulo, SP, Brazil, 05652‐900 |
Differences between protocol and review
There are several differences between the protocol (Estcourt 2015) and this review due to lack of data.
There were no completed studies and therefore we could not:
Report on any of the primary or secondary outcomes of the review.
Perform a 'Risk of bias' assessment.
Assess the quality of the evidence or produce a 'Summary of findings' table.
Assess publication bias.
Perform any analyses or subgroup analyses.
Contributions of authors
Lise Estcourt: protocol development, searching, selection of studies, eligibility and quality assessment, data extraction and analysis
and content expert.
Michael Desborough: protocol development, searching, selection of studies, eligibility and quality assessment, data extraction and analysis and content expert.
Sally Hopewell: protocol development and methodological expert.
Carolyn Doree: protocol development, searching and selection of studies.
Simon Stanworth: protocol development and content expert.
Sources of support
Internal sources
-
NHS Blood and Transplant, Research and Development, UK
Funds the work of the Systematic Review Initiative (SRI)
External sources
-
Cochrane Haematological Malignancies Group, Department for Internal Medicine, Germany
Editorial support
-
National Institute for Health Research (NIHR) Cochrane Programme Grant, UK
Provides funding for systematic reviewers and methodological support from the Centre for Statistics in Medicine, Oxford
Declarations of interest
Lise Estcourt: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. Michael Desborough: none known. Sally Hopewell: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Carolyn Doree: none known.
Simon Stanworth: none known.
Edited (no change to conclusions)
References
References to studies excluded from this review
Adorno 1999 {published data only}
- Adorno G, Zinnio F, Bruno A, Lanti A, Ballatore G, Masi M, et al. Femoral catheters: safety and efficacy in peripheral blood stem cell collection. The International Journal of Artifical Organs 1999;22(10):710-12. [PubMed] [Google Scholar]
Amarapurkar 2014a {published data only}
Amarapurkar 2014b {published data only}
Barrera 1996 {published data only}
- Barrera R, Mina B, Huang Y, Groeger JS. Acute complications of central line placement in profoundly thrombocytopenic cancer patients. Cancer 1996;78(9):2025-30. [PubMed] [Google Scholar]
Barrett 2004 {published data only}
- Barrett AM, Imeson J, Leese D, Philpott C, Shaw ND, Pizer BL, et al, United Kingdom Children's Cancer Study Group and the Paediatric Oncology Nurses Forum of the Royal College of Nursing. Factors influencing early failure of central venous catheters in children with cancer. Journal of Pediatric Surgery 2004;39:1520-3. [DOI] [PubMed] [Google Scholar]
Blumberg 2002 {published data only}
- Blumberg N, Heal JM, Rowe JM. Platelet transfusion and clinical outcome in acute leukemia in adults. In: Transfusion. Vol. 42. 2002:5S.
Napolitano 2012 {published data only}
- Napolitano G, Valvano MR, Caruso N, Merla A, Lauriola WA Ippolito A, et al. Hemorrhagic complications following invasive procedures in cirrhotic patients with normal or abnormal coagulopathy. A prospective study. In: Journal of Hepatology. Vol. 56. 2012:S262.
Napolitano 2013 {published data only}
- Napolitano M, Malato A, Raffaele F, Palazzolo M, Lo Iacono G, Pinna R, et al. Ultrasonography-guided central venous catheterisation in haematological patients with severe thrombocytopenia. Blood Transfusion 2013;11:506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Natalia 2012 {published data only}
- Natalia N, Garcia SJ, Guillen H, Martin Y, Callejas M, Gil FJJ, et al. A team-based, multidisciplinary approach to reducing peripherally-inserted central catheters (PICC) complications in haematological patients: a prospective study. In: 17th Congress of the European Hematology Association Amsterdam Netherlands. Haematologica. 2012.
OPTIMAL {published data only}
- NCT01615146. Outpatient platelet transfusions in myelodysplastic syndromes and leukemia: the OPTIMAL pilot. http://ClinicalTrials.gov/show/NCT01615146. Accessed 25 Feb. 2015.
Potet 2013 {published data only}
- Potet J, Thome A, Curis E, Arnaud FX, Weber-Donat G, Valbousquet L, et al. Peripherally inserted central catheter placement in cancer patients with profound thrombocytopaenia: a prospective analysis. European Journal of Radiology 2013;23:2042-8. [DOI] [PubMed] [Google Scholar]
Ray 1997 {published data only}
- Ray CE Jr, Shenoy SS. Patients with thrombocytopenia: outcome of radiologic placement of central venous access devices. Radiology 1997;204:97-9. [DOI] [PubMed] [Google Scholar]
Schiffer 2011 {published data only}
- Schiffer CA. What to do if there is no evidence? The issue of surgical procedures in patients with thrombocytopenia. Transfusion 2011;51:2262-4. [DOI] [PubMed] [Google Scholar]
Slichter 2011 {published data only}
- Slichter SJ. Prophylactic platelet transfusion. In: ANZSBT abstracts presented at the HSANZ/ANZSBT/ASTH ISH Annual Scientific Meeting 2011 held 30 October to 2 November 2011 in Sydney, Australia. Transfusion Medicine. Vol. 22. 2012:221.
Weigand 2009 {published data only}
- NCT00448188. Bleeding risk in CVCs. Clinical.Trials.gov [Accessed 25 Feb. 2015].
- Weigand K, Encke J, Meyer FJ, Hinkel UP, Munder M, Stremmel W, et al. Low levels of prothrombin time (INR) and platelets do not increase the risk of significant bleeding when placing central venous catheters. Medizinische Klinik (Munich) 2009;104(5):331-5. [DOI] [PubMed] [Google Scholar]
Zeidler 2011 {published data only}
- Zeidler K, Arn K, Senn O, Schanz U, Stussi G. Optimal preprocedural platelet transfusion threshold for central venous catheter insertions in patients with thrombocytopenia. Transfusion 2011;51(11):2269-76. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
POCKET (NCT02311985) {published data only}
- Rocha LL, Correa TD. Comparison of three transfusion strategies for central venous catheterization in cirrhotics (POCKET). Clinicaltrial.gov [Accessed 25 Feb. 2015] 2014.
Additional references
BCSH 2003
- BCSH. British Committee for Standards in Haematology: guidelines for the use of platelet transfusions. British Journal of Haematology 2003;122(1):10-23. [DOI] [PubMed] [Google Scholar]
Benson 2009
- Benson AB, Moss M, Silliman CC. Transfusion-related acute lung injury (TRALI): a clinical review with emphasis on the critically ill. British Journal of Haematology 2009;147(4):431-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bishop 2007
- Bishop L, Dougherty L, Bodenham A, Mansi J, Crowe P, Kibbler C, et al. Guidelines on the insertion and management of central venous access devices in adults. International Journal of Laboratory Hematology 2007;29:261-78. [DOI] [PubMed] [Google Scholar]
Blumberg 2009
- Blumberg N, Spinelli SL, Francis CW, Taubman MB, Phipps RP. The platelet as an immune cell - CD40 ligand and transfusion immune modulation. Immunology Research 2009;45:251-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton‐Maggs 2012
- Bolton-Maggs PHB (Ed), Cohen H, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2011 Annual SHOT Report. Serious Hazards of Transfusion (SHOT), 2012. [Google Scholar]
Bosly 2007
- Bosly A, Muylle L, Noens L, Pietersz R, Heim D, Hübner R, et al. Guidelines for the transfusion of platelets. Acta Clinica Belgica 2007;62(1):36-46. [DOI] [PubMed] [Google Scholar]
Cameron 2007
- Cameron B, Rock G, Olberg B, Neurath D. Evaluation of platelet transfusion triggers in a tertiary-care hospital. Transfusion 2007;47(2):206-11. [DOI] [PubMed] [Google Scholar]
Cavanna 2010
- Cavanna L, Civardi G, Vallisa D, Di Nunzio C, Cappuciati L, Berte R, et al. Ultrasound-guided central venous catheterization in cancer patients improves the success rate of cannulation and reduces mechanical complications: a prospective observational study of 1,978 consecutive catheterizations. World Journal of Surgical Oncology 2010;8:91. [DOI: 10.1186/1477-7819-8-91] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
DistillerSR [Computer program]
- DistillerSR: Data Management Software. Ottawa, ON: Evidence Partners, 2015. https://systematic-review.ca/.
Estcourt 2012
- Estcourt LJ, Birchall J, Lowe D, Grant-Casey J, Rowley M, Murphy MF. Platelet transfusions in haematology patients: are we using them appropriately? Vox Sanguinis 2012;103(4):284-93. [DOI] [PubMed] [Google Scholar]
Foster 1992
- Foster P, Moore L, Sankary H, Hart M, Ashmann M, Williams J. Central venous catheterization in patients with coagulopathy. Archives of Surgery 1992;127(3):273-5. [DOI] [PubMed] [Google Scholar]
GMA 2009
- The Board of the German Medical Association on the recommendation of the Scientific Advisory Board. Platelet transfusions. Transfusion Medicine and Hemotherapy 2009;36:372-82. [Google Scholar]
Greeno 2007
- Greeno E, McCullough J, Weisdorf D. Platelet utilisation and the transfusion trigger: a prospective analysis. Transfusion 2007;47(2):201-5. [DOI] [PubMed] [Google Scholar]
Haas 2010
- Haas B, Chittams J, Trerotola S. Large-bore tunneled central venous catheter insertion in patients with coagulopathy. Journal of Vascular and Interventional Radiology 2010;21(2):212-7. [DOI] [PubMed] [Google Scholar]
Heddle 2009
- Heddle NM, Webert K. Investigation of acute transfusion reactions. In: Murphy MF, Pamphilion DH, editors(s). Practical Transfusion Medicine. 4th edition. Blackwell, 2009:63-89. [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hind 2003
- Hind D, Calvert N, McWilliams R, Davidson A, Paisley S, Beverley C, et al. Ultrasonic locating devices for central venous canulation: meta-analysis. BMJ 2003;327(7411):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hong Pheng Loh 2007
- Hong Pheng Loh A, Hon Chui C. Port-A-Cath insertions in acute leukemia: does thrombocytopenia affect morbidity? Journal of Pediatric Surgery 2007;42:1180-4. [DOI] [PubMed] [Google Scholar]
Kaufman 2015
- Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Annals of Internal Medicine 2015;162(3):205-13. [DOI] [PubMed] [Google Scholar]
Khan 2007
- Khan H, Belsher J, Yilmaz M, Afessa B, Winters J, Moore S, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest 2007;131(5):1308-14. [DOI] [PubMed] [Google Scholar]
Knowles 2011
- Knowles S, Cohen H, Serious Hazards of Transfusion (SHOT) Steering Group. The 2010 Annual SHOT Report. Serious Hazards of Transfusion (SHOT), 2011. [Google Scholar]
Lau 2006
- Lau J, Ioannidis J, Terrin N, Schmid C, Olkin I. The case of the misleading funnel plot. BMJ 2006;333(7568):597-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-Analyses: the PRISMA Statement. Annals of Internal Medicine 2009;151(4):264-9. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar M, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pearce 2011
- Pearce S, Rowe GP, Field SP. Screening of platelet for bacterial contamination at the Welsh Blood Service. Transfusion Medicine 2011;21(1):25-32. [DOI] [PubMed] [Google Scholar]
Pendry 2011
- Pendry K, Davies T. An audit of use and wastage in the north west of England and North Wales: where have all the platelets gone? Blood and Transplant Matters 2011;34:17-9. [Google Scholar]
Popovsky 1985
- Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion 1985;25:573-7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al, Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Silliman 2003
- Silliman CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood 2003;101(2):454-62. [DOI] [PubMed] [Google Scholar]
Smith 2013
- Smith RN, Nolan JP. Central venous catheters. BMJ 2013;347:f6570. [DOI: 10.1136/bmj.f6570] [DOI] [PubMed] [Google Scholar]
Stanworth 2013
- Stanworth SJ, Walsh TS, Prescott RJ, Lee RJ, Watson DM, Wyncoll DLA. Thrombocytopenia and platelet transfusion in UK critical care: a multicenter observational study. Transfusion 2013;53(5):1050-8. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Taylor 2010
- Taylor C, Cohen H, Mold D, Jones H, Ball J, Mistry H, et al, Serious Hazards of Transfusion (SHOT) Steering Group. The 2009 Annual SHOT Report. Serious Hazards of Transfusion (SHOT), 2010. [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(16). [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed]
References to other published versions of this review
Estcourt 2015
- Estcourt LJ, Desborough M, Hopewell S, Trivella M, Doree C, Stanworth S. Comparison of different platelet transfusion thresholds prior to insertion of central lines in patients with thrombocytopenia. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011771. [DOI: 10.1002/14651858.CD011771] [DOI] [PMC free article] [PubMed] [Google Scholar]


