Abstract
Background
Trivalent oral poliovirus vaccine (OPV) is known to interfere with monovalent rotavirus vaccine (RV1) immunogenicity. The interference caused by bivalent and monovalent OPV formulations, which will be increasingly used globally in coming years, has not been examined. We conducted a post hoc analysis to assess the effect of coadministration of different OPV formulations on RV1 immunogenicity.
Methods
Healthy infants in Matlab, Bangladesh, were randomized to receive 3 doses of monovalent OPV type 1 or bivalent OPV types 1 and 3 at either 6, 8, and 10 or 6, 10, and 14 weeks of age or trivalent OPV at 6, 10, and 14 weeks of age. All infants received 2 doses of RV1 at about 6 and 10 weeks of age. Concomitant administration was defined as RV1 and OPV given on the same day; staggered administration as RV1 and OPV given ≥1 day apart. Rotavirus seroconversion was defined as a 4-fold rise in immunoglobulin A titer from before the first RV1 dose to ≥3 weeks after the second RV1 dose.
Results
There were no significant differences in baseline RV1 immunogenicity among the 409 infants included in the final analysis. Infants who received RV1 and OPV concomitantly, regardless of OPV formulation, were less likely to seroconvert (47%; 95% confidence interval, 39%–54%) than those who received both vaccines staggered ≥1 day (63%; 57%–70%; P < .001). For staggered administration, we found no evidence that the interval between RV1 and OPV administration affected RV1 immunogenicity.
Conclusions
Coadministration of monovalent, bivalent, or trivalent OPV seems to lower RV1 immunogenicity.
Clinical Trials Registration
Keywords: Rotarix, rotavirus vaccine, oral polio vaccine, OPV, vaccine interference
In 2008, rotavirus disease was responsible for >450 000 deaths and >2 million hospitalizations in children <5 years of age, with 90% of rotavirus-associated deaths occurring in low-income countries in Asia and Africa [1, 2]. Based on large safety and efficacy trials conducted initially in Europe, Latin America, and the United States, followed by studies in sub-Saharan Africa and Asia, the World Health Organization has recommended the use of 2 live oral rotavirus vaccines (RVs)—a pentavalent vaccine containing 5 human-bovine reassortant viruses (RotaTeq; RV5) and a monovalent vaccine containing a single attenuated human rotavirus strain (Rotarix; RV1)—for infants worldwide [1]. Though different in composition, the 2 vaccines have been shown to be similarly efficacious in preventing rotavirus-associated gastroenteritis in high-income regions such as the United States and Europe and middle-income regions such as Brazil in Latin America [1, 3]. However, the efficacy of both RVs has been lower in low-income than in high- and middle-income countries [1, 3, 4].
The interference with concomitant administration of trivalent oral poliovirus vaccine (tOPV) is one reason for the lower immune response to both RV1 and RV5, particularly the first vaccine dose, in low-income countries [5–11]. Because type 2 poliovirus in tOPV replicates at much higher levels than types 1 and 3 Sabin viruses [12, 13], particularly after the first tOPV dose, the type 2 component was potentially considered the principal Sabin virus responsible for the interference of tOPV with RV response. As part of the polio endgame strategy, the World Health Organization has recommended a switch from tOPV to bivalent oral poliovirus vaccine (bOPV) containing only poliovirus types 1 and 3 in routine immunization schedules [14, 15]. Because there are no data on coadministration of bOPV and RV1 or RV5, it is important to understand the effect on RV response with impending changes in use of oral poliovirus vaccine (OPV) formulations for routine immunization.
The objective of this post hoc analysis was to compare serum immunoglobulin A (IgA) responses to 2 doses of RV1 among infants in Bangladesh who also received monovalent OPV type 1 (mOPV1), bOPV, or tOPV. Another objective was to examine potential interference in RV1 immunogenicity with mOPV1, bOPV, or tOPV by comparing serum IgA responses in infants who received concomitant or staggered (separated by ≥1 day) RV1 and OPV administration.
METHODS
Study Design
We performed a post hoc analysis of an open-label randomized clinical trial of OPV in healthy infants conducted at 2 sites in Bangladesh, urban Mirpur and rural Matlab, from May through October 2012, as reported elsewhere [16]. The study was registered at clinicaltrials.gov (NCT01633216). Briefly, eligible healthy infants were randomized to 1 of 5 arms, receiving 3 doses of mOPV1 or bOPV (types 1 and 3) at either 6, 8, and 10 or 6, 10, and 14 weeks of age or 3 doses of tOPV at 6, 10, and 14 weeks of age. Infants also received other vaccines according to the routine immunization schedule in Bangladesh; a birth dose of OPV was not given. RV1 was administered by vaccinators from the Bangladesh Program for Immunization at 6 and 10 weeks of age only in Matlab district and administered only through bimonthly outreach immunization sessions scheduled for the infant’s village of residence. Therefore, the dates that infants received RV1 and other routine immunizations were not linked to the study OPV dates, allowing infants to receive OPV the same day or several days apart from other routine vaccines, including RV1. Concomitant administration was defined as either dose of RV1 and OPV given on the same day, and staggered administration as both doses of RV1 and OPV given ≥1 day apart. Blood samples were collected before administration of OPV at 6 weeks of age and 4 weeks after the last OPV dose (ie, at 14 or 18 weeks of age).
Laboratory Testing
The IgA antibody titers against RV were determined using end point enzyme-linked immunosorbent assay, as described elsewhere [17, 18]. Briefly, 96-well plates were coated with rabbit hyperimmune serum containing antibodies to rhesus rotavirus overnight at 4°C and incubated with BLOTTO buffer (5% skim milk in phosphate-buffered saline) and clarified RV1 at 1:10 dilution for 1 hour at 37°C. After washing, serum samples were serially diluted from 1:20 to 1:10 240 in diluent buffer (phosphate-buffered saline supplemented with 1% skim milk and 0.5% [vol/vol] of 10% polyoxthelyene ether W1), added to wells, and incubated for 2 hours at 37°C. After another washing, biotinylated goat anti–human IgA antibodies (KPL) were added, followed by incubation at 37°C for 1 hour, addition of ExtrAvidin (Sigma Aldrich) to the washed plates, and incubation at 37°C for 1 more hour. Substrate was added after washing, and the reaction was stopped with tetramethylbenzidine/hydrochloric acid mixture (KPL). The IgA titer in serum was calculated as the reciprocal of the highest dilution that yielded a mean optical density greater than the cutoff value (5 standard deviations above the mean optical density of the wells containing only BLOTTO buffer). Rotavirus IgA seropositivity at baseline was defined as an IgA titer ≥40 before the first RV1 dose, and rotavirus IgA seroconversion was defined as a ≥4-fold rise in IgA titer from baseline to ≥3 weeks after the second RV1 dose.
Statistical Analysis
IgA response rates and geometric mean titers (GMTs) were calculated with 95% confidence intervals (CIs) at each blood sampling point. Infants were included in the final analysis of rotavirus IgA response if they (1) received both RV1 doses, (2) had both baseline and post–dose 2 IgA titers, and (3) received the second RV1 dose ≥3 weeks before final blood collection. Fisher exact tests, χ2 tests, and 2-sided 95% CIs were used to compare rotavirus IgA seroconversion rates among groups, and nonparametric Mann–Whitney U tests and 2-sided 95% CIs were used to compare rotavirus IgA antibody titers, because the distribution was not normal. Univariate logistic regression was used to obtain odds ratios when comparing rotavirus IgA seroconversion rates. Between-group differences were considered significant at P ≤ .05. SAS 9.3 (SAS Institute) and SPSS 21 (SPSS) software were used for data analysis.
RESULTS
Study Population
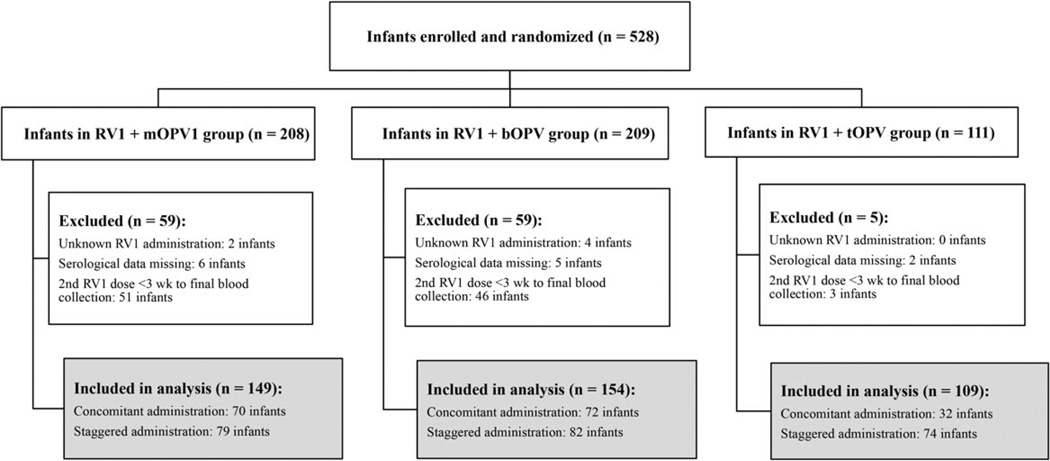
From the 528 infants who received RV1 + mOPV1, RV1 + bOPV, or RV1 + tOPV, we excluded 6 (1%) who received <2 or an unknown number of RV1 doses, 13 (5%) who were missing IgA serological titers, and 100 (19%) who received the second dose of RV1 <3 weeks before the final blood collection (Figure 1). Thus, the final analysis included 409 infants—149 in the RV1 + mOPV1 arm, 154 in the RV1 + bOPV arm, and 106 in the RV1 + tOPV arm.
Figure 1.
Enrolled subjects and final study population. Abbreviations: bOPV, bivalent oral poliovirus vaccine; mOPV1, monovalent oral poliovirus vaccine type 1; RV1, monovalent RV; tOPV, trivalent oral poliovirus vaccine.
There were no statistically significant differences in age, sex, mother’s education, malnutrition, and breastfeeding between study arms (Table 1). The mean rotavirus IgA seropositivity rates at baseline did not differ significantly among study arms; these rates were 32% (95% CI, 24%–39%) in the RV1 + mOPV1, 30% (23%–37%) in the RV1 + bOPV, and 35% (26%–44%) in the RV1 + tOPV arm. There were also no significant differences between the arms in baseline rotavirus IgA GMTs, which were 13 (95% CI, 9–17) in the RV1 + mOPV1, 10 (8–14) in the RV1 + bOPV, and 14 (10–18) in the RV1 + tOPV arm.
Table 1.
Baseline Characteristics of Study Population by Study Arm
| Characteristic | RV1 + mOPV1 (n = 149) | RV1 + bOPV (n = 154) | RV1 + tOPV (n = 106) | Total (N = 409) |
|---|---|---|---|---|
| Sex, No. (%) | ||||
| Male | 70 (47) | 84 (54) | 61 (58) | 215 (53) |
| Female | 79 (53) | 70 (46) | 45 (43) | 194 (47) |
| Baseline age, d | ||||
| Mean (SD) | 45.9 (2.5) | 45.6 (2.4) | 45.3 (2.5) | 45.6 (2.5) |
| Median (range) | 46.0 (42–50) | 45.6 (42–50) | 45.0 (42–50) | 46.0 (42–50) |
| Mother’s educational level <5 y, No. (%) | 66 (44) | 77 (50) | 45 (43) | 188 (46) |
| Malnutrition, No. (%)a | ||||
| Stunting at any study visit | 15 (10) | 21 (14) | 18 (17) | 54 (13) |
| Wasting at any study visit | 60 (40) | 62 (40) | 37 (35) | 159 (39) |
| Full breastfeeding, No. (%) | 149 (100) | 154 (100) | 105 (99) | 408 (99) |
| Rotavirus IgA titer | ||||
| Baseline seropositivity rate (95% CI), % | 32 (24–39) | 30 (23–37) | 35 (26–44) | 32 (27–36) |
| Baseline GMT (95% CI) | 13 (9–17) | 10 (8–14) | 14 (10–19) | 12 (10–14) |
Abbreviations: bOPV, bivalent oral poliovirus vaccine; CI, confidence interval; GMT, geometric mean titer; IgA, immunoglobulin A; mOPV1, monovalent oral poliovirus vaccine type 1; RV1, monovalent rotavirus vaccine; SD, standard deviation; tOPV, trivalent oral poliovirus vaccine.
Length for age and weight for length were compared with the standard distribution of an international reference population recommended by the World Health Organization [16]. Stunting (low height for age) and wasting (low weight for height) were defined as ≤2 standard deviations below the mean for the reference population during any visit [16].
Anti-Rotavirus IgA Response by OPV Type
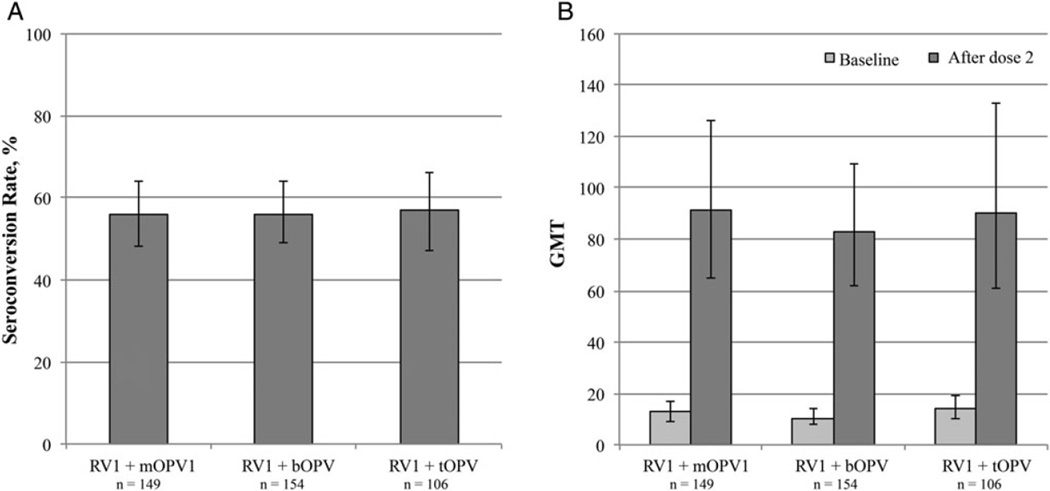
The rotavirus IgA seroconversion rate for the total population was 56% (95% CI, 51%–61%). No significant differences were observed among the study arms, with rates of 56% (95% CI, 48%–64%) in the RV + mOPV1, 56% (49%–64%) in the RV1 +bOPV, and 57% (47%–66%) in the RV1 + tOPV group (Figure 2). Likewise, rotavirus IgA GMTs ≥3 weeks after vaccine administration did not differ significantly among study arms, at 91 (95% CI, 65–126) in the RV + mOPV1, 83 (62–112) in the RV1 + bOPV, and 90 (61–133) in the RV1 + tOPV group.
Figure 2.
Rotavirus immunoglobulin A (IgA) seroconversion and geometric mean titers (GMTs) by study arm. A, Rotavirus IgA seroconversion. Seroconversion was defined as a ≥4 fold rise in IgA titer from baseline to after dose 2. B, Rotavirus IgA GMTs at baseline and after dose 2. Abbreviations: bOPV, bivalent oral poliovirus vaccine; mOPV1, monovalent oral poliovirus vaccine type 1; RV1, monovalent rotavirus vaccine; tOPV, trivalent oral poliovirus vaccine.
Anti-Rotavirus IgA Response by Vaccine Coadministration
Infants who received RV1 and OPV the same day were less likely to seroconvert to rotavirus (81 of 174; 47% [95% CI, 39%–54%]) than those who received RV1 and OPV ≥1 day apart (149 of 235; 63% [57%–70%]; odds ratio, 0.50 [.34–.75]; P < .001) (Table 2). Seroconversion was significantly lower when RV1 and OPV were administered concomitantly than when they were administered ≥1 day apart for RV1 + bOPV (47% vs 65%, respectively; P = .04) and RV1 + tOPV (seroconversion, 41% vs 65%; P = .04) (Table 2).
Table 2.
Rotavirus Immunoglobulin A Seroconversion and Geometric Mean Titers by Concomitant Versus Staggered Oral Poliovirus Vaccine Administrationa
| Study Arm and Timing of RV1 and OPV Administration |
Infants, No. | Rotavirus IgA Seroconversion (95% CI), % |
P Valueb | Rotavirus IgA Titer, GMT (95% CI) |
P Valuec |
|---|---|---|---|---|---|
| Total (n = 409) | |||||
| Concomitant | 174 | 47 (39–54) | .001 | 60 (45–80) | .001 |
| Staggered | 235 | 63 (57–70) | 116 (91–149) | ||
| RV1 + mOPV1 (n = 149) | |||||
| Concomitant | 70 | 49 (37–60) | .10 | 63 (40–102) | .04 |
| Staggered | 79 | 62 (51–73) | 126 (79–195) | ||
| RV1 + bOPV (n = 154) | |||||
| Concomitant | 72 | 47 (36–59) | .04 | 54 (35–83) | .01 |
| Staggered | 82 | 65 (53–75) | 123 (83–182) | ||
| RV1 + tOPV (n = 106) | |||||
| Concomitant | 32 | 41 (24–59) | .04 | 68 (33–135) | .23 |
| Staggered | 74 | 64 (52–74) | 102 (63–162) |
Abbreviations: bOPV, bivalent oral poliovirus vaccine (OPV); CI, confidence interval; GMT, geometric mean titer; IgA, immunoglobulin A; mOPV1, monovalent OPV type 1; RV1, monovalent rotavirus vaccine; tOPV, trivalent OPV.
Seroconversion was defined as a ≥4 fold rise in IgA titer from baseline to post dose 2. Concomitant administration was defined as any RV1 dose given together with any OPV dose. Staggered administration was defined as RV1 and OPV doses given ≥1 day apart.
Based on χ2 tests.
Based on Mann–Whitney tests.
Infants who received RV1 and OPV concomitantly also showed lower rotavirus IgA GMTs ≥3 weeks after the second RV1 dose (GMT, 60; 95% CI, 45–80) than when RV1 and OPV administration was staggered (116; 91–149; P = .001) (Table 2). RV1 IgA GMTs were significantly lower after concomitant administration versus staggered administration in the RV1 + bOPV group (54 vs 123, respectively; P = .01) and the RV1 + mOPV1 group (63 vs 126; P = .04).
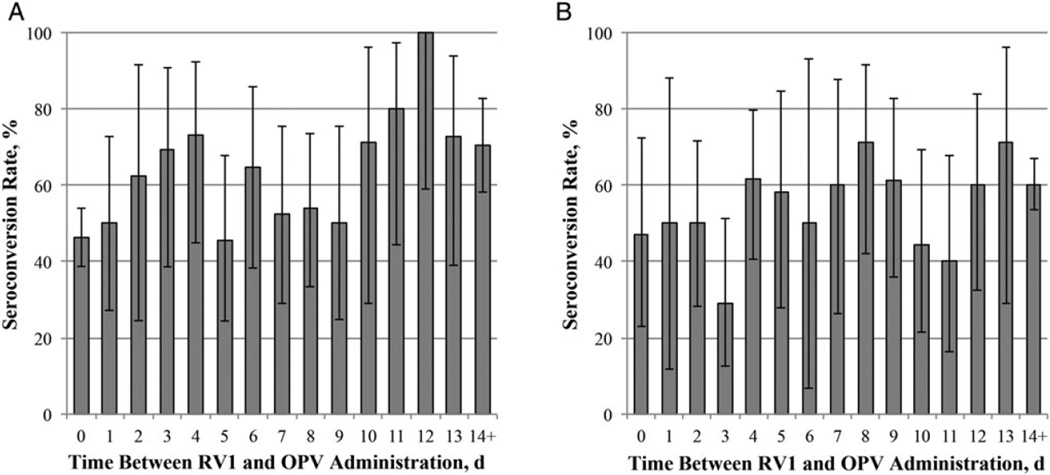
When we considered differences in RV1 immunogenicity by the length of time between vaccine administrations, we observed higher RV1 seroconversion rates as the length of time between vaccine administrations increased (Figure 3). Conversely, infants who seroconverted generally had a longer time between RV1 and OPV administration than those who did not seroconvert (Table 3). However, when we excluded infants who received the vaccine concomitantly, we found no evidence that the length of time between RV1 and OPV administration affected RV1 immunogenicity. These differences were similar for all 3 OPV formulations but were not statistically significant (data not shown).
Figure 3.
Rotavirus immunoglobulin A (IgA) seroconversion rates by length of time between administration of monovalent rotavirus vaccine (RV1) and oral poliovirus vaccine (OPV) (disregarding the order of vaccine administration). A, Seroconversion rates by length of time between the first RV1 and OPV doses. B, Seroconversion rates by length of time between second RV1 and OPV doses.
Table 3.
Rotavirus Immunoglobulin A Seroconversion by Interval Between Administration of Monovalent Rotavirus Vaccine and Oral Poliovirus Vaccinea
| Population and Doses Compared | Rotavirus IgA Seroconversion |
Infants, No. | Interval Between Administration of RV1 and OPV, Median (IQR) d |
P Valueb |
|---|---|---|---|---|
| Total (n = 409) | ||||
| 1st doses of OPV and RV1 | No | 179 | 1 (0–7) | <.001 |
| Yes | 230 | 5 (0–11) | ||
| 2nd doses of OPV and RV1 | No | 179 | 11 (4–18) | .04 |
| Yes | 230 | 14 (7–19) | ||
| Staggered administration only (n = 235) | ||||
| 1st doses of OPV and RV1 | No | 86 | 7 (5–9) | .06 |
| Yes | 149 | 8 (5–13) | ||
| 2nd doses of OPV and RV1 | No | 86 | 12 (9–18) | .11 |
| Yes | 149 | 14 (9–20) |
Abbreviations: IgA, immunoglobulin A; IQR, interquartile range (25%–75%); OPV, oral poliovirus vaccine; RV1, monovalent rotavirus vaccine.
Rotavirus seroconversion and its relationship with the interval between administration of RV1 and OPV were assessed in the total population and among infants who received the vaccines staggered ≥1 day apart for doses 1 and 2 (regardless of which vaccine was administered first).
Based on Mann–Whitney tests.
Anti-Rotavirus IgA Response by Other Factors
When we assessed RV1 IgA seroconversion by other factors that may lower immunogenicity, we found that infants who were IgA seropositive at baseline were less likely to seroconvert than those who were seronegative at baseline (odds ratio, 0.34; 95% CI, .22–.53; P < .001) (Supplement).
DISCUSSION
We observed no differences in rotavirus IgA seroconversion rate and GMTs between mOPV1, bOPV, and tOPV. However, concomitant administration of RV1 with each of the 3 OPV formulations resulted in lower rotavirus IgA responses than staggered administration (≥1 day apart) of the 2 vaccines. These observations indicate that mOPV1, bOPV and tOPV have similar inhibitory effects on RV1, which disagrees with the current hypothesis that the presence of Sabin poliovirus type 2 in OPV is the main driver of vaccine interference with the live attenuated RV [11].
Interestingly, when we excluded infants who received the RV1 and OPV concomitantly, there was no evidence that the length of time between RV1 and OPV administration affected RV1 immunogenicity. This suggests that some early events in the viral replication cycles may primarily contribute to the inhibitory effect of OPV on RV1 immunogenicity. One possible mechanism for this interference may be the competition for receptors between multiple viruses, which can inhibit the efficiency of viral entry into the same cell. In a study by Wang et al [19], mixed astrovirus, enterovirus, and rotavirus infections resulted in reduced rotavirus replication and protein expression compared with single rotavirus infection in vitro, suggesting multiple mechanisms of viral interference, including interference during entry.
Another explanation for this interference may be the result of immune responses against multiple vaccine components. Both cellular immunity (predominantly a T-helper [Th] 1 response) and humoral immunity (predominantly a Th2 response) are necessary for overcoming rotavirus disease, with humoral immunity being the best immune marker for protection [20]. Cytokines are also critical for initiating the adaptive immune response against rotavirus disease and may be important for the initial response against rotavirus infection [21, 22]. In a study looking at the effect of OPV coadministration on live attenuated BCG vaccine, Sartono et al [23] reported reduced Th1 and Th2 cytokine responses when infants received OPV and BCG concomitantly, suggesting that OPV may down-regulate cellular immune responses to other vaccine targets. It is possible that OPVs intrinsically elicits an earlier, stronger or specific immune response that can interfere with the response to RV1.
Our study was subject to several limitations. First, the results presented are from an ad hoc analysis in a subset of participants in a study aimed to evaluate immunogenicity of different OPV formulations; the sample size was not powered for comparisons on rotavirus immunogenicity among study arms. Insufficient study power may also have limited our ability to detect a significant association of the length of time between RV1 and OPV administration with RV1 immunogenicity. Second, our definitions of concomitant and staggered administration were different from those in previous studies, which defined staggered administration as both RV1 doses given ≥2 weeks before or after OPV administration [8–11]. In the current study, most of the concomitant administration occurred with the first RV1 dose compared with the second RV1 dose, with <20 infants receiving both RV1 doses concomitantly with OPV. This limitation, however, allowed us to evaluate the time frame between vaccines administrations and their relationship with rotavirus immunogenicity, although most variability in administration dates was around the first RV1 dose. A potential next step is to conduct a clinical trial to identify the optimal interval between RV1 and bOPV.
A third limitation was that we could measure only rotavirus IgA antibodies in serum, which are used as a marker for protection but are not directly correlated with protection. Therefore, we cannot associate changes in immunogenicity with efficacy [20, 21, 24]. Finally, the constraints in our study design did not allow us to assess other factors that may lower RV1 immunogenicity, including maternal antibodies, concurrent infections, and gut microflora [11, 25–28]. Although we found no effect of malnutrition on rotavirus IgA immunogenicity, the high baseline IgA seropositivity rate in this population, most likely due to prevalent wild-type rotavirus circulating during the study period [29],was a possible confounder in our analysis, coupled with the fact that nearly all infants were breastfed. It would be interesting to evaluate the immune responses to RV5 when given with mOPV1/mOPV3 or mOPVs and bOPV, because tOPV has been associated with lower RV5 IgA titers in settings similar to our study [30].
Several strengths should also be noted. We demonstrated baseline rotavirus IgA titers and seroconversion rates comparable to those in studies assessing RV1 and tOPV coadministration in South Africa, Latin America, urban Bangladesh, and India, despite different assays used to measure rotavirus IgA responses [8–10,31].Interestingly, the post hoc study in India demonstrated slightly higher RV1 immunogenicity among infants receiving RV1 and OPV together, although seroconversion rates were analyzed for infants who were initially seronegative before RV1 vaccination [31]. Conversely, studies in high-income countries looking at RV1 immunogenicity in children receiving inactivated poliovirus vaccine and other childhood vaccines had much higher seroconversion rates and GMTs than in our study [32, 33]; these observations support our hypothesis that OPV interference may be a potential factor in the lower RV1 immunogenicity in low- and middle-income countries.
In conclusion, this post hoc analysis is the first to examine the potential impact of mOPV1 and bOPV on the immune response to RV1. Coadministration of mOPV1, bOPV, or tOPV seems to lower RV1 immunogenicity similarly. The fact that interference seemed greatest when both vaccines were given concomitantly suggests an inhibitory effect of OPV on RV1 in early stages of virus replication, although the mechanism of interference still needs to be elucidated. These findings, however, do not detract from continuing administration of OPV in routine immunization to sustain the gains of polio eradication and expand introduction of RVs to reduce the burden of severe rotavirus disease in low-income countries [1, 34, 35]. Inactivated poliovirus vaccine and new parenteral RVs could also improve the efficacy of the current RVs worldwide.
Supplementary Material
Acknowledgments
We thank all participants in this study.
Financial support. This research was funded by the CDC (grant 00846 to the International Centre for Diarrhoeal Disease Research) and D. M. E. received appointment to the Emerging Infectious Diseases Fellowship Program administered by the Association of Public Health Laboratories.
Footnotes
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Rotavirus vaccines WHO position paper—January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 3.Jiang V, Jiang B, Tate J, Parashar UD, Patel M. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010;6:532–542. doi: 10.4161/hv.6.7.11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares-Weiser K, Maclehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2012;11:1–178. doi: 10.1002/14651858.CD008521.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Rennels MB. Concurrent oral poliovirus and rhesus-human assortant rotavirus vaccination: effects on immune responses to both vaccines and on efficacy of rotavirus vaccines. J Infect Dis. 1996;173:306–313. doi: 10.1093/infdis/173.2.306. [DOI] [PubMed] [Google Scholar]
- 6.Rennels MB. Influence of breast-feeding and oral poliovirus vaccine on immunogenicity and efficacy of rotavirus vaccine. J Infect Dis. 1996;174(suppl 1):S107–S111. doi: 10.1093/infdis/174.supplement_1.s107. [DOI] [PubMed] [Google Scholar]
- 7.Ciarlet M, San-Grosso R, Yuan G, et al. Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral polio vaccine. Pediatr Infect Dis J. 2008;27:874–880. doi: 10.1097/INF.0b013e3181782780. [DOI] [PubMed] [Google Scholar]
- 8.Zaman K, Sack DA, Arifeen SE, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27:1333–1339. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 9.Steele AD, De Vos B, Tumbo J, et al. Co-administration study of South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. 2010;28:6542–6548. doi: 10.1016/j.vaccine.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Tregnaghi MW, Abate HJ, Valencia A, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30:e103–e108. doi: 10.1097/INF.0b013e3182138278. [DOI] [PubMed] [Google Scholar]
- 11.Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine. 2012;30S:A30–A35. doi: 10.1016/j.vaccine.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado YA, Pena-Cruz V, de la Luz Sanchez M, et al. Host and viral factors affecting the decreased immunogenicity of Sabin type 3 vaccine after administration of trivalent oral polio vaccine to rural Mayan children. JID. 1997;175:545–553. doi: 10.1093/infdis/175.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okayasu H, Sutter RW, Czerkinsky C, Ogra PL. Mucosal immunity and poliovirus vaccines: impact on wild poliovirus infection and transmission. Vaccine. 2011;29:8205–8214. doi: 10.1016/j.vaccine.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Rec. 2014;9:73–92. [PubMed] [Google Scholar]
- 15.World Health Organization. Geneva, Switzerland: WHO Press; 2013. Polio eradication & endgame strategic plan 2013–2018. [Google Scholar]
- 16.Estivariz CF, Anand A, Gary HE, Jr, et al. Immunogenicity of three doses of bivalent, trivalent, or type 1 monovalent oral poliovirus vaccines with a 2 week interval between doses in Bangladesh: an open-label, non-inferiority, randomized, control trial. Lancet Infect Dis. 2015;15:898–904. doi: 10.1016/S1473-3099(15)00094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon SS, Wang Y, Shane AL, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010;29:919–923. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groome MJ, Moon SS, Velasquez D, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ. 2014;92:238–245. doi: 10.2471/BLT.13.128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Moon S, Wang Y, Jiang B. Multiple virus infection alters rotavirus replication and expression of cytokines and toll-like receptors in intestinal epithelial cells. Virus Res. 2012;167:48–55. doi: 10.1016/j.virusres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 21.Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol. 2012;2:419–425. doi: 10.1016/j.coviro.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang B, Snipes-Magaldi L, Dennehy P, et al. Cytokines as mediators for or effectors against rotavirus disease in children. Clin Vaccine Immunol. 2003;10:995–1001. doi: 10.1128/CDLI.10.6.995-1001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartono E, Lisse IM, Terveer EM, et al. Oral polio vaccine influences the immune response to BCG vaccination: a natural experiment. PLoS One. 2010;5:e10328. doi: 10.1371/journal.pone.0010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis. 2013;208:284–294. doi: 10.1093/infdis/jit166. [DOI] [PubMed] [Google Scholar]
- 25.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most. J Infect Dis. 2009;200(suppl 1):S39–S48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appaihgari MB, Glass R, Singh S, et al. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine. 2014;32:651–656. doi: 10.1016/j.vaccine.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Velasquez D, Bautista A, Esparza M, et al. Association between breastfeeding and immunogenicity of Rotarix in Mexican infants [abstract W12-8]. Scientific program and abstracts of the 33rd Annual Meeting of the American Society for Virology; American Society for Virology; (Fort Collins). Toledo, OH. 2014. p. 110. [Google Scholar]
- 28.Becker-Dreps SSV, Velasquez D, Moon SS, Hudgens MG, Zambrana LE, Jiang B. Rotavirus specific IgG antibodies from mother’s serum may inhibit infant immune responses to the pentavalent rotavirus vaccine. Pediatr Infect Dis. 2015;34:115–116. doi: 10.1097/INF.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaman K, Yunus MD, Faruque ASG, et al. Surveillance of rotavirus in a rural diarrhoea treatment centre in Bangladesh, 2000–2006. Vaccine. 2009;27S:F31–F34. doi: 10.1016/j.vaccine.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 30.Shin S, Anh DD, Zaman K, et al. Immunogenicity of the pentavalent rotavirus vaccine among infants in two developing countries in Asia, Bangladesh and Vietnam. Vaccine. 2012;30S:A106–A113. doi: 10.1016/j.vaccine.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 31.Narang A, Bose A, Pandit AN, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5:414–419. doi: 10.4161/hv.5.6.8176. [DOI] [PubMed] [Google Scholar]
- 32.Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics. 2008;122:e1062–e1066. doi: 10.1542/peds.2008-1059. [DOI] [PubMed] [Google Scholar]
- 33.Vesikari T, Karvonen A, Prymula R, et al. Immunogenicity and safety of the human rotavirus vaccine Rotarix co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine. 2010;28:5272–5279. doi: 10.1016/j.vaccine.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 34.Tate JE, Patel MM, Steele AD, et al. Global impact of rotavirus vaccines. Expert Rev Vaccines. 2010;9:395–407. doi: 10.1586/erv.10.17. [DOI] [PubMed] [Google Scholar]
- 35.O’Ryan M, Lucero Y, Linhares AC. Rotarix: vaccine performance 6 years postlicensure. Expert Rev Vaccines. 2011;10:1645–1616. doi: 10.1586/erv.11.152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.